This study analyzed the relationship between latent iron deficiency evaluated by ferritin, and the myelination of the central nervous system evaluated through the brainstem evoked response audiometry test.
MethodA total of 109 full-term newborns, born without anemia and risk factor for hearing deficiency, were enrolled. After delivery, umbilical cord blood was collected to determine ferritin and hematocrit levels. The brainstem evoked response audiometry test was carried out in the first 28 days of life. Analysis was performed between the control group (n=71) with ferritin greater than 75ng/mL, and the latent iron deficiency group (n=38) with ferritin between 11 and 75ng/mL. Results were presented as mean±standard deviation. Statistical analysis was performed using GraphPad prism7 and SPSS with a significance level of 5%.
ResultsA significant higher V-wave (p=0.02) and interpeak intervals I–III (p=0.014), I–V (p=0.0003), and III-V (p=0.0002) latencies were found in the latent iron deficiency group, as well as a significant inversely proportional correlation between ferritin and the same wave and intervals (p=0.003, p=0.0013, p=0.0002, p=0.009, respectively). Multiple correlation analysis showed a significant correlation of latent iron deficiency with all interpeak intervals, even taking into account newborn gestational age.
ConclusionIron deficiency anemia is a prevalent pathology; this study showed auditory delayed maturation associated to intrauterine iron deficiency, even in its latent form. This reinforces the importance of adopting effective measures, on a global scale, to prevent and treat this pathology in different life periods, especially in the most vulnerable population.
Este estudo analisou a relação entre deficiência de ferro latente avaliada pela ferritina e a mielinização do sistema nervoso central avaliada pelo teste de Potenciais Evocados Auditivos de Tronco Encefálico.
MétodoForam incluídos no estudo 109 recém-nascidos a termo, nascidos sem anemia e fator de risco para deficiência auditiva. Após o parto, o sangue do cordão umbilical foi coletado para determinar os níveis de ferritina e hematócrito. O teste de Potenciais Evocados Auditivos de Tronco Encefálico foi realizado nos primeiros 28 dias de vida. A análise foi realizada entre o grupo controle (n=71) com ferritina acima de 75ng/mL e o grupo com deficiência de ferro latente (n=38) com ferritina entre 11 e 75ng/mL. Os resultados foram apresentados como média±desvio-padrão. A análise estatística foi realizada utilizando o software GraphPad prism7 e SPSS com nível de significância de 5%.
ResultadosLatências significativamente maiores da onda V (p=0,02) e dos intervalos interpicos I-III (p=0,014), I-V (p=0,0003) e III-V (p=0,0002) foram encontradas no grupo de deficiência de ferro latente, assim como uma correlação significativa inversamente proporcional entre a ferritina e a mesma onda e intervalos (p=0,003, p=0,0013, p=0,0002, p=0,009, respectivamente). A análise de correlação múltipla mostrou uma correlação significativa da deficiência de ferro latente com todos os intervalos interpicos, mesmo se levarmos em consideração a idade gestacional do recém-nascido.
ConclusãoA anemia ferropriva é uma patologia prevalente e este estudo demonstrou maturação auditiva tardia associada à deficiência intrauterina de ferro, mesmo em sua forma latente. Isso reforça a importância da adoção de medidas efetivas, em escala global, para prevenir e tratar essa patologia em diferentes períodos da vida, principalmente na população mais vulnerável.
