To present the currently available evidence of the effects of congenital Zika virus infection on infant growth, to discuss possible intervening factors, and to describe preliminary data on this growth in a cohort of exposed children.
Source of dataNon-systematic review in PubMed, BVS, CAPES, Scopus, Web of Science, Cochrane and Google Scholar databases in the last 5 years, using the terms infection/disease by Zika virus and growth/nutrition/nutritional status/infant nutrition and nutritional needs. Additionally, the anthropometric data of the first 2.5 years of a cohort of children exposed to the Zika virus during pregnancy were reviewed.
Synthesis of dataBoth intrauterine growth restriction and low birth weight were reported in series of cases of children with congenital Zika syndrome. The postnatal growth deficit of these children appears to be directly proportional to the degree of neurological impairment. The etiology is multifactorial, and nutritional and non-nutritional factors are probably involved. The data from the present cohort show that the head circumference evolution depends on this measurement at birth and that weight-height growth has a trend toward lower weight and length in children with congenital microcephaly and normocephalic at birth who develop some neurological abnormality.
ConclusionsThe few existing data suggest that, in children with congenital Zika, the greater the degree of neurological impairment, the greater the impact on growth, whether or not associated with microcephaly at birth.
Apresentar as evidências atualmente disponíveis das repercussões da infecção congênita pelo vírus Zika no crescimento infantil, discutir possíveis fatores intervenientes e descrever dados preliminares desse crescimento em uma coorte de crianças expostas.
Fonte dos dadosRevisão não sistemática nos portais de banco de dados PubMed, BVS, Capes, Scopus, Web of Science, Cochrane e Google Scholar nos últimos cinco anos, com o uso dos termos infecção/doença pelo vírus Zika e crescimento/nutrição/status nutricional/nutrição infantil e necessidades nutricionais. Além disso, foram revistos os dados antropométricos dos primeiros dois anos e meio de uma coorte de crianças expostas ao vírus Zika durante a gestação.
Síntese dos dadosTanto a restrição do crescimento intrauterino como o baixo peso ao nascer têm sido relatados em séries de casos de crianças com síndrome de Zika congênita. O déficit de crescimento pós-natal dessas crianças parece ser diretamente proporcional ao grau de comprometimento neurológico. A etiologia é multifatorial com fatores nutricionais e não nutricionais provavelmente envolvidos. Os dados de nossa coorte mostram que a evolução do perímetro cefálico é dependente do valor dessa medida ao nascimento e que o crescimento pondero-estatural apresenta uma tendência de menor peso e comprimento em crianças com microcefalia congênita e normocefálicas ao nascimento, mas com alguma anormalidade neurológica evolutiva.
ConclusõesOs poucos dados existentes sugerem que em crianças com Zika congênita, o impacto sobre o crescimento será tanto maior quanto maior for o grau de comprometimento neurológico, associado ou não à microcefalia ao nascimento.
In October 2015, the Brazilian Ministry of Health notified the World Health Organization (WHO) of a significant increase in cases of congenital microcephaly in northeastern Brazil, in association with the epidemic of Zika virus infection in the region.1 In February 2016, the WHO declared this association a Public Health Emergency of International Concern.2
Evidence has been accumulating in favor of the association between Zika virus infection during pregnancy and the development of congenital microcephaly and other neurological abnormalities in newborns.3–5 Public health authorities in Europe and the United States have warned of the risk of worldwide dissemination of the Zika virus and the importance of increased surveillance, as well as disease prevention and control measures.6
While microcephaly was the most striking clinical sign for the monitoring of this group of children, it is now believed that the spectrum of congenital infections by Zika virus can reach far beyond microcephaly, requiring special attention from pediatricians and the health care teams.7,8
Children with microcephaly and chronic non-progressive encephalopathy, as observed in congenital Zika syndrome (CZS), usually grow less, having shorter stature and weighing less than their healthy peers of the same age.9 The etiology of this growth deficit is multifactorial and may be related to both nutritional and non-nutritional factors, secondary to brain malformations.9
Regardless of microcephaly status, reports on the growth of children exposed to the Zika virus during pregnancy are still scarce. To date, studies have given more emphasis to their development. Thus, the aim of this review was to present the currently available evidence of the effects of congenital Zika virus infection on infant growth, to discuss possible intervening factors, and to describe preliminary data on this growth in a cohort of exposed children.
