To report the prevalence of lymphatic filariasis and intestinal parasitic infections in school-aged children living in a filariasis endemic area and discuss about the therapeutic regimen adopted in Brazil for the large-scale treatment of filariasis.
MethodsA cross-sectional study including 508 students aged 5-18 years old, enrolled in public schools within the city of Olinda, Pernambuco. The presence of intestinal parasites was analyzed using the Hoffman, Pons and Janer method on 3 stool samples. The diagnosis of filarial infection was performed using the rapid immunochromatographic technique (ICT) for the antigen, and the polycarbonate membrane filtration for the presence of microfilariae. Descriptive statistics of the data was performed using EpiInfo version 7.
ResultsThe prevalence of filariasis was 13.8% by ICT and 1.2% by microfilaraemia, while intestinal parasites were detected in 64.2% of cases. Concurrent diagnosis of filariasis and intestinal parasites was 9.4%, while 31.5% of students were parasite-free. Among individuals with intestinal parasites, 55% had one parasite and 45% had more than one parasite. Geohelminths occurred in 72.5% of the parasited individuals. In the group with filarial infection the prevalence of soil-transmitted helminthiasis was 54.5%.
ConclusionsThe simultaneous diagnosis of filariasis and intestinal parasites as well as the high frequency of geohelminths justify the need to reevaluate the treatment strategy used in the Brazilian filariasis large-scale treatment program.
Descrever a prevalência de infecção filarial e de parasitoses intestinais em escolares numa área endêmica de filariose e refletir sobre a opção terapêutica utilizada no Brasil no tratamento coletivo para filariose.
MétodosEstudo transversal envolvendo 508 alunos na faixa etária de 5-18 anos cadastrados em escolas públicas do município de Olinda-PE. Realizou-se a investigação da parasitose intestinal em três amostras de fezes, analisadas pelo método de Hoffmann, Pons e Janer. A investigação filarial foi feita com teste antigênico pela técnica de imunocromatográfica rápida (ICT) e pesquisa de microfilárias, utilizando filtração em membrana de policarbonato. Para análise de dados utilizou-se a estatística descritiva através do programa EpiInfo versão 7.
ResultadosA prevalência de filariose por ICT foi de 13,8% e por microfilaremia de 1,2%, enquanto a de parasitoses intestinais foi 64,2%. A concomitância do diagnóstico filarial e de parasitoses intestinais foi de 9,4% e, 31,5% eram isentos de ambas as parasitoses. Entre os indivíduos com parasitoses intestinais, 55% eram monoparasitados e 45% poliparasitados. A presença de geohelmintos ocorreu em 72,5% dos parasitados. No grupo com infecção filarial a ocorrência de geohelmintíase foi de 54,5%.
ConclusõesO diagnóstico simultâneo de infecção filarial e parasitose intestinal, bem como a elevada frequência de geohelmintos justificam uma reavaliação da estratégia terapêutica do tratamento coletivo no programa de filariose no Brasil.
Neglected diseases (ND) are a set of diseases caused by parasitic agents that lead to significant physical, cognitive and socioeconomic harms in children and adolescents, mainly among low income communities.1 They represent a public health challenge, particularly those, such as filariasis, which impact on morbidity and can cause severe and long term disability.2
The geographic distribution and development of the ND are closely related to poverty in consequence to the scarcity of basic sanitation, and are associated to other health problems.3,4 The World Health Organization (WHO) considers as a public health problem a set of 17 different ND distributed in 148 countries. Of these, 100 are endemic for two or more of these diseases, and six countries for six or more ND.5 Nine of them are present in Brazil2,6 and seven of these diseases are considered as priorities by the Health Ministry (dengue, Chagas disease, leishmaniasis, malaria, schistosomiasis, leprosy and tuberculosis).6 The state of Pernambuco has developed intervention strategies for the reduction and eradication of the following diseases: Chagas disease, leprosy, schistosimiasis, trachoma, lymphatic filariasis, geohelminthiasis and tuberculosis.7
In Brazil, lymphatic filariasis is endemic only in the metropolitan region of Recife, state of Pernambuco.8 The efforts to eradicate this disease must focus on the prevention, the early treatment of infected individuals and the control or stabilization of the morbid complications of the infection.9
Infections with soil-transmitted helminths (geohelminthiasis) impose a great burden on the poor populations worldwide. The WHO considers priorities for large-scale treatment programs the parasitic diseases caused by Ascaris lumbricoides, Ancylostoma duodenale, Necator americanus and Trichuris trichiura.10 Schistosomiasis is another disease that causes harm to the exposed population, and the collective treatment is also considered a control strategy by the WHO.10