Nutrition is critical for the normal development of the central nervous system (CNS) and is particularly important throughout pregnancy and childhood. These are essential time periods for brain growth and for establishing adequate cognitive, motor, and psychosocial development in childhood, as well as in adulthood. Thus, nutritional deficiencies during these periods have the potential to impair cognition, behavior, and productivity in school years and adulthood. Among the nutritional deficiencies, iron deficiency occupies a prominent place.1
Iron plays a fundamental role in neural development, participating in the myelination and synaptogenesis processes.2 Regarding embryonic life, the importance of iron in the first three months is mostly related to the embryogenesis of the nervous system, whereas in the last trimester of pregnancy the fetus needs to form its own iron stores. Studies suggest that the peak of iron uptake in the CNS coincides with the myelination peak, especially in the late fetal and early postnatal stages.3
Iron deficiency (ID) is the most common nutritional deficiency in the world, affecting about a quarter of the world population, and these numbers increase even more during pregnancy, reaching 59%.4 In addition, some maternal and fetal pathologies may affect the newborn's (NB) iron stores, such as hypertension, diabetes mellitus, intrauterine fetal growth restriction, smoking during pregnancy, and premature birth, making the perinatal period especially susceptible to nutritional deficiency of this element.2
ID anemia has well-known impacts on CNS, such as reduced nerve conduction velocity and cognitive-behavioral changes.5 Iron deficiency without anemia, which has a prevalence 3–5 times higher than that of iron deficiency anemia, can also harm the CNS.3 Oligodendrocytes, the cells responsible for myelin production, are particularly sensitive to iron deficiency6 and it has been shown that NBs submitted to intrauterine iron deficiency presented long-term CNS damage, reaching lower language scores and motor development at 5 years of age compared to those born with normal iron stocks.2
Being part of the CNS, the developing auditory system is particularly vulnerable to nutritional changes during early fetal and postnatal life, especially due to the accelerated process of neuronal growth and development, myelination, and establishment of the synaptic network.7 Neural maturation of the auditory system progresses in the caudal–rostral direction and myelination of the auditory nerve pathway is considered an index of cerebral myelination.8
Brainstem evoked response audiometry (BERA) consists of recording the electrical stimulus from the inner ear to the brainstem in response to an acoustic stimulus.9 This test evaluates the electrophysiological activity of the auditory system at the level of the brainstem from the cochlear nerve, cochlear nuclei, superior olivary complex, and the bridge to the inferior-mesencephalic colliculus.10
The waves evaluated by BERA comprise three larger waves (I, III, and V) that can be reliably measured in newborns with a gestational age of more than 34 weeks. The absolute latencies for each of the waves and their intervals, as determined by BERA, are influenced by the degree of myelination, neuronal development, synaptic function, and axonal growth of the auditory nervous system; they are a measure of the nerve conduction velocity at different levels of the auditory pathway.11 As the relationship between these latencies and myelination is negative, less latency means greater (better) myelination and vice versa.12
Although data from the literature show the close relationship between the development of the auditory system and iron homeostasis, the changes that ID without anemia cause, also called latent iron deficiency (LID), are still not completely clear.13–15
Considering the possible negative repercussions of LID on the CNS myelination process, this study aimed to analyze the relationship between LID through serum ferritin of the umbilical cord, and myelination of the auditory nerve through BERA in NBs of gestational age (GA) equal to or greater than 37 weeks.
MethodsThis study was performed at the Santa Casa de Misericordia Maternity Hospital in a city of 240,000 inhabitants of Sao Paulo state (Brazil), from August 2016 to January 2018. The Santa Casa de Misericordia Maternity Hospital is currently the only institution responsible for all the births in the city and its micro region.
The project was approved by the Institutional Research Human Ethics Committee (Process 791,511). Only NBs whose parents or legal representatives signed the informed consent were included in the study.
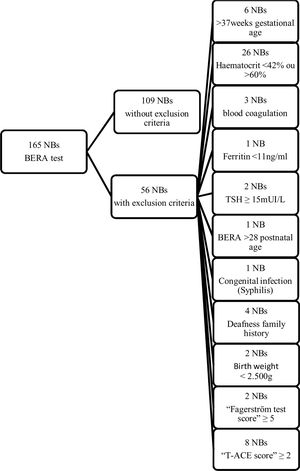
One-hundred sixty five newborns (NBs) had their blood collected and underwent otologic evaluation with BERA, and 109 full-term NBs with weight >2500g, born without fetal distress (Apgar≥7), who fulfilled the inclusion criteria, were fully studied (Supplementary Fig. 1). All the mothers answered a sociodemographic questionnaire designed by the researchers. Maternal and NB data were obtained from hospital clinical records.
After delivery, umbilical cord blood was collected to determine the ferritin and hematocrit levels. The samples were then kept at room temperature until their determination, up to 4h after collection. All NBs underwent an otologic evaluation within the first 28 days after birth. Two tests were carried out: the otoacoustic emission test (OAE) and BERA. OAE was performed before hospital discharge, between 24h and 48h after birth, and BERA was carried out after hospital discharge, up to 28 days. The audiological evaluations were performed by a single qualified phonoaudiologist, who did not have prior access to the blood test results.