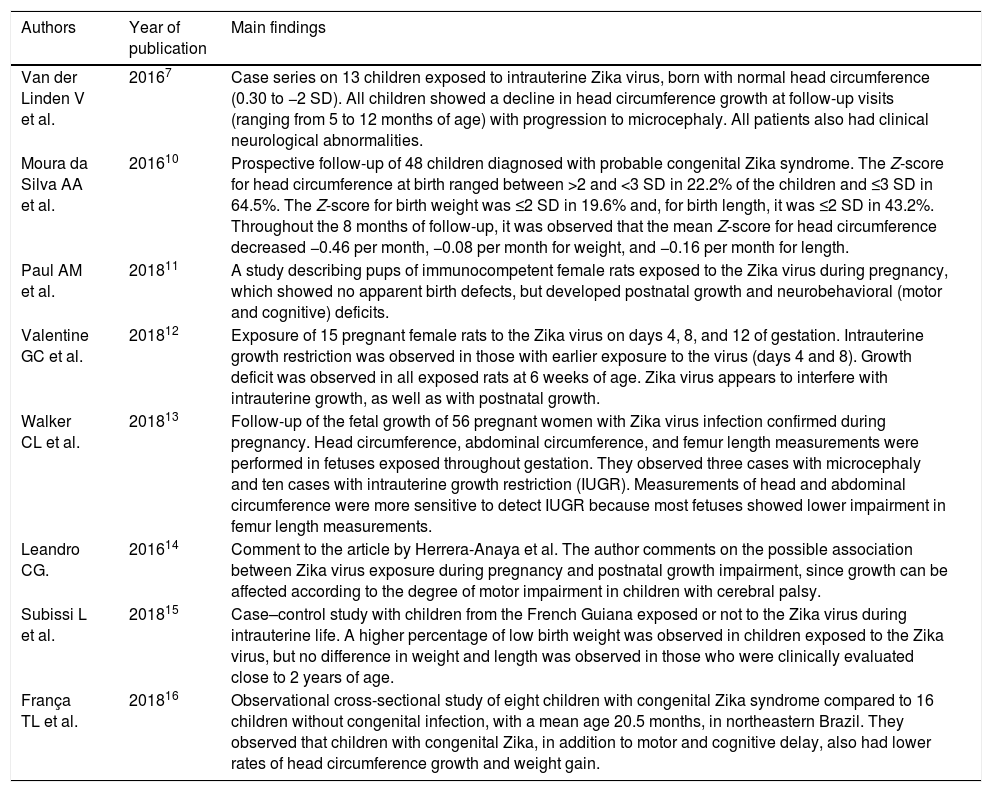
Source of dataA non-systematic review was carried out at PubMed, BVS, CAPES, Scopus, Web of Science, and Cochrane databases, comprising the last 5 years, using the terms (“Zika Virus Infection”, “ZikV Infection”, “Zika Virus Disease”) and (“Growth”, “Failure to Thrive”, “Growth and Development”, “Nutrition Assessment”, “Nutritional Status”, “Deficiency Diseases”, “Child Nutrition”, “Infant Nutrition”, “Recommended Dietary Allowances”, “Diet, Food and Nutrition”, “Nutritional Requirements”, “Nutrition Status”, “Nutrition Assessment”). The search was also carried out in Google Scholar. A total of 182 articles were found. After careful evaluation by two authors (MMM and ABG), based on their association to the proposed theme, 171 articles were excluded after reading the title and six after reading the abstract; thus, a total of eight articles that analyzed the growth of children exposed to intrauterine infection by the Zika virus were (Table 1).7,10–16
Selected scientific articles that addressed the growth of children exposed to intrauterine infection by Zika virus.
| Authors | Year of publication | Main findings |
|---|---|---|
| Van der Linden V et al. | 20167 | Case series on 13 children exposed to intrauterine Zika virus, born with normal head circumference (0.30 to −2 SD). All children showed a decline in head circumference growth at follow-up visits (ranging from 5 to 12 months of age) with progression to microcephaly. All patients also had clinical neurological abnormalities. |
| Moura da Silva AA et al. | 201610 | Prospective follow-up of 48 children diagnosed with probable congenital Zika syndrome. The Z-score for head circumference at birth ranged between >2 and <3 SD in 22.2% of the children and ≤3 SD in 64.5%. The Z-score for birth weight was ≤2 SD in 19.6% and, for birth length, it was ≤2 SD in 43.2%. Throughout the 8 months of follow-up, it was observed that the mean Z-score for head circumference decreased −0.46 per month, −0.08 per month for weight, and −0.16 per month for length. |
| Paul AM et al. | 201811 | A study describing pups of immunocompetent female rats exposed to the Zika virus during pregnancy, which showed no apparent birth defects, but developed postnatal growth and neurobehavioral (motor and cognitive) deficits. |
| Valentine GC et al. | 201812 | Exposure of 15 pregnant female rats to the Zika virus on days 4, 8, and 12 of gestation. Intrauterine growth restriction was observed in those with earlier exposure to the virus (days 4 and 8). Growth deficit was observed in all exposed rats at 6 weeks of age. Zika virus appears to interfere with intrauterine growth, as well as with postnatal growth. |
| Walker CL et al. | 201813 | Follow-up of the fetal growth of 56 pregnant women with Zika virus infection confirmed during pregnancy. Head circumference, abdominal circumference, and femur length measurements were performed in fetuses exposed throughout gestation. They observed three cases with microcephaly and ten cases with intrauterine growth restriction (IUGR). Measurements of head and abdominal circumference were more sensitive to detect IUGR because most fetuses showed lower impairment in femur length measurements. |
| Leandro CG. | 201614 | Comment to the article by Herrera-Anaya et al. The author comments on the possible association between Zika virus exposure during pregnancy and postnatal growth impairment, since growth can be affected according to the degree of motor impairment in children with cerebral palsy. |
| Subissi L et al. | 201815 | Case–control study with children from the French Guiana exposed or not to the Zika virus during intrauterine life. A higher percentage of low birth weight was observed in children exposed to the Zika virus, but no difference in weight and length was observed in those who were clinically evaluated close to 2 years of age. |
| França TL et al. | 201816 | Observational cross-sectional study of eight children with congenital Zika syndrome compared to 16 children without congenital infection, with a mean age 20.5 months, in northeastern Brazil. They observed that children with congenital Zika, in addition to motor and cognitive delay, also had lower rates of head circumference growth and weight gain. |
Additionally, the anthropometric data of a cohort of children followed-up at the Follow-up Outpatient Clinic at the UFRJ School Maternity Unit were reviewed as part of a research protocol approved by the institution's Research Ethics Committee (REC) under No. 1,541,109 (CAAE 55465616.0.0000.5275). In this cohort, all participating children were found to be exposed to the Zika virus during pregnancy, based on: (1) positive result of polymerase chain reaction (PCR) test for Zika virus in maternal blood; (2) presence of positive IgM for Zika virus in maternal blood, or (3) probable congenital infection with Zika virus, according to the clinical-epidemiological criteria of the Brazilian Ministry of Health.