There are few studies on the prevalence of intestinal parasitosis in Pernambuco. In 2005 the National Plan for Surveillance and Control of Intestinal Parasitic Diseases identified few studies, conducted with different methods and heterogeneous populations, which showed prevalence ranging from 23.3% and 66.3% among school-aged children or those who attended public health services.11
The control of the diseases caused by helminths, as well as other agents, aims to alleviate suffering, reduce the poverty and to support equal opportunities for men and women.10 Prophylactic chemotherapy represents the main strategy for control of the ND, using the available anti-helminthic drugs, either alone or in combination, to reduce morbidity and the sustained transmission. Since some of these are broad-spectrum drugs that can simultaneously treat several diseases, their use represents an operationally feasible strategy, which is more effective than the individual treatment.10
The transmission of lymphatic filariasis occurs only in three urban areas of the Metropolitan region of Recife (PE): Recife, Jaboatão dos Guararapes and Olinda.12 Recife was the first Brazilian city to join the mass treatment program,13 followed by Olinda in 2005.13 The treatment regimen adopted in Brazil was the collective treatment with a single drug (dietilcarmabazine), not in combination with an anti-helminthic drug (albendazol) which is recommended by the WHO.9
Thus, this study aimed to report the occurrence of intestinal parasitosis and filarial infection in a filariasis endemic area, and to discuss about the therapeutic regimen adopted in Brazil for the collective treatment of filariasis.
MethodsThe study was conducted in the Sapucaia neighborhood of Olinda city (PE). This municipal district is located six kilometers from the state capital and has an area of 37,9km2 (98.13% of it is an urban area), where 377,779 inhabitants live in 123,954 permanent private households and 25,523 in subnormal agglomerates. Regarding the urban infrastructure, 105,546 households are served by the public water supply, 103,398 have their garbage collected by a cleaning service, 45,613 have an exclusive bathroom and sewage system, 102,907 have electric energy provided by an electric power company (with measure system) and 32,370 have a nominal monthly income from one half to one minimum wage.14 We choose the Sapucaia neighborhood because it is a unit of collective treatment of the lymphatic filariasis global eradication program of the municipal district.13
We conducted a cross sectional study. The study population was constituted of 5-18 year old school students enrolled in public schools in the Sapucaia neighborhood from 2009 to 2010. The Secretary of Education provided a listing of the 508 students aged 5-18 years and enrolled in the public municipal schools in the neighborhood. Sample size was calculated based on this population of 508 students; estimating a prevalence of 1.1% microfilaremia for the municipal district, a design effect=1.0, a standard error=1.6% and a 95% confidence interval we obtained n=124. Taking into account the possibility of drop outs, we estimated a sample size of 149 students.
Inclusion criteria were: belonging to the pre-determined age group and agreement of the child and his/her legal guardian to participate on the study by signing an informed consent. In order to enroll a larger number of participants we conducted educational lectures for the teachers, parents and students.
Venous blood samples were drawn from 11 p.m. and 1 a.m. The diagnosis of filariasis was established by immunochromatographic rapid test (ICT Diagnosticx, Binax NOW®) that uses polyclonal and specific monoclonal antibodies15 and by the polycarbonate membrane filtration technique.
For the ICT card test we used 100μL of blood on the specified region of the card, which was closed after approximately 30seconds. The card was read after 10minutes. When the W. bancroft antigen is present in the sample, it is captured by the AD12 monoclonal antibody present in the nitrocellulose strip and depicted as a pink bar. The test was considered positive when two pink lines were identified, and negative when only the control line was displayed.15
The search and quantification of circulating microfilariae were performed using the filtration technique with a 3μm pore polycarbonate membrane. When the results with 1mL blood were negative, further 9mL were subsequently filtered for confirmation of negativity. The membrane was fixed and stained by Carazzi's haematoxylin, and then read by optic microscopy (160x). The filtration technique was considered negative when no microfilaria was identified in 10mL of blood, and positive in the presence of 1 ≥ microfilaria.