Inclusion criteria: NBs of both genders with 37 weeks or more of GA, weight >2500g, born at the Santa Casa de Misericordia Maternity Hospital during the study period, from the public or supplementary health care system. Only NBs with a normal hematocrit value (≥42% and ≤60%) were included in the study. NBs whose mothers had arterial hypertension or diabetes mellitus were included in the study, given the higher probability of these NBs being iron deficient.
Exclusion criteria: NBs at risk of hearing impairment (family history of congenital hearing loss in first-degree relatives), those with congenital infections (toxoplasmosis, rubella, cytomegalovirus, herpes, syphilis, HIV), genetic syndromes/chromosomal abnormalities, perinatal problems (hemolytic disease, hypoglycaemia, prolonged jaundice, neonatal sepsis), intensive care unit (ICU) permanence, and presence of craniofacial abnormality, as any of these comorbidities could alone cause changes in BERA results. NBs with a 1 or 5min Apgar score less than 7 and ferritin <11ng/ml, as well as children of mothers with a T-ACE score equal or greater than 2 and a Fagerström test score equal or greater than 5 during gestation were excluded as well. T-ACE is an alcohol screening questionnaire adapted to identify risk drinking during pregnancy and the Fagerström test is a standard instrument for assessing the level of addiction to nicotine. NBs with increased TSH (thyroid stimulating hormone) values in neonatal screening program (blood spot TSH≥15mUI/L) (cut-off APAE-SP) were also excluded.
NBs were divided into two groups: Group 1 (control): value of ferritin in umbilical cord >75ng/mL, infants without iron deficiency; Group 2 (LID: value of ferritin in umbilical cord ≥11 and ≤75ng/mL, infants with LID.13,15–17
Hematocrit was measured using the method proposed by Wintrobe et al. in 1987. Ferritin determination was performed by a solid phase chemiluminescent immunoassay enzyme assay (Immulite/Immulite 1000 Ferritin – Siemens Healthcare Diagnostics, United Kingdom).
The OAE was done using a “pass or fail” screening mode and the BERA using a diagnostic mode. Only infants who underwent auditory screening using the OAE, and passed, were recruited for BERA. The OAE was carried out by the same phonoaudiologist responsible for the BERA test.
The equipment used for BERA was the Bio-Logic Navigator Pro® (stimulus: click; transducer: insertion earphones; polarity: alternating). The test was run while the NB was asleep in a quiet environment with electrical insulation and dimmed light, and did not require sedation. Headphones with monaural stimulation were used. Sound stimulus with an intensity of 80 dBNA, at a rate of 27.7clicks/second (Hood Standardization – 1998) was used. The impedance between the electrodes was considered to be less than 3kOhms, according to the equipment's instruction manual. The parameters utilized were: alternating polarity clicks, low pass filter 100Hz and high pass 1500Hz, a total of 2000 stimuli, a presentation rate of 27.7stimuli/s and a window analysis of 10.66ms. Each record was duplicated to ensure reproducibility of the results. For each patient, the lower latency of each wave and interpeak interval between the two ears was selected.15
To analyze the effect of mothers’ diabetes mellitus (DM) and/or hypertension on BERA values, the authors designed a variable named patho that was categorized as: 0=no pathology and 1=hypertension or/and DM, for statistical calculations. Accounting for the main role of ferritin as a cutoff variable (determining two significant different groups: higher or lower than 75ng/mL), the authors analyzed LID's contribution to BERA results through the variable named LID, where 0=no LID and 1=presence of LID.
Basic statistical analysis was performed by using GraphPad prism, version 7 for Windows (GraphPad Software, CA, USA) and multivariate models by SPSS (SPSS for Windows, Chicago, USA). Results were presented as mean±standard deviation (SD), median (minimum–maximum), or percentage, depending on the variable. The statistical significance of the differences was determined by the nonparametric t-test (Mann–Whitney) or the Fisher's exact test, as needed. The linear correlation among all variables was calculated by the Spearman method. Multivariate regression models were utilized for multiple correlation analysis in two ways: (1) the variables that were significant at p<0.10 in the univariate model (Spearman correlation) were included in the multiple model to evaluate the contribution of each one of these variables; (2) analysis in a stepwise processes where the non-contribute predictors were excluded by the statistical process, highlighting the most significant correlates of BERA values. Analysis of covariance was carried out to evaluate the influence of LID on BERA results, taking into account NB gestational age. The significance level adopted was 5%.
