To describe the characteristics of children aged 0–14 years diagnosed with diabetic ketoacidosis and compare the following outcomes between children with prior diagnosis of type 1 diabetes mellitus and children without prior diagnosis of type 1 diabetes mellitus length of hospital stay, severity on admission, insulin dosage, time of continuous insulin use, volume of fluids infused during treatment, and complications.
MethodsA retrospective descriptive study with review of medical records of patients admitted to the pediatric intensive care unit of a referral hospital from June 2013 to July 2015. The following data regarding 52 admissions were analyzed: age, sex, weight, body surface area, signs, symptoms and severity on admission, blood gas, blood glucose, glycated hemoglobin, serum osmolarity, and index of mortality. The insulin dosage, time of continuous insulin use, volume administered in the expansion phase and in the first 24h, length of stay, and complications such as electrolyte disturbances, hypoglycemia, cerebral edema, and death were compared between the two groups.
ResultsPatients without a previous diagnosis of DM1 were younger at admission, with mean age of 8.4 years (p<0.01), reported more nausea or vomiting, polydipsia and polyuria, and showed more weight loss (p<0.01). This study also observed a higher prevalence of hypokalemia (p<0.01) and longer hospital stay in this group.
ConclusionsNo differences in severity between groups were observed. The study showed that children without prior diagnosis of type 1 diabetes mellitus were younger at admission, had more hypokalemia during the course of treatment, and had greater length of hospital stay.
Descrever as características de pacientes de zero a 14 anos admitidos com diagnóstico de Cetoacidose Diabética e comparar desfechos entre os pacientes com diabete melito tipo 1 prévio e aqueles sem diabete melito tipo 1 prévio: tempo de internação, gravidade na admissão, dose de insulina utilizada, tempo de insulinização contínua, volume de líquido infundido durante o tratamento e complicações.
MétodosEstudo descritivo retrospectivo com revisão de prontuários de pacientes internados na UTI pediátrica de um hospital de referência no período de junho de 2013 a julho de 2015. Analisamos os seguintes dados referentes a 52 internações: idade, sexo, peso, superfície corporal, sinais, sintomas, gravidade na admissão, gasometrias, glicemia, hemoglobina glicada, osmolaridade sérica e índice de mortalidade. As crianças com diabete já diagnosticado foram comparadas com aquelas sem diagnóstico prévio quanto à dose de insulina, tempo de insulinização continua, volume infundido na fase de expansão e nas primeiras 24 horas, tempo de internação e complicações como distúrbios hidroeletrolíticos, hipoglicemia, edema cerebral e morte.
ResultadosOs pacientes sem diagnóstico prévio de DM I eram mais jovens no momento da admissão, com média de idade de 8,4 anos (p<0,01). Relataram mais sintomas como vômitos, polidipsia e poliúria e apresentaram mais perda de peso (p<0,01). Observamos maior prevalência de hipocalemia (p<0,01) e maior tempo de internação no grupo acima citado.
ConclusõesNão observamos diferenças quanto a gravidade entre os grupos. Pacientes diabéticos prévios eram mais jovens na admissão, apresentaram mais hipocalemia durante o tratamento e permaneceram mais tempo internados.
Diabetic ketoacidosis (DKA) is a potentially severe and common condition in emergency rooms and pediatric intensive care units (PICU). It is one of the major complications in patients with type 1 diabetes mellitus (DM1). In Brazil, approximately 20% of patients with previously undiagnosed DM1 initially present with DKA. It is more common in children under 4 years of age and affects 10/100,000 children.1,2 As a complication in children already diagnosed with DM1, DKA occurs in 1–10% of cases.2
Hospital da Criança Conceição (HCC), located in the city of Porto Alegre, state of Rio Grande do Sul, Brazil, is a reference center for the treatment of children with DM1. Patients admitted for DKA are referred for treatment in the PICU of the institution, and after the initial management and resolution of acidosis, they are followed at the Outpatient Clinic of the Institute for Children with Diabetes (ICD). Although it is a reference for the care of these patients, the service does not have a care protocol.
DKA treatment has been widely studied and described in the literature; however, there are few studies comparing the clinical characteristics and outcomes between patients admitted for DKA with previously diagnosed DM1 and those with no prior diagnosis of DM1. The objective of this study is to evaluate the characteristics and outcomes of patients admitted for DKA in the intensive care unit of HCC, comparing the following variables between the patient with a DM1 diagnosis and those without previous diagnosis: length of hospital stay, severity on admission, prognostic index (Pediatric Index of Mortality II), insulin dose, time of continuous insulin use, liquid volume infused during treatment, and complications.
MethodsA descriptive, retrospective study was carried out, based on a review of medical records of patients with DKA diagnosis admitted to the PICU of HCC, Porto Alegre, from June 2013 to July 2015. A total of 52 admissions of patients aged between zero and 14 years admitted for DKA treatment were analyzed. DKA diagnosis was defined according to the criteria established by the International Society for Pediatric and Adolescent Diabetes (ISPAD) Consensus of 2014: venous or arterial blood gas pH<7.3 and/or bicarbonate <15mmol/L, glucose or hemoglucotest >200mg/dL, presence of ketonemia or ketonuria.3 Patients with other diseases in addition to metabolic acidosis were excluded. DKA cases were identified from the PICU database. Data collection was performed using a standardized tool that contained the following variables: age, gender, weight, body surface area, initial diagnosis of DM1 or previous DM1, signs and symptoms on admission, blood gas data (pH, pCO2, HCO3−, BE) on admission and after withdrawal of continuous insulin use, time of continuous insulin use, administered insulin dose, initial blood glucose and during the course of treatment, glycated hemoglobin levels, serum osmolality, prognostic index, infused volume at the expansion phase and in the first 24h of treatment, and complications (hypoglycemia, hypercalcemia, hypokalemia, hyponatremia, cerebral edema, and death).
DKA was classified as mild (pH<7.3 or HCO3−<15), moderate (pH<7.2 and HCO3−<10), or severe (pH<7.1 and HCO3−<5) according to the ISPAD Consensus of 2014.3 Hypoglycemia was defined as blood glucose<60mg/dL, hypokalemia as serum potassium<3.5mEq/L,4 hyperkalemia as serum potassium>5.0mEq/L, hyponatremia as serum sodium<135mEq/L,4 uncorrected by blood glucose value. Serum osmolality was calculated using the following formula: [(2×sodium)+(glucose/18)+(Urea/5.6)].4 The prognostic index used in this PICU is the Pediatric Index of Mortality (PIM2).5
The data were entered into an Excel spreadsheet for