To summarize and differentiate abdominal ultrasound findings of necrotizing enterocolitis and food protein-induced enterocolitis syndrome.
MethodsFrom January 2017 to December 2018, the abdominal ultrasound results of 304 cases diagnosed necrotizing enterocolitis or food protein-induced enterocolitis syndrome were retrospectively analyzed. The presence of pneumatosis intestinalis, portal venous gas, bowel wall thickening, intestinal motility, focal fluid collections and hypoechoic change of gallbladder wall were calculated, and the results were compared and analyzed.
ResultsPneumatosis intestinalis, portal venous gas, bowel wall thickening, intestinal motility weakened/absent, focal fluid collections and hypoechoic change of gallbladder wall can be found in both necrotizing enterocolitis and food protein-induced enterocolitis syndrome infants. However, in infants with necrotizing enterocolitis, intestinal motility was weakened/absent in whole abdomen, and in food protein-induced enterocolitis syndrome, it only involved isolated segment of bowel. The positive rates of above signs in necrotizing enterocolitis infants were significantly higher than those in food protein-induced enterocolitis syndrome (p<0.01). Moreover, it was observed that the rate of weakened intestinal motility besides the lesion segment of bowel in necrotizing enterocolitis infants was 100%, and in food protein-induced enterocolitis syndrome infants, it was 0%, which is supposed to be a main sign for identification.
ConclusionIn the early stage, abdominal ultrasound can be used to differentiate necrotizing enterocolitis and food protein-induced enterocolitis syndrome.
Food protein-induced enterocolitis syndrome (FPIES) is a non-immunoglobulin E (Ig E)-mediated gastrointestinal food hypersensitivity. Current diagnosis relies mainly on medical history, clinical manifestations, oral food provocation test and symptomatic relief after dietary avoidance.1 Diagnosis of FPIES is challenging for several reasons. An overall lack of familiarity with the illness, in conjunction with symptoms that often mimic other diseases, for example, necrotizing enterocolitis, when presenting in neonates, frequently leads to misdiagnoses. Necrotizing enterocolitis (NEC) is a common gastrointestinal emergency, in neonatal intensive care units (NICUs), the incidence is 1–7.7%.2
In the early stage, it is difficult to differentiate FPIES from NEC. They share a lot of similar clinical symptoms, such as vomiting, abdominal distention, diarrhea, bloody stool, feeding difficulties, sleepiness, apnea and even shock. By abdominal radiograph, both show intestinal dilatation, pneumatosis intestinalis (PI) and portal venous gas(PVG).3 But their treatments are quite different. NEC requires fasting and antibiotics, whereas for FPIES rests with dietary modification. If FPIES is not identified in a timely manner, illness will lead to repeated attacks, resulting in progressive changes in intestinal structure and even, surgical treatment. On the other hand, fasting and antibiotics use will aggravate the allergic state and bring unnecessary harm to infants. Therefore, it is necessary to identify FPIES from NEC in the early stage. Recent studies have shown abdominal ultrasound (US) plays an important role in the diagnosis and prognosis of NEC.4,5 At present, there is no report comparing the abdominal US and differentiate these two conditions.
The purpose of this study was to summarize and compare the sonographic manifestations of NEC and FPIES, to differentiate them by abdominal US to help clinicians to make early diagnosis and choose appropriate treatments.