ResultsBlood samples, OEA, and BERA were carried out on 109 NBs. OAE was normal in all individuals.
After dividing the groups, 71 NBs had normal serum ferritin (>75ng/mL) and comprised the control group; 38 NBs presented LID (≥11 and ≤75ng/mL) and were included in the LID group (Table 1). There was no significant difference between the groups regarding maternal age, number of pregnancies, previous abortions, and number of prenatal consultations. The LID group presented a higher number of pregnant women presenting DM and/or hypertension (p=0.06; Table 1).
Maternal and neonatal characteristics of control and LID groups.
| Control group(n=71) | LID group(n=38) | p-Value | |
|---|---|---|---|
| Maternal age(mean±SD) | 30.6±5.8 | 32.0±6.1 | 0.28a |
| Supplemental health care (%) | 56.3 | 50 | 0.55b |
| Public health care (%) | 43.7 | 50 | |
| Number of pregnanciesmedian (min–max) | 2 (1–4) | 2 (1–6) | 0.59a |
| Number of abortionsmedian (min–max) | 0 (0–3) | 0 (0–4) | 0.98a |
| Number of prenatal visitsmedian (min–max) | 10 (4–15) | 10 (7–15) | 0.36a |
| Maternal DM or/and hypertension (%) | 7 | 21.1 | 0.06b |
| Fagerström test (%) | Smoker 4Non-smoker 96 | Smoker 0Non-smoker 100 | 0.55b |
| T-ACE scoremedian (min–max) | 0 (0–1) | 0 (0–1) | 0.59a |
| Apgar score 1′median (min–max) | 9 (7–10) | 9 (7–10) | 0.51a |
| Apgar score 5′median (min–max) | 10 (9–10) | 10 (9–10) | 0.80a |
| Birth weight (g)mean±SD | 3273±343.9 | 3573±374.7 | <0.0001a,c |
| Birth length (cm)mean±SD | 48.3±2.6 | 48.9±2.1 | 0.12a |
| Female (%) | 46.5 | 34.2 | 0.23b |
| Male (%) | 53.5 | 65.8 | |
| Gestational age (days)mean±SD | 274.5±5.9 | 272.1±6.5 | 0.04a,c |
| Gestational age test (days)mean±SD | 289.1±8.1 | 287.3±7.1 | 0.21a |
| Postnatal age test (days)mean±SD | 14.6±5.9 | 15.3±5.7 | 0.40a |
| TSH (mUI/L)mean±SD | 5.1±2.7 | 4.8±2.5 | 0.68a |
DM, diabetes mellitus; Fagerström test, standard instrument for assessing the level of addiction to nicotine; LID, latent iron deficiency; SD, standard deviation; T-ACE, alcohol screening questionnaire adapted to identify risk drinking during pregnancy; max, maximum; min, minimum; TSH, thyroid stimulating hormone.
Birth weight was significantly higher and the GA significantly lower in the LID group compared to the control group. The birth weight for the control group was 3273±343.9g and the LID group 3573±374.7g (p<0.0001). The mean GA of the control group was 274.5±5.9 days and for the LID group, 272.1±6.5 (p=0.04). Postnatal age and the corrected gestational age for BERA testing were not significantly different between groups, as well as the Apgar scores, birth length, and gender distribution (Table 1).
Hematocrit values were 47.0±3.6 for the control group and 48.4±4.3 for LID group (p=0.10). Ferritin values were 160.1±94.2 for the control group and 49.6±17.1 for LID group. Ferritin values were significantly different between groups as a result of the study design (p<0.0001).
The V-wave latency values (p=0.02), intervals I–III (p=0.014), I–V (p=0.0003) and III–V (p=0.002) were significantly higher in the LID group compared to the control group (Table 2). All these parameters presented a significant nonparametric negative correlation with ferritin and positive with LID: V-wave (r=−0.29; p=0.003; r=−0.29; p=0.003), interval I–III (r=−0.24; p=0.013; r=−0.29; p=0.003), interval I–V (r=−0.35; p=0.0002; r=−0.29; p=0.003) and interval III–V (r=−0.25; p=0.009; r=−0.29; p=0.003), respectively (Table 3).