These patients were followed-up from birth to 2.5 years of age, from February 2016 to August 2018. At each consultation, weight, length, and head circumference measurements were performed by a previously trained team. A calibrated digital scale, stadiometer, and non-extensible measuring tape were used for measuring the head circumference. The measured values were entered in the WHO charts according to age and gender. This cohort was divided into three distinct groups: (1) microcephalic, (2) normocephalic, with some neurological abnormality detected during the follow-up, (3) normocephalic, without neurological abnormalities.
Synthesis of data and discussionCongenital Zika syndromeSmall case series have described some congenital and postnatal characteristics of intrauterine infection by the Zika virus. In addition to microcephaly, this group of children may present intrauterine growth retardation, reduced brain volume, brain malformations (especially subcortical calcifications, ventriculomegaly and cortical migration defects), craniofacial disproportion, skin accumulation in the occipital region, hyperexcitability, hypertonia, irritability, epilepsy, dysphagia, and arthrogryposis.17–19
During the evolution follow-up, there is a delay in the acquisition of developmental milestones, with difficulty in sustaining cervical and appendicular hypertonia. The persistence of primitive reflexes is also observed, in addition to pyramidal (hypertonia, hyperreflexia, clonus, and persistence of primitive reflexes), and extrapyramidal (fluctuating tonus and symmetrical dyskinesia of the extremities during wakefulness) signs and symptoms10,20 in children with and without microcephaly at birth.7,8 Ocular abnormalities, such as the presence of pigmented macules, chorioretinal atrophy in the macular region, and loss of visual function, probably associated with cortical visual loss, have also been described in 21–55% of the cases.21–23
Although they have an important impact, these changes reported as part of CZS are found mainly in children who have microcephaly at birth. However, there has been an increasing interest by researchers and healthcare teams regarding the consequences of congenital Zika infection in non-microcephalic newborns, and the first reports on the evolution of this group are beginning to appear.7,8 Animal studies have demonstrated the occurrence of growth and developmental changes in oligosymptomatic animals at birth,11 which appears to be associated with the gestational period during which the infection occurred.12
Intrauterine growthIntrauterine growth restriction, represented by fetal weight <10th percentile in the standard curves used, has been observed in approximately 10–18% of the pregnancies that report infection of the pregnant woman by the Zika virus.13,24 In these cases, ultrasonography studies present a pattern of head and abdominal circumference reduction, with relative preservation of the femoral length, leading to a disproportion in the head circumference/femoral length and abdominal circumference/femoral length ratios (<10th percentile).13
Regarding cerebral growth impairment, which leads to microcephaly, reports have shown that the earlier in the pregnancy the maternal infection occurs, the greater the risk of microcephaly.24 Sophisticated bench studies using mini-brains originated from induced pluripotent stem cells (iPS), infected early with Zika virus, demonstrated the great destructive power of the virus on the formation of neural spheres and cortical neural precursors.25,26
Birth weightThe case series of children exposed to Zika virus during pregnancy show that the prevalence of low birth weight and of infants born small for gestational age may be twice the usual rate.10,24,27,28 Children with low birth weight or those who are small for gestational age usually achieve lower weight, height, and head circumference during the growth process, in addition to having less lean mass and a higher percentage of body fat mass.14,29
Postnatal growth and intervening factorsIn a cohort of 45 children with probable congenital Zika syndrome, Moura da Silva et al.10 observed short length in 43.2% and low weight in 19.6% of newborns as early as at birth. Moreover, during the prospective follow-up of these children, the latter maintained short length and low weight at 8 months of age.