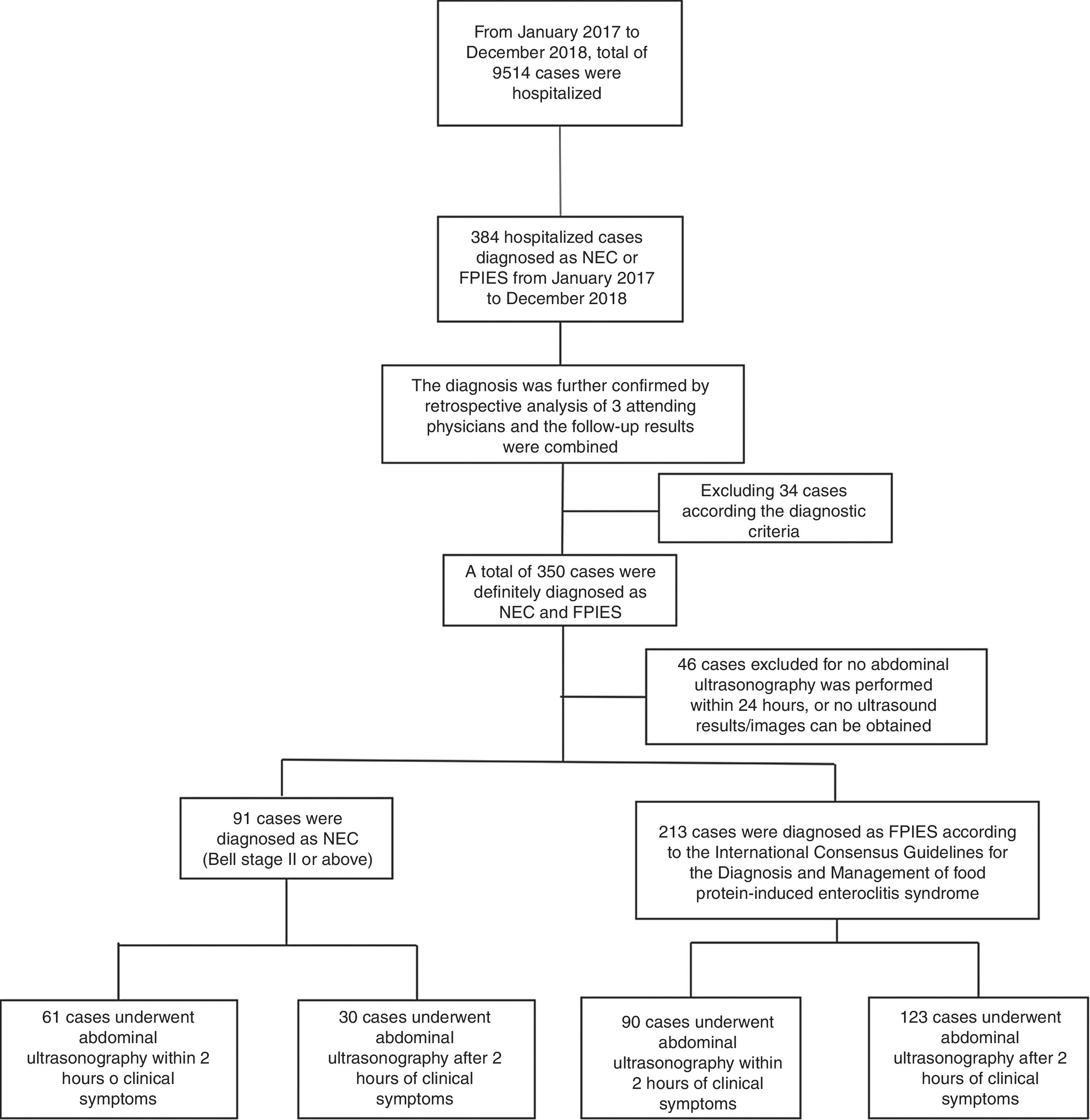
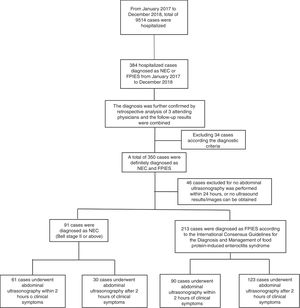
Materials and methodsInclusion and exclusion criteriaFrom January 2017 to December 2018, 384 cases were diagnosed as NEC or FPIES in the Department of Neonatology, the First Hospital of Jilin University. The cases were retrospectively analyzed by three attending physicians in consensus according to Bell staging criteria6 and the International Consensus Guidelines for the Diagnosis and Management of FPIES, 1 and follow-up results considered. In the course of data collection, 46 cases were excluded (27 cases because no abdominal US was performed within 24h, 19 cases because no ultrasound results/images were able to be obtained), and 304 cases were included. The process of case inclusion is shown in Fig. 1. The research ethics board approved the abdominal US was required for treatment and a confidentiality agreement was signed by all authors.