BERA values for control and LID groups.
| Control(n=71) | LID(n=38) | p-Value | |
|---|---|---|---|
| I-Wavemean±SD(median) | 1.8±0.1(1.7) | 1.7±0.1(1.7) | 0.33a |
| III-Wavemean±SD(median) | 4.4±0.1(4.4) | 4.4±0.2(4.4) | 0.59a |
| V-wavemean±SD(median) | 6.9±0.1(6.9) | 7.0±0.2(7.0) | 0.02 a,b |
| Interval I–IIImean±SD(median) | 2.6±0.1(2.6) | 2.7±0.1(2.7) | 0.01a,b |
| Interval I–Vmean±SD(median) | 5.1±0.1(5.1) | 5.2±0.2(5.2) | 0.0003a,b |
| Interval III–Vmean±SD(median) | 2.4±0.1(2.4) | 2.5±0.1(2.5) | 0.002a,b |
BERA, brainstem evoked response audiometry test; LID, latent iron deficiency group; SD, standard deviation.
Correlation coefficient (r) and level of significance (p) of correlation analysis (Spearman) of V-wave, interval I–III, interval I–V, interval III–V, and all relevant variables.
| V-wave | Interval I–III | Interval I–V | Interval III–V | |||||
|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | |
| Maternal age | −0.047 | 0.629 | −0.134 | 0.165 | −0.042 | 0.668 | 0.049 | 0.612 |
| Number of pregnancies | 0.009 | 0.924 | −0.150 | 0.120 | 0.072 | 0.459 | 0.163 | 0.091 |
| Number of abortions | −0.002 | 0.981 | −0.067 | 0.490 | 0.025 | 0.793 | 0.150 | 0.120 |
| Number of prenatal visits | 0.214 | 0.025a | 0.020 | 0.839 | 0.165 | 0.087 | 0.079 | 0.412 |
| Patho | 0.084 | 0.385 | 0.124 | 0.198 | 0.150 | 0.120 | 0.136 | 0.159 |
| Gestational age (days) | −0.246 | 0.010a | −0.033 | 0.731 | −0.221 | 0.021a | −0.150 | 0.118 |
| Birth weight (g) | 0.034 | 0.729 | 0.043 | 0.657 | 0.117 | 0.224 | 0.219 | 0.022a |
| Birth length (cm) | −0.016 | 0.871 | 0.089 | 0.358 | 0.042 | 0.664 | 0.114 | 0.238 |
| Apgar score 1′ | −0.177 | 0.066 | 0.040 | 0.677 | −0.217 | 0.023a | −0.262 | 0.006a |
| Apgar score 5′ | 0.040 | 0.682 | 0.125 | 0.196 | 0.052 | 0.590 | −0.058 | 0.546 |
| Hematocrit (%) | −0.056 | 0.562 | −0.113 | 0.244 | 0.071 | 0.461 | 0.174 | 0.070 |
| Ferritin (ng/mL) | −0.287 | 0.003a | −0.237 | 0.013a | −0.351 | 0.000a | −0.249 | 0.009a |
| LID | 0.240 | 0.012a | 0.265 | 0.005a | 0.365 | 0.000a | 0.294 | 0.002a |
| Gestational age test (days) | −0.316 | 0.001a | −0.039 | 0.687 | −0.294 | 0.002a | −0.151 | 0.116 |
| Postnatal age test (days) | −0.193 | 0.045a | 0.018 | 0.849 | −0.170 | 0.077 | −0.059 | 0.541 |
| I-Wave | 0.575 | 0.000a | −0.106 | 0.272 | −0.006 | 0.954 | 0.018 | 0.849 |
| III-Wave | 0.730 | 0.000a | 0.595 | 0.000a | 0.388 | 0.000a | 0.018 | 0.853 |
| V-Wave | 1 | 0.000a | 0.324 | 0.001a | 0.743 | 0.000a | 0.491 | 0.000a |
| Interval I–III | 0.324 | 0.001a | 1 | 0.000a | 0.485 | 0.000a | −0.065 | 0.500 |
| Interval I–V | 0.743 | 0.000a | 0.485 | 0.000a | 1 | 0.000a | 0.626 | 0.000a |
| Interval III–V | 0.491 | 0.000a | −0.065 | 0.500 | 0.626 | 0.000a | 1 | 0.000a |
LID, variable accounting for the role of ferritin as a cutoff (where 0=no latent iron deficiency and 1=latent iron deficiency); Patho, variable that account for the effect of mothers diabetes mellitus (DM) or hypertension (categorized as 0=no pathology. 1=hypertension and/or DM); p, p-value; r, correlation coefficient.