Poor dietary intake is one of the main factors leading to inadequate nutritional status in children with some type of neurological impairment.9 Only 20% of these children receive adequate caloric intake. The energy requirements of this group of children is associated with body composition, motor impairment degree, and physical activity level, including rehabilitation, which may increase the daily energy requirements.30 These patients usually require more energy for ambulation, while those who do not ambulate require approximately 60–70% of the energy needs when compared to children without neurological impairment of the same age group and gender.31 In general, multiple factors can affect the adequate dietary intake of these children, even with different degrees of neurological impairment. Sensory factors related to the texture and taste of food may lead to a limited consumption of certain food groups. Fatigue is another contributing factor to an inadequate food intake, both the fatigue that can be observed before meals and that which occurs due to the effort during the feeding process of these patients.32 Meals that last a very long time result in stress and fatigue to both the child and the parents, leading to negative behaviors being associated with feeding moments. Other factors include disturbances in the feeling of hunger and satiety, inability to communicate about one's nutritional needs, dental caries, dental malocclusion, and secondary conditions such as gastroesophageal reflux and constipation.33 Micronutrient deficiencies (calcium, iron, zinc, selenium, and vitamins C, D, and E) are also common in these children with neurological impairment and may contribute to growth deficit.34–36
Other non-nutritional factors may affect the weight-height growth of children with CZS-associated microcephaly. Children with intellectual disability are at increased risk of malnutrition and growth deficits, especially when the intellectual disability is associated with cerebral palsy; this association is observed in approximately 50% of cases. Obesity may also be a nutritional problem in this population, especially in those with low physical activity and who do not have swallowing dysfunction.37
The use of antiepileptic drugs, common in children with microcephaly due to CZS, is also associated with weight gain, and can be observed in up to 57% of patients with chronic use of valproic acid, probably due to metabolic changes due to the decrease in the beta-oxidation of fatty acids and increase in insulin and leptin levels.38 In turn, weight loss is commonly observed with chronic use of topiramate, possibly involving a reduction in the caloric intake, hormonal involvement (especially adiponectin), and changes in lipid and glucose metabolism.39