Data collection and imaging examinationsThe first abdominal US results of the 304 infants were analyzed. According to the interval between clinical symptoms and abdominal US, the patients were divided into early group (within two hours) and later group (two hours later). The abdominal US was performed by a B-mode ultrasound machine, Mindray 7, 10MHz linear array probe, and by a pediatric radiologist (with US experience of 19 years) at the bedside. Portal venous gas (punctate or strip shape gas echoes in the hepatic portal vein and its branches), pneumatosis intestinalis (punctate hyperechoic phenomena in the bowel wall), bowel wall thickening (loss of “gut signature”), intestinal motility (peristalsis was assessed according to the presence or absence of bowel contractions during 1min), focal fluid collections and hypoechoic change of gallbladder wall were observed carefully. The radiologist was unaware of the clinical diagnosis of the infants during the examination.
Statistical analysisSPSS software was used to analyze the data. Chi-square test was used to compare the positive rate of the abdominal sonography signs. If p<0.05, the difference was significant.
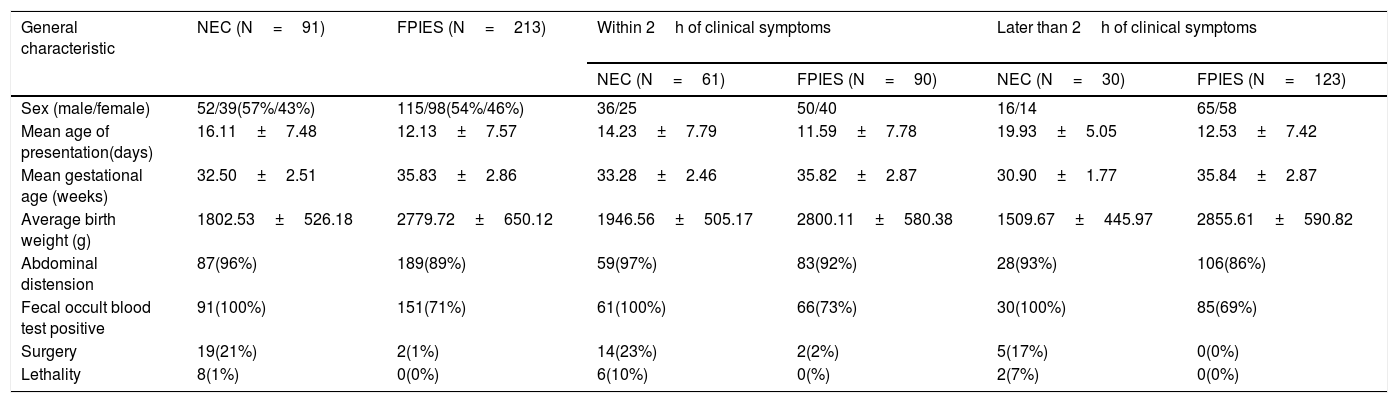
ResultsA total of 304 cases were included, 91 NEC and 213 FPIES, the gestational age ranged from 27 to 40 weeks, birth weight 580−4050g, presentation at 2–38 days of age, and comprise of 167 males and 137 females. The general characteristics are shown in Table 1.
The general characteristics.
| General characteristic | NEC (N=91) | FPIES (N=213) | Within 2h of clinical symptoms | Later than 2h of clinical symptoms | ||
|---|---|---|---|---|---|---|