For the investigation of intestinal parasites, three stool samples obtained on different days and kept in 10% formaldeid were analyzed using the Hoffmann, Pons and Janer method. The test was considered positive if one or more parasites were found in any of the samples.
Data entry and validation were done in the Epi Info database, version 6.04d, with double input and correction of the identified differences. Analysis of the descriptive statistics (mean and distribution of frequencies) was done with Epi Info version 7.
The study was approved by the Research Ethics Committee of Aggeu Magalhães Research Center/Fiocruz - PE (CAAE 0069.0 095 000-06). The tests results were notified to the children's’ guardians by the schools, and all those with filarial infection or intestinal parasites were given the specific treatment.
ResultsTests for filarial and intestinal parasites were concomitantly performed for 159 children. Mean age was 9.8 years (5-18); 53.4% (85/159) were male and 46.6% (74/159) were female. Intestinal parasites were identified in 64.2% children (102/159), and filariasis was diagnosed in 13.8% by the ICT technique (22/159).
Concurrent filarial and intestinal parasites were diagnosed in 9.4% (15/159) students, and 31.5% (50/159) were free from any parasites. From the total, 87 (54.7%) individuals were positive for intestinal parasites and negative for filariasis, and 7 (4.4%) were negative for intestinal parasites and positive for filariasis.
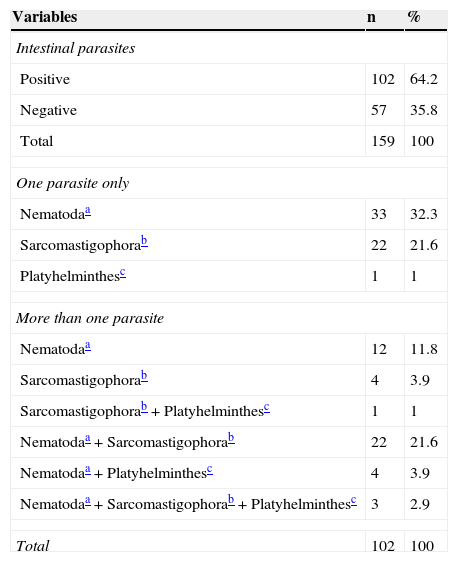
Among the individuals who were positive for intestinal parasites, 45% (46/102) had more than one parasite identified in stools. Geohelminths occurred in 72.5% (74/102), with A. lumbricoides and T. trichiura being the most prevalent parasites. For the Ancilostomatidae, Enterobius vermicularis and Strongyloides stercoralis cases, most of them were observed among the individuals with more than one intestinal parasite. Two cases of Schistosoma mansoni and one of Taenia sp were diagnosed, all of them in individuals with more than one intestinal parasite. The prevalence of intestinal protozoa was 51% (52/102), with Giardia lamblia being the most common among the Sarcomastigophora (Table 1).
Intestinal parasites in children and adolescent students in Olinda, Pernambuco, 2009-2010.
| Variables | n | % |
|---|---|---|
| Intestinal parasites | ||
| Positive | 102 | 64.2 |
| Negative | 57 | 35.8 |
| Total | 159 | 100 |
| One parasite only | ||
| Nematodaa | 33 | 32.3 |
| Sarcomastigophorab | 22 | 21.6 |
| Platyhelminthesc | 1 | 1 |
| More than one parasite | ||
| Nematodaa | 12 | 11.8 |
| Sarcomastigophorab | 4 | 3.9 |
| Sarcomastigophorab+Platyhelminthesc | 1 | 1 |
| Nematodaa+Sarcomastigophorab | 22 | 21.6 |
| Nematodaa+Platyhelminthesc | 4 | 3.9 |
| Nematodaa+Sarcomastigophorab+Platyhelminthesc | 3 | 2.9 |
| Total | 102 | 100 |
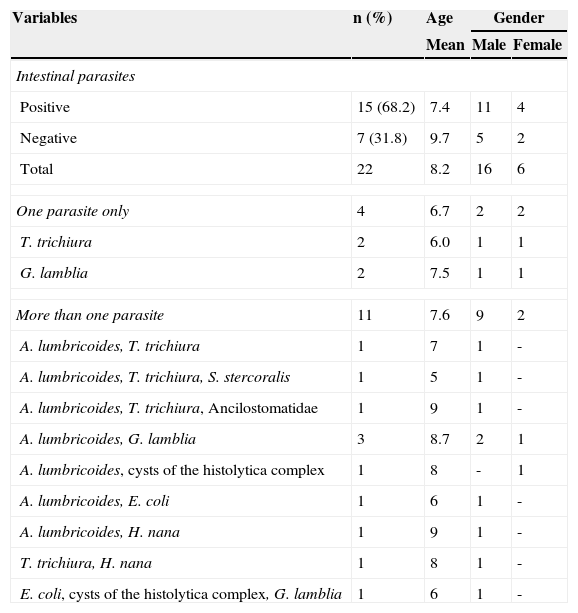
Regarding the diagnosis of filariasis, among the 22 cases diagnosed by the ICT card test, 20 were negative and 2 positive for the search of microfilariae (in 10mL of blood). Thus, the prevalence of microfilaremia was 1.2% (2/159), with parasite quantifications of 5mf/3mL and 25mf/mL. From the 137 children with negative rapid test, 118 had the night filtration test performed, which confirmed the negative results. The analysis of the distribution of filarial infection according to the age and gender showed a greater prevalence among males (Table 2), but the difference was not statistically significant (p>0.05).