Growth curvesThe Brazilian Ministry of Health recommends monitoring the growth of children with congenital Zika virus infection by using the growth curves of The International Fetal and Newborn Growth Consortium for the 21st Century (Intergrowth 21st) for preterm infants up to 64 weeks of corrected gestational age and the WHO growth curves for children born full-term and post-term.40,41 The equations proposed by Stevenson allow the evaluation of stature in children aged 2–12 years who are unable to stand upright.42
Weight-length and head circumference growth in a cohort exposed to intrauterine infection by Zika virusDue to the lack of data in the literature on the growth of children whose mothers were at risk for Zika virus infection during pregnancy, the authors present the evolution of the anthropometric data of a cohort of 29 patients divided as follows: (a) five children with congenital microcephaly and severe neurological impairment; (b) 11 children normocephalic at birth, with some mild neurological abnormality detected during follow-up; and (c) 13 children normocephalic at birth, with no neurological abnormalities detected during follow-up (Table 2).
Evolution of a cohort of 29 patients whose mothers were proven to have been exposed to the Zika virus during pregnancy.
| Case | HC at birth | GA at the infection (weeks) | GA at birth (weeks) | Weight at birth (g) | Length at birth (cm) | HC at birth (cm) | Apgar 5th minute | Z-score for growth | Neuroimaging (findings) | Development (Gesell) | Treatment |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Microcephalic | 10 | 39 | 2690 | 47 | 30 | 9 | Weight: −1.23 to −0.86 Length: −1.15 to −0.43 HC: -3.53 to −4.43 | Findings “a” | Important overall developmental delay | Physical therapy, speech therapy, and stimulation. Use of clonazepam and topiramate. |
| 2 | Microcephalic | 12 | 38+1 | 3140 | 45 | 29.5 | 9 | Weight: −0.52 to −1.29 Length: −2.58 to −1.68 HC: −3.91 to −4.81 | Findings “b” | Important overall developmental delay | Physical therapy, speech therapy, and stimulation. |
| 3 | Microcephalic | No symptoms | 39 | 3570 | 48.5 | 31.5 | 9 | Weight: −0.51 to −0.98 Length: −0.73 to −1.38 HC: −2.33 to −3.15 | Findings “c” | Important overall developmental delay | Physical therapy, speech therapy, and stimulation. |
| 4 | Microcephalic | No symptoms | 37+1 | 2110 | 43 | 27.5 | 9 | Weight: −2.95 to −2.06 Length: −3.64 to −1.73 HC: −5.48 to −5.46 | Findings “d” | Important overall developmental delay | Physical therapy, speech therapy, and stimulation. |
| 5 | Microcephalic | 12 | 38+3 | 2005 | 40 | 29 | 9 | Weight: −3.09 to −4.74 Length: −4.37 to −3.10 HC: −4.12 to −6.48 | Findings “e” | Important overall developmental delay | Physical therapy, speech therapy, and stimulation. |
| 6 | Normocephalic | 25 | 31+3 | 1620 | 38.5 | 28.5 | 9 | Weight: −0.02 to −1.26 Length: −1.21 to −3.83 HC: −0.27 to −1.64 | Normal | Adaptive, fine motor and language delay | Physical therapy, speech therapy, and stimulation. |
| 7 | Normocephalic | 38+5 | 39 | 3395 | 51 | 35 | 9 | Weight: 0.36 to 1.17 Length: 0.99 to 1.01 HC: 0.95 to −0.17 | Normal | Adaptive, fine motor language and personal-social delay | Physical therapy, speech therapy, and stimulation. |