Multiple correlation analysis of the significant variables for each BERA interval and V-wave are represented in Table 4. Utilizing the stepwise approach, the most significantly predictor of V-wave values was test gestational age (standardized β=0.255, p=0.007); for interval I–III was LID (standardized β=0.233, p=0.015); for interval I–V were LID (standardized β=0.249, p=0.08) and Apgar 1minute score (standardized β=0.206, p=0.027); for interval III–V they were LID (standardized β=0.229, p=0.013) and Apgar 1minute score (standardized β=0.263, p=0.05). The analysis of covariance showed that LID contributed to the results of all BERA intervals, even taking into account the gestational age: interval I–III (F=5.087, p=0.026), interval I–V (F=6.436, p=0.013) and interval III–V (F=5.856, p=0.017). The significance for V-wave was borderline (F=2.909, p=0.91).
Multiple correlation analyses for V-wave and intervals I–III, I–V, III–V.
| Variable | Multiples correlationsSignificant independent variables | Multiples correlationsStepwise | |||
|---|---|---|---|---|---|
| Standardized β coefficient | p-Value | Predictors of the variable | |||
| Standardized β coefficient | p-Value | ||||
| V-Wave | Number of prenatal visits | 0.141 | 0.143 | ||
| Birth weight | 0.66 | 0.531 | |||
| Ferritin | −0.047 | 0.676 | |||
| LID | 0.125 | 0.300 | |||
| Gestational age test | −0.226 | 0.073b | −0.255 | 0.007a | |
| Postnatal age test | −0.003 | 0.979 | |||
| Interval I–III | Birth weight | −0.059 | 0.563 | ||
| Ferritin | −0.012 | 0.916 | |||
| LID | 0.249 | 0.042a | 0.233 | 0.015a | |
| Interval I–V | Birth weight | 0.087 | 0.394 | ||
| Apgar score 1′ | −0.191 | 0.041a | −0.206 | 0.027a | |
| Ferritin | 0.017 | 0.879 | |||
| LID | 0.206 | 0.084b | 0.249 | 0.008a | |
| Gestational age | −0.027 | 0.829 | |||
| Gestational age test | −0.175 | 0.164 | |||
| Interval III–V | Birth weight | 0.189 | 0.061b | ||
| Apgar score 1′ | −0.255 | 0.006a | −0.263 | 0.005a | |
| Ferritin | 0.087 | 0.430 | |||
| LID | 0.185 | 0.114 | 0.229 | 0.013a | |
| Gestational age | −0.123 | 0.196 | |||
LID, variable accounting for the role of ferritin as a cutoff (where 0=no latent iron deficiency and 1=latent iron deficiency).