| NEC (N=61) | FPIES (N=90) | NEC (N=30) | FPIES (N=123) | |||
| Sex (male/female) | 52/39(57%/43%) | 115/98(54%/46%) | 36/25 | 50/40 | 16/14 | 65/58 |
| Mean age of presentation(days) | 16.11±7.48 | 12.13±7.57 | 14.23±7.79 | 11.59±7.78 | 19.93±5.05 | 12.53±7.42 |
| Mean gestational age (weeks) | 32.50±2.51 | 35.83±2.86 | 33.28±2.46 | 35.82±2.87 | 30.90±1.77 | 35.84±2.87 |
| Average birth weight (g) | 1802.53±526.18 | 2779.72±650.12 | 1946.56±505.17 | 2800.11±580.38 | 1509.67±445.97 | 2855.61±590.82 |
| Abdominal distension | 87(96%) | 189(89%) | 59(97%) | 83(92%) | 28(93%) | 106(86%) |
| Fecal occult blood test positive | 91(100%) | 151(71%) | 61(100%) | 66(73%) | 30(100%) | 85(69%) |
| Surgery | 19(21%) | 2(1%) | 14(23%) | 2(2%) | 5(17%) | 0(0%) |
| Lethality | 8(1%) | 0(0%) | 6(10%) | 0(%) | 2(7%) | 0(0%) |
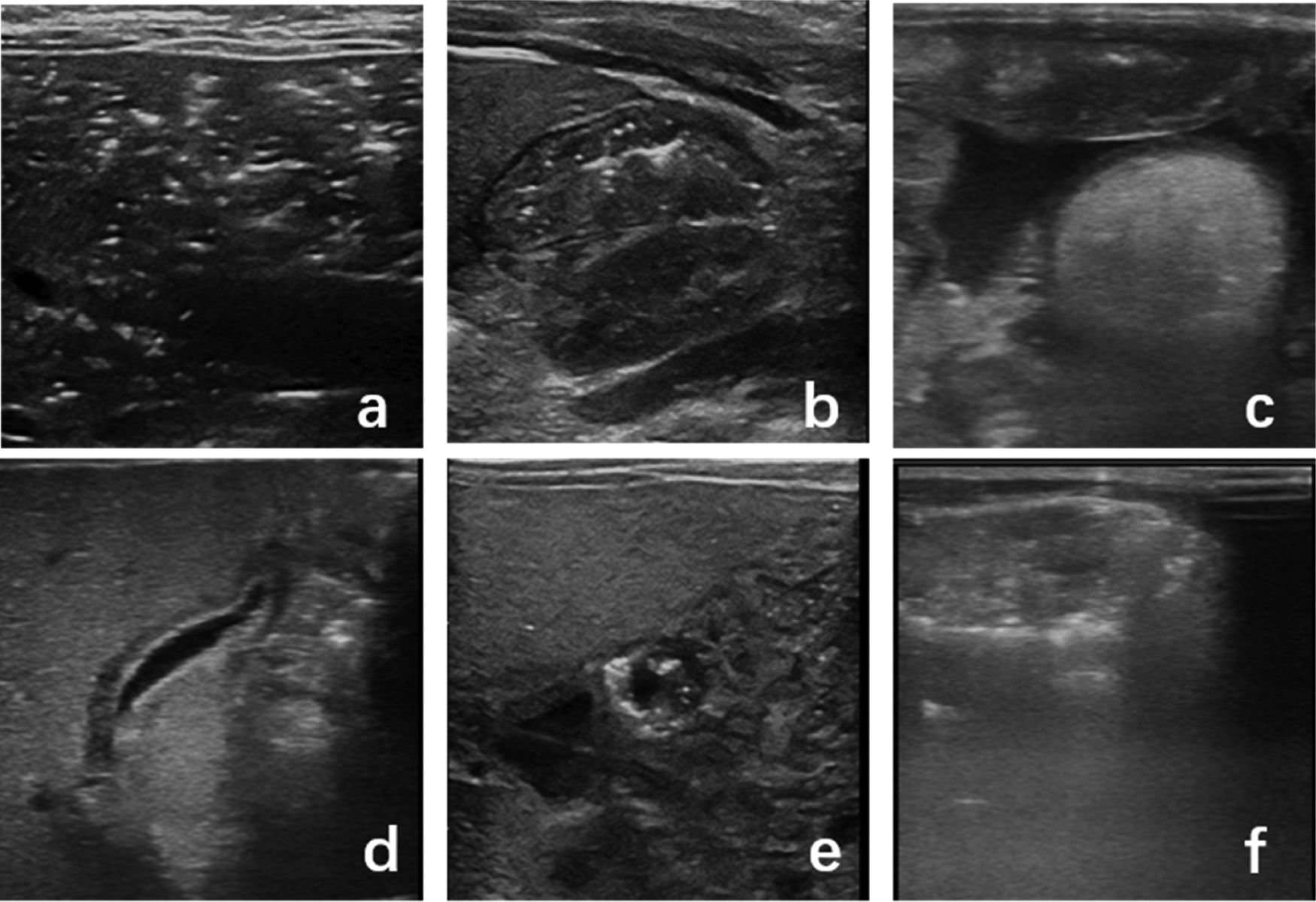
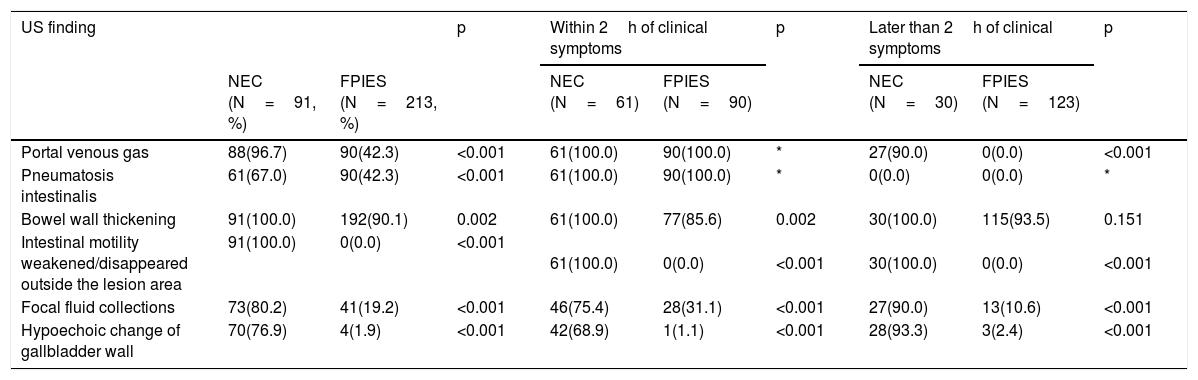
The main US manifestations of NEC included bowel wall thickening (100%), PI (67%), PVG (96.7) and intestinal motility weakening/absent (100%). Most patients had abdominal effusion and hypoechoic change of gallbladder wall (76.9%). In comparison, the main manifestations of FPIES were PI (42.3%), PVG (42.3%) and bowel wall thickening (90.1%) in the lesion area. Intestinal motility was normal outside the lesion area, and peritoneal effusion was present in some cases (19.2%). Only a few cases had hypoechoic change of gallbladder wall (1.9%). The positive rates of the above indicators were significantly different between NEC and FPIES (Table 2). US images are shown in Fig. 2. US findings in the early stage are different from those in the later stage. The positive rate of PI and PVG was 100% in the early stage, but decreased in the later stage. Especially for PI, the positive rate was 0% in the later examination. In NEC cases, the positive rate of PVG did not change significantly, while in PFIES, it was 0% in the later stage.