| 8 | Normocephalic (twin 1) | 21 | 32 | 1905 | 44 | 30 | 8 | Weight: 0.74 to 0.92 Length: 0.78 to 1.23 HC: 0.51 to 0.23 | Normal | Language delay | Speech therapy and stimulation. |
| 9 | Normocephalic (twin 2) | 21 | 32 | 1650 | 40.5 | 31.5 | 8 | Weight: −0.88 to 0.02 Length: −1.05 to −0.55 HC: 0.59 to 1.48 | Normal | Language delay | Speech therapy and stimulation. |
| 10 | Normocephalic | 21 | 39+1 | 3540 | 48.5 | 34 | 9 | Weight: 0.31 to 0.83 Length: −0.73 to 0.40 HC: −0.36 to 0.29 | Normal | Adaptive, fine motor language and Personal-Social delay | Physical therapy, speech therapy and stimulation. |
| 11 | Normocephalic | 12+5 | 40+4 | 3570 | 49 | 36 | 9 | Weight: 1.18 to 0.55 Length: −0.08 to 0.74 HC: 1.79 to 1.68 | Subcortical calcification in frontal and parietal lobes | Normal | Stimulation. |
| 12 | Normocephalic | 37+3 | 39 | 2995 | 47.5 | 35 | 9 | Weight: −0.73 to −0.31 Length: −1.26 to −0.43 HC: 0.42 to −0.31 | Normal | Fine motor delay | Physical therapy and stimulation. |
| 13 | Normocephalic | 34 | 39 | 2870 | 46 | 35 | 9 | Weight: −0.75 to −0.99 Length: −1.69 to −0.85 HC: −0.99 to −1.04 | Myelination is more evident than usual | Normal | Stimulation. |
| 14 | Normocephalic | 8 | 38+4 | 2785 | 48 | 34 | 7 | Weight: −0.75 to −0.39 Length: −0.62 to −0.20 HC: 0.10 to 0.56 | Slight increase in the frontal CSF space with mild ectasia of lateral ventricles | Language delay | Speech therapy and stimulation. |
| 15 | Normocephalic | 16+2 | 39+4 | 3080 | 49.5 | 33 | 10 | Weight: −0.29 to 1.06 Length: 0.19 to 1.63 HC: 3.48 to 2.70 | Normal | Normal | Stimulation. |
| 16 | Normocephalic | 35 | 39 | 3340 | 50 | 33.5 | 9 | Weight: 0.15 to −0.57 Length: 0.46 to −0.92 HC: −0.32 to −0.08 | Discrete prominent perivascular spaces in peritrigonal regions | Normal | Stimulation. |
| 17 | Normocephalic | 28+4 | 38+2 | 2620 | 47 | 34 | 9 | Weight: −1.655 to −0.67 Length: −1.52 to −0.25 HC: −0.36 to −0.50 | Normal | Normal | Stimulation. |
| 18 | Normocephalic | 26+6 | 37 | 2855 | 48.5 | 33.5 | 9 | Weight: −0.75 to −1.68 Length: −0.35 to −0.91 HC: −0.32 to −0.43 | Normal | Normal | Stimulation. |
| 19 | Normocephalic | 21 | 40+4 | 3350 | 50.5 | 34.5 | 9 | Weight: 0.36 to 1.50 Length: 0.73 to 1.00 HC: 0.52 to 2.43 | Not performed | Normal | Stimulation. |
| 20 | Normocephalic | 5 | 40+4 | 3750 | 51 | 35 | 8 | Weight: 1.18 to 1.40 Length: 0.99 to 1.34 HC: 0.95 to 1.66 | Not performed | Normal | Stimulation. |
| 21 | Normocephalic | 9 | 40+1 | 3535 | 50.5 | 34.5 | 9 | Weight: 0.78 to 0.42 Length: 0.73 to 0.33 HC: 0.52 to 1.4 | Normal | Normal | Stimulation. |
| 22 | Normocephalic | 9 | 38 | 2160 | 43.4 | 31.7 | 9 | Weight: −2.53 to −0.31 Length: −3.09 to −1.86 HC: −1.84 to 1.24 | Normal | Normal | Stimulation. |
| 23 | Normocephalic | 9 | 39+5 | 3490 | 48 | 34 | 9 | Weight: 0.57 to 0.56 Length: −0.62 to −0.07 HC: 0.10 to 1.50 | Normal | Normal | Stimulation. |