The developing brain and auditory system are particularly vulnerable to nutritional changes during early fetal and postnatal life, especially due to the accelerated process of neuronal growth and development, myelination, and formation of the cerebral synaptic network.3 Perinatal ID results in deficiency of the redox reactions of the hippocampus and frontal cortex energetic metabolism, modifications of the glutamate and dopamine concentrations, and of the neuronal myelination pattern.18
This study showed that LID determined a significant increase in V-wave and all interpeak interval latencies in BERA, with an inverse proportional correlation between serum ferritin concentrations and BERA parameters. In animal studies, iron deficiency without anemia resulted in a decreased number of synapses and in a lower wave amplitude in BERA, with increased susceptibility to noise-induced hearing loss.19 Other animal studies also reported significant functional consequences on BERA as a result of borderline iron deficiency, and in Long–Evans rats, the severity of the changes in BERA correlated with the level of serum iron.14 In 2012, Lee et al. analyzed BERA in iron-deficient female rats and showed a significant decrease in nerve conduction velocity at 40 days of life.6 Other studies on rodents have demonstrated that neurobehavioral changes resulting from iron deficiency during pregnancy and lactation persist even when iron concentrations are normalized after weaning.20
Studies on humans also suggest that LID causes damage to the neuropsychomotor development of preterm and full-term infants. In 2002, Tamura et al. reported lower motor skills and language development at 5 years of age in NBs with serum ferritin in the lower quartile compared to those with ferritin above this value.21 In another study, infants of diabetic mothers with a low serum ferritin level in the cord serum had a lower auditory recognition memory at birth and lower psychomotor development scores at 1 year of age.22
In a study that showed a relationship between LID and BERA waves, 33 NBs with normal ferritin compared to 12 NBs with LID, with a gestational age ≥35 weeks, finding significantly increased latency in individuals with LID, as was shown in the present study.15 Another study evaluated NBs with a gestational age ≥34 weeks and the results indicated a higher V-wave and I–V and III–V interpeak intervals latency in the LID group.13 In 2016, Lou also showed that at 10 months, infants born with LID still had a decrease in the V-wave amplitude and an increase in I-wave latency.16 All these studies, including the, demonstrated changes in the I–V interval, which may mean that the whole auditory pathway can be affected by intrauterine iron deficiency. The degree of reversibility of BERA changes secondary to LID in utero is not well established and there are a lack of studies that define to what extent and until when the changes caused by LID can last, just as the factors that affect the reversibility of these alterations.16 For example, in 2011 Berglund et al. realized an iron intervention trial and did not show a significant auditory effect of postnatal iron supplementation in marginally low birth-weight infants.23
In this study, there was a significant difference in gestational age between the two groups, which could be a confounding factor for the analysis, although the whole group studied was at term and the BERA age test and the postnatal age test were not significantly different between groups. The correlation among GA and BERA values (wave V, intervals I–III and I–V) showed that, even in term NBs, the gestational age have an influence on BERA values. Nevertheless, this study and recent studies with a similar methodological design that included NBs ≥35 weeks15 or ≥34 weeks13 demonstrated a significant relationship between LID and higher latencies in BERA, even after controlling GA as a variable.
The authors used ferritin concentration to define a state of LID, a parameter also utilized in recent studies on the subject.16 Although there are newer iron store assays, in addition to ferritin, they have not been standardized for use on neonates and are not readily available.17
An unanswered question is whether the alterations in myelination of the auditory pathways are representative of the development of white matter in other brain areas.24 It is also unclear whether infants at risk for iron deficiency should be routinely assessed for their iron status shortly after birth.25 This may be critical for populations in which maternal iron deficiency during pregnancy is very common, such as the Brazilian population. Currently, iron supplementation is not recommended for full-term infants during the first months after birth26,27 and there is no evidence that early iron replacement in the neonatal period is capable of reversing the changes in the CNS development and the auditory system.23,28,29
One limitation of this study is that, as in other observational studies, a causal relationship could not be established between BERA changes and LID, and currently there is no data regarding subsequent follow up of these NBs.16 More research is needed to answer these questions.
In conclusion, iron deficiency anemia is still a very prevalent pathology, and this research, in addition to other studies on the subject, points to serious and perhaps irreversible auditory maturation damage related to LID in utero. Therefore, it is important to take effective measures for the prevention and adequate management of this nutritional challenge in the different phases of life, such as iron supplementation during pregnancy, control of diseases that pose a risk for ID during the gestational period, and screening and treatment of anemia at any stage of life, especially in the most vulnerable and at risk individuals, such as NBs and pregnant women.
Compliance with ethical standardsThe research was approved by the Human Research Ethics Committee at the Federal University of São Carlos (process 791,511).
FundingThis work was supported by the São Paulo Research Foundation (Fundação de Amparo a Pesquisa do Estado de São Paulo [FAPESP]; grant number 2015/15471-4).
Conflicts of interestThe authors declare no conflicts of interest.
To all the families who took part in this research, as well as the Santa Casa de Misericordia Maternity Hospital. They authors also wish to thank the Sao Paulo Research Foundation (FAPESP) for the funding to carry out this research project.
Please cite this article as: Pallone LV, Jesus FA, Gonçalves GA, Navarra LC, Melo DG, Ferreira RA, et al. Effects of intrauterine latent iron deficiency on auditory neural maturation in full-term newborns. J Pediatr (Rio J). 2020;96:202–9.