Intestinal parasites among children and adolescents with filarial infection diagnosed by the ICT test in Olinda, Pernambuco, 2009-2010.
| Variables | n (%) | Age | Gender | |
|---|---|---|---|---|
| Mean | Male | Female | ||
| Intestinal parasites | ||||
| Positive | 15 (68.2) | 7.4 | 11 | 4 |
| Negative | 7 (31.8) | 9.7 | 5 | 2 |
| Total | 22 | 8.2 | 16 | 6 |
| One parasite only | 4 | 6.7 | 2 | 2 |
| T. trichiura | 2 | 6.0 | 1 | 1 |
| G. lamblia | 2 | 7.5 | 1 | 1 |
| More than one parasite | 11 | 7.6 | 9 | 2 |
| A. lumbricoides, T. trichiura | 1 | 7 | 1 | - |
| A. lumbricoides, T. trichiura, S. stercoralis | 1 | 5 | 1 | - |
| A. lumbricoides, T. trichiura, Ancilostomatidae | 1 | 9 | 1 | - |
| A. lumbricoides, G. lamblia | 3 | 8.7 | 2 | 1 |
| A. lumbricoides, cysts of the histolytica complex | 1 | 8 | - | 1 |
| A. lumbricoides, E. coli | 1 | 6 | 1 | - |
| A. lumbricoides, H. nana | 1 | 9 | 1 | - |
| T. trichiura, H. nana | 1 | 8 | 1 | - |
| E. coli, cysts of the histolytica complex, G. lamblia | 1 | 6 | 1 | - |
Table 2 shows the frequency and distribution of the intestinal parasites identified among children with positive filarial rapid test (ICT card test). Geohelminths were the most prevalent parasites with 54.5% (12/22), with A. lumbricoidis being the most frequent one. Cases of Ancilostomidae and S. stercoralis were only observed among those individuals with more than one intestinal parasite. No cases of E. vermicularis, S. mansoni or Taenia sp were diagnosed. The prevalence of intestinal protozoa among these children was 36.4% (8/22), and Giardia lamblia was the most common among the Sarcomastigophora.
DiscussionConcurrent infection with both lymphatic filariasis and intestinal parasites was observed in nearly 10% students. The evaluation of the association between intestinal helminths and filarial infection in the studied area is nevertheless limited because, in addition to the low frequency of filarial infection, the distribution of both diseases is different within the age groups: while the prevalence and intensity of A. lumbricoides and T. trichiura tend to increase among pre-school children, peak among school-aged children and to decline in adulthood, the highest frequency of lymphatic filariasis is observed among adults.4 The difficulties in establishing comparisons among the studies conducted in different countries on the frequency of lymphatic filariasis and helminthiasis has been previously reported, and justified due to the different epidemiological methods used by the researchers.4
Lymphatic filariasis and intestinal helminthic infections are two of the seven most prevalent ND among the chronic infections in the world.16 Moreover, it is generally acknowledged that the ND do not occur in isolation. Geographical overlap of populations multi-infested with one or more soil-transmitted helminths, schistosomiasis and filarial worms has been reported in many countries,17,18 particularly among the poorest populations,19 a situation that adversely affects the growth and physical fitness in childhood.