| 24 | Normocephalic | 19+4 | 38+4 | 3055 | 47.5 | 34 | 9 | Weight: −0.29 to −0.14 Length: −0.88 to 0.42 HC: 0.10 to 0.93 | Not performed | Normal | Stimulation. |
| 25 | Normocephalic | 18 | S/I | 4130 | 52 | 36 | 9 | Weight: 1.76 to 1.35 Length: 1.53 to 1.97 HC: 1.79 to 2.43 | Normal | Normal | Stimulation. |
| 26 | Normocephalic | 11+4 | 36+3 | 2990 | 46.5 | 33 | 9 | Weight: −0.52 to 1.50 Length: −1.42 to 0.59 HC: −0.74 to 1.13 | Normal | Normal | Stimulation. |
| 27 | Normocephalic | 10 | 38+3 | 3220 | 48 | 35 | 9 | Weight: 0.15 to 0.33 Length: −0.62 to 0.72 HC: 0.95 to 0.18 | Normal | Normal | Stimulation. |
| 28 | Normocephalic | 16 | 41+1 | 3850 | 46.7 | 35.7 | 9 | Weight: 1.08 to 0.37 Length: −1.68 to −0.28 HC: 0.97 to 1.20 | Normal | Stimulation. | |
| 29 | Normocephalic | 12 | 37+6 | 3200 | 48 | 36 | 9 | Weight: −0.30 to 1.86 Length: −1.00 to 0.75 HC: 1.21 to 2.56 | Normal | Normal | Stimulation. |
HC, head circumference; GA, gestational age; Neuroimaging (findings): a) frontal oligogyria, frontal subcortical calcifications, corpus callosum hypoplasia, ventriculomegaly; b) oligogyria, reduction of white matter volume, frontal, parietal, occipital, and temporal subcortical calcifications, cystic images near the lateral ventricle walls, corpus callosum hypoplasia, discrete reduction of the brainstem volume, marked supratentorial ventriculomegaly, increased subarachnoid space; c) underdeveloped brain, oligogyria, polymicrogyria, cortical-subcortical calcifications of the frontal and parietal lobes, subependymal cystic formations in the temporal poles, occipital and frontal regions, corpus callosum, brainstem and cerebellum hypoplasia, ventriculomegaly; (d) oligogyria, reduction of the white matter volume, cortical-subcortical calcifications around the lateral ventricles and in the lentiform nuclei, corpus callosum hypoplasia, malacia along the lateral horn of the lateral ventricles, volumetric reduction of the brainstem, ventriculomegaly; e) oligogyria, global reduction of white matter volume, parenchymal calcifications, with predominance of the subcortical region of the cerebral hemispheres, corpus callosum hypoplasia, reduced hippocampus, ventriculomegaly; f) questionable reduction of the geniculocalcarine tract thickness, presence of small cysts in the right choroid plexus, the two largest ones measuring 1.1 × 0.8cm (ventricular atrium) and 0.5 × 0.3cm (ventricular body).
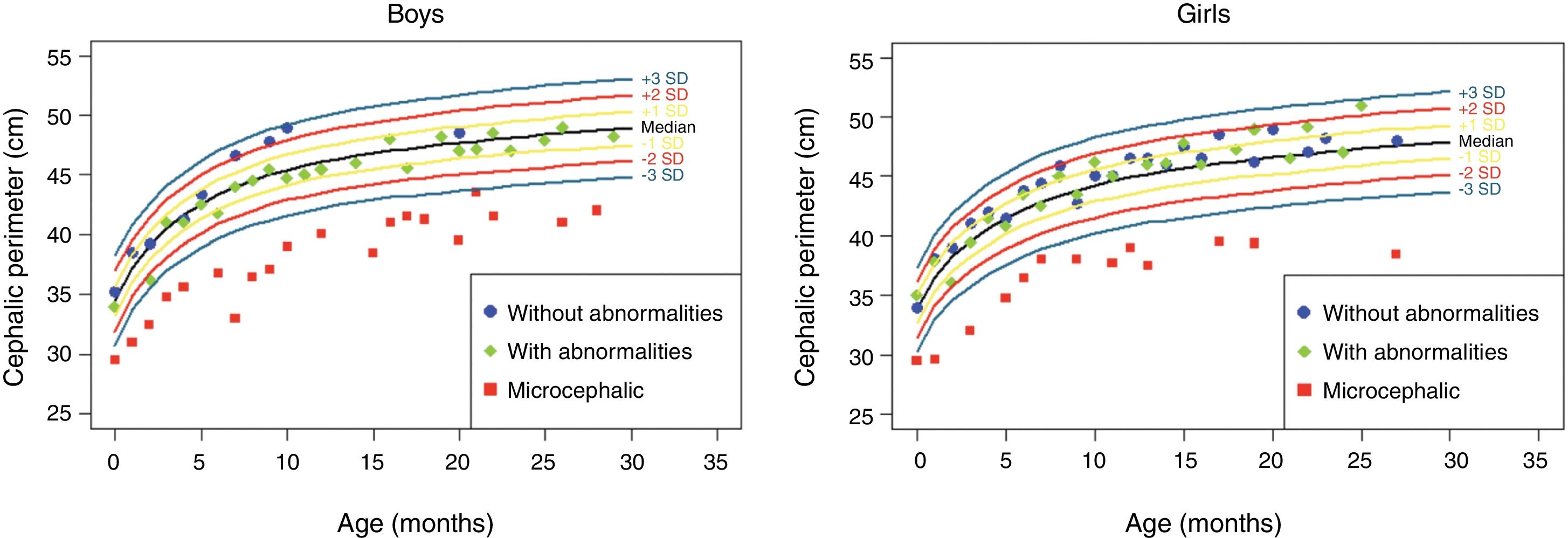
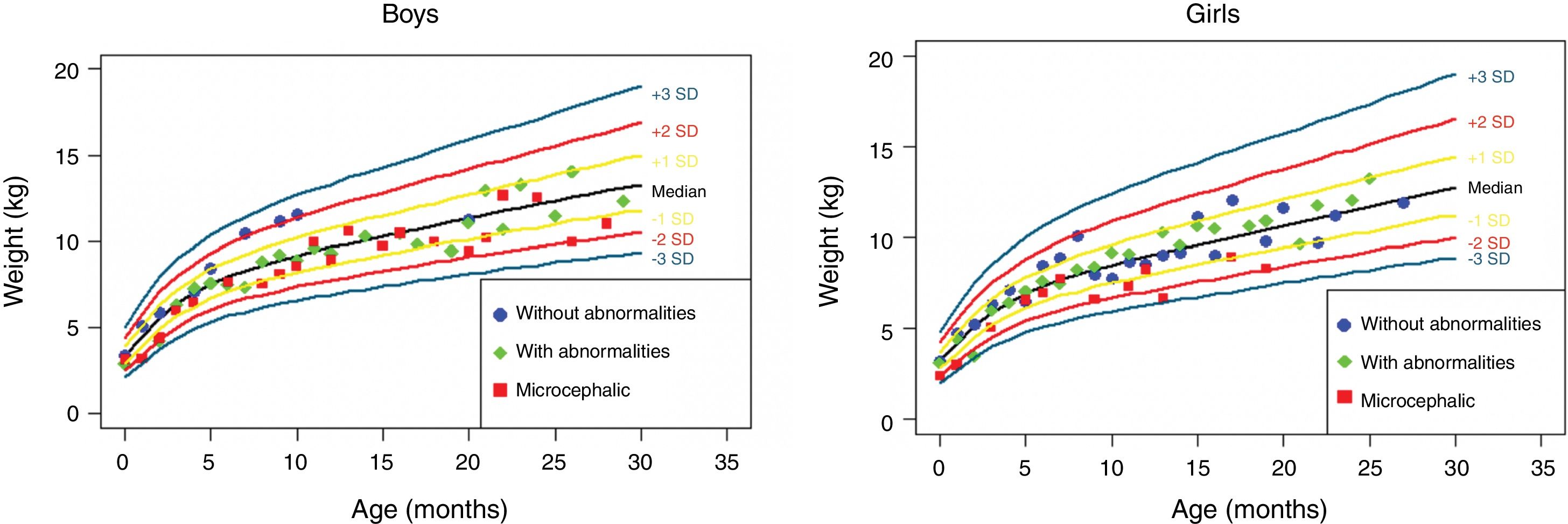
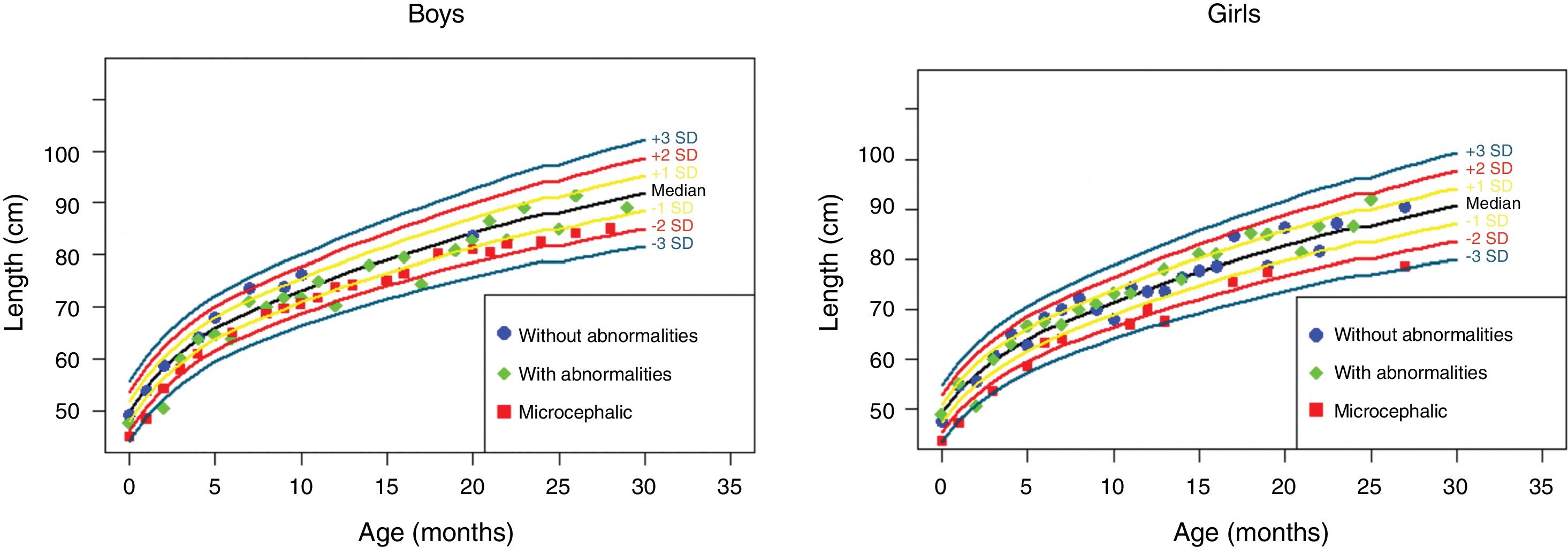
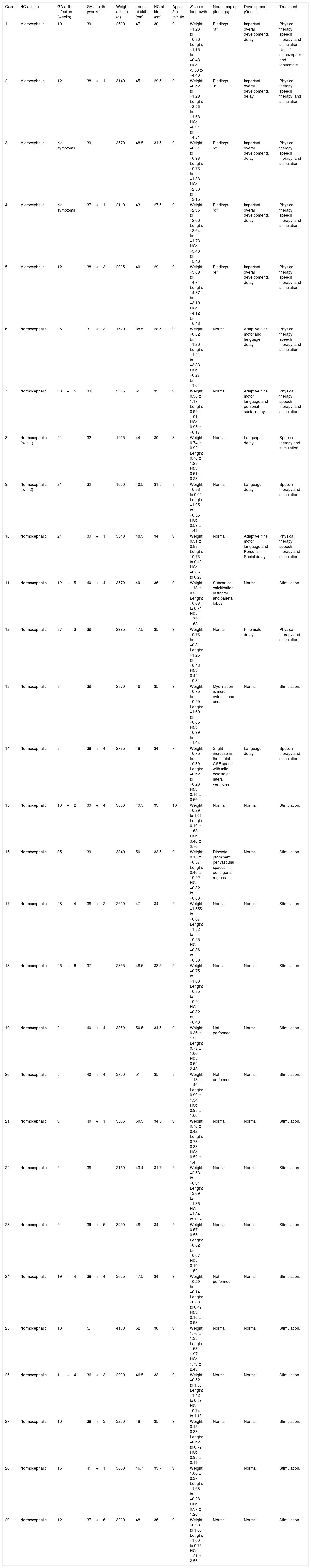
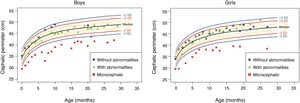
The anthropometric data (head circumference and length [in cm] and weight [in kg]) obtained during the prospective follow-up of these children were plotted into the following WHO charts:41 head circumference × age (months), weight × age (months) and length × age (months) for boys and girls (Figs. 1–3).