The ultrasound observation indicators in NEC and FPIES.
| US finding | p | Within 2h of clinical symptoms | p | Later than 2h of clinical symptoms | p | ||||
|---|---|---|---|---|---|---|---|---|---|
| NEC (N=91, %) | FPIES (N=213, %) | NEC (N=61) | FPIES (N=90) | NEC (N=30) | FPIES (N=123) | ||||
| Portal venous gas | 88(96.7) | 90(42.3) | <0.001 | 61(100.0) | 90(100.0) | * | 27(90.0) | 0(0.0) | <0.001 |
| Pneumatosis intestinalis | 61(67.0) | 90(42.3) | <0.001 | 61(100.0) | 90(100.0) | * | 0(0.0) | 0(0.0) | * |
| Bowel wall thickening | 91(100.0) | 192(90.1) | 0.002 | 61(100.0) | 77(85.6) | 0.002 | 30(100.0) | 115(93.5) | 0.151 |
| Intestinal motility weakened/disappeared outside the lesion area | 91(100.0) | 0(0.0) | <0.001 | 61(100.0) | 0(0.0) | <0.001 | 30(100.0) | 0(0.0) | <0.001 |
| Focal fluid collections | 73(80.2) | 41(19.2) | <0.001 | 46(75.4) | 28(31.1) | <0.001 | 27(90.0) | 13(10.6) | <0.001 |
| Hypoechoic change of gallbladder wall | 70(76.9) | 4(1.9) | <0.001 | 42(68.9) | 1(1.1) | <0.001 | 28(93.3) | 3(2.4) | <0.001 |
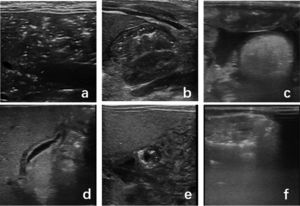
Several main signs of abdominal US. a: Portal venous gas: massive punctate gas echo in hepatic portal vein; b: Intestinal wall thickening, pneumatosis intestinalis: intestinal wall thickening, punctate gas echo in intestinal wall. c: Peritoneal effusion. d: Gallbladder wall thickening, hypoechoic; e: Pneumatosis intestinalis in short axis. f: Intestine dilatation, pneumatosis intestinalis.