The prevalence of intestinal parasites was high (64.2%), and poliparasitism was observed in 45% of cases. Helminths were the most frequent observed parasites, which confirms that the geohelminthiasis still represent a significant health problem.20 Among the helminths, the greatest prevalence were observed for A. lumbricoides and T. trichiura, similarly to what has been shown in other studies conducted in Brazil.20,21
The frequency of filarial infection when analyzed only by the search for microfilariae in blood was low (1.2%); when associated with the ICT it improved to 13.8%. The use of the former technique as the only diagnostic tool for detection of filarial infection can result in the under diagnosis of individuals with low parasitic load (as observed in children), and also of those who are infected but who show no filariae in the blood (asymptomatic infection),22 but have the potential to contribute to further transmission. The ICT test is more sensitive than the search for microfilariae in the blood and can detect the adult forms of W. bancrofti. It is currently indicated as the diagnostic method of choice for mapping the distribution, as well as to check on the eradication of lymphatic filariasis.15
The age group analyzed has been historically considered as a group with lower infection rates than those of young adults,23 which is attributed to the limitation of the techniques previously used (direct search of microfilariae in blood) and to the subclinical manifestations in the initial phases of the infection, which leads to difficulties in the identification and to subsequent under-representation of children in epidemiological studies.23 Furthermore, although the collective treatment of the disease had not been implemented by the Health Secretary of Olinda by the time of the study, the individual investigation and treatment were already being conducted, which may have contributed to the low frequency of filarial infection the area.
Due to the geographic overlapping of endemic diseases distribution, combined strategies of eradication or control of ND have been proposed, particularly for countries of the sub-Saharan Africa, using a combination of drugs with therapeutic effect against multiple parasites.24
In 1997 the WHO launched a global program for the eradication of filariasis as a public health issue up to 2020.25 One of the elements of the strategy consists in blocking the transmission by collectively treating all the populations living in risk zones. The treatment schedule proposed by the WHO, with the association of two drugs (dietilcarbamazine and albendazol) assures a wide spectrum combination for the treatment of lymphatic filiariasis and intestinal geohelminths, which have been recognized as co-endemic diseases. This strategy simplifies the treatment, improves adhesion and can be easily adopted by the existing public health services without overloading them.26
In co-endemic communities, programs for lymphatic filariasis control that use combined therapy have resulted in greater treatment adhesion than a single drug therapy, due to the most obvious benefits, such as the visible elimination of A. lumbricoidis worms.27 A systematic review that analyzed the use of albendazol for the treatment and control of lymphatic filariasis concluded that the effect of this drug on filarial parasites needs further investigation; however, it was observed that other health benefits consequent to the use of albendazol can improve the adhesion to the collective treatment of filariasis.28 Also, several mass treatment programs that included albendazol for the control of lymphatic filariasis have shown that this inclusion results in a significant and continuous decline in the prevalence of helminthic infection.29
Side effects that could preclude the association of albendazol to the treatment regimen, such as intestinal occlusion, have not been reported. Furthermore, there is no evidence of increased side effects when the association with albendazol is compared with the treatment with dietilcarbamazine alone. In countries such as Indonesia, where the prevalence of intestinal helminths infection is high, the use of the combination of dietilcarbamazine plus albendazol in the program for control of lymphatic filariasis resulted in a supplemental impact on the program for control of intestinal helminthic infections.30
In conclusion, the results of this study confirm the association between intestinal geohelminths and lymphatic filariasis infections, which may result in the reevaluation by the Health Secretaries of the Metropolitan Region of Recife and the Health Ministry on the association of albendazol with dietilcarbazamine in the areas where the mass treatment is to be implemented, as a combined strategy for the control of both endemic diseases.
FundingConselho Nacional de Desenvolvimento Científico e Tecnológico (process no: 476336/2008-2).
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Aguiar-Santos AM, Medeiros Z, Bonfim C, Rocha AC, Brandão E, Miranda T, et al. Epidemiological assessment of neglected diseases in children: lymphatic filiriasis and soil-transmitted helminthiasis. J Pediatr (Rio J). 2013;89:250–5.