Head circumference evolution in the first 2.5 years of life in a cohort of 29 children whose mothers were exposed to Zika virus during pregnancy, five of whom had congenital microcephaly and severe neurological impairment, 11 normocephalic children at birth, with some type of neurological abnormality detected during follow-up, and 13 normocephalic children at birth, without neurological abnormalities during follow-up.
Weight gain in the first 2.5 years of life in a cohort of 29 children whose mothers were exposed to Zika virus during pregnancy, five of whom had congenital microcephaly and severe neurological impairment, 11 normocephalic children at birth, with some mild neurological abnormality detected during follow-up, and 13 normocephalic children at birth, without neurological abnormalities during follow-up.
Height evolution in the first 2.5 years of life in a cohort of 29 children whose mothers were exposed to Zika virus during pregnancy, five of whom had congenital microcephaly and severe neurological impairment, 11 normocephalic children at birth, with some mild neurological abnormality detected during follow-up, and 13 normocephalic children at birth, without neurological abnormalities during follow-up.
Children born with microcephaly maintained a head circumference measurement below three standard deviations (SD) during the first 2 years of life. Normocephalic children had adequate head circumference growth during the same period. The weight gain, however, remained within the range of +1SD to −2SD for microcephalic boys and for normocephalic children with neurological abnormalities, whereas normocephalic children without neurological abnormalities remained between the median and +2SD. In girls, no difference was observed in weight gain between normocephalic patients with or without neurological abnormalities, whereas lower weight gain was observed in microcephalic girls (between the median and -3DP). Regarding length, the evolution during the first 2 years of life was similar between boys and girls, with values ranging from −3SD to +2SD, but with a tendency toward lower values in microcephalic children (below the median) and more intense in girls.
These data suggest there is an association between the presence of neurological impairment and weight-height deficit in children exposed to Zika virus during pregnancy. The probable risk for growth deficiency appears to be associated with the presence of some degree of neurological impairment, even in children born without microcephaly. In the present cohort, head circumference growth during the first 2 years of life was associated with head circumference at birth. Children born with microcephaly remained microcephalic and normocephalic children remained with normal values for age. This behavior is probably associated with the higher degree of brain structure impairment in children born with microcephaly, since cranial growth depends on adequate brain growth and the latter is lower in the presence of more severe brain malformations. Weight–height growth was not as poor as the head circumference growth, but there was a trend toward lower weight and length during the first 2 years of life in children with congenital microcephaly and in normocephalic children who presented minor neurological abnormalities (more often observed in girls than in boys). Children with some degree of neurological impairment have a higher risk of weight-height deficit, directly related to the degree of neurological impairment, which is in agreement with the recent literature on the subject.9,30,43 Nutritional and non-nutritional factors may contribute to weight-height deficits in these children. Nevertheless, the children in the present cohort did not show significant weight–height deficit. This may be due to the fact that they were followed-up since birth at a multiprofessional service of a university hospital, where different professionals in the areas of pediatrics, child neurology, physical therapy, nutrition, and speech therapy contributed to the prevention and early approach of factors that could contribute to the weight-height deficit in this group of children.
ConclusionsTo date, there are scarce data in the literature demonstrating that congenital Zika virus infection can affect infant weight–height growth. The few existing data suggest that, in children with congenital Zika, the greater the degree of neurological impairment, the greater the impact on growth, whether or not associated with microcephaly at birth.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Prata-Barbosa A, Martins MM, Guastavino AB, Cunha AJ. Effects of Zika infection on growth. J Pediatr (Rio J). 2019;95:S30–S41.
Please add 2 more stars here connected to: Study conducted at Universidade Federal do Rio de Janeiro (UFRJ), Rio de Janeiro, RJ, Brazil.