With an incidence of 0.34%,7 FPIES is thought to be a rare disease, but some data suggest its incidence may be increasing.8,9 It is unclear if this rise is due solely to an increased awareness of the disease or true rise in incidence. In our study, the incidence of FPIES in neonates is higher than previously reported in children. The diagnostic criteria and incidence of FPIES in neonates need further study.
FPIES is difficult to differentiate in the early stage of onset and is often misdiagnosed as NEC.10 This study summarized and compared the abdominal US findings of NEC and FPIES, and found they could be differentiated. In recent studies11,12 abdominal US is increasingly recognized as an important imaging tool for evaluating NEC and provides more details than plain radiography.5 The main ultrasonographic features of NEC include PVG, PI, bowel wall thickening, focal fluid collections, and decreased intestinal motility.4,5,13 In this group of cases, all the above-mentioned signs appeared in the NEC cases, which is consistent with the literature reports. However, there is no report on the US diagnosis and evaluation of FPIES. In this study, it was found that many FPIES infants also showed signs of bowel wall thickening, PI, PVG and focal fluid collections. However, the intestinal motility was normal or active in the non-affected part of all infants with FPIES, and the incidence of other signs was different. After dietary management (changing to extensively hydrolyzed protein or amino acid-based formula, or breastfeeding with maternal dietary modifications), the clinical symptoms and feeding tolerance were improved. It confirming a diagnosis of FPIES rather than misdiagnosis of NEC reduces unnecessary fasting and antibiotic treatment.
In this study, 96.7% of NEC cases had PVG, and some cases had PI at the same time. PVG and PI were also found in some infants with FPIES (42.3%). It is presumably that both diseases cause intestinal mucosal injury in the involved intestines, and cause PVG and PI. Various hypotheses have been proposed to explain the pathogenesis of PI, including mechanics14,15 and bacteriology.16–18 In the case of incomplete intestinal mucosa, gas enters the intestinal wall and can diffuse to a distant place through the mesenteric vein, resulting in PVG. Some cases with PVG did not detect PI, which may be related to the time of examination. Among infants who underwent repeated US examinations, it was found that PVG was absorbed in 4−6h in most cases with stable condition, and the duration of PI might be shorter. The positive rate of PI and PVG was 100% in the early stage of FPIES, but decreased significantly in the later group, which suggests these two signs last for a short time. In NEC cases, the incidence of PVG in early and later stage did not change significantly (100 vs 90%), this may because the lesions of NEC are more extensive than FPIES, and the damage of intestinal mucosa is more serious. After grouping according to the examination time, it was found the positive rate of each US sign was different in the early and later stages of the disease, which suggests US manifestations would change with the course of the disease, so the control of examination time is very important. At present, there is no uniform standard for the choice of examination time for FPIES and as such, further research in this area is warranted.
Both NEC and FPIES may have local intestinal wall thickening and absence of intestinal motility. However, there were significant differences in intestinal motility outside the lesion area. This may because FPIES, as a non-IgE-mediated digestive tract allergic disease, only involves specific areas of the digestive tract, while NEC has severe systemic symptoms. Besides the local changes of the intestinal tract, the motility of the unaffected intestine is generally weakened or even absent due to the influence of systemic conditions. The positive rate of reduced intestinal motility outside the lesion area in NEC and FPIES was 100% and 0% respectively, which could be used as a direct marker for the differential diagnosis of NEC and FPIES.
In addition to the above signs, gallbladder wall thickening and hypoechoic was also observed in NEC. Four cases of FPIES with hypoechoic gallbladder wall were accompanied by fever and increased CRP, and the incidence of NEC was closely related to the infection factors. Therefore, it was inferred that the change of gallbladder wall echogenicity may be a clue to neonatal infection.
This study had some limitations. This is a retrospective study, and the time of US were different in the cases. In different periods, the ultrasonographic manifestations of the disease were different. Only the results of the first abdominal US were included in the study, and there were no dynamic observations.
In conclusion, both NEC and FPIES can show signs of PI, PVG, intestinal wall thickening, and focal fluid collections in abdominal US in the early stage. In FPIES, the duration of PVG is shorter than NEC, and the intestinal motility outside the lesion area is different, so they can be differentiated. This plays an important role in guiding the choice of clinical treatment applied in NEC and FPIES cases.
Conflict of interestThere authors declare no conflicts of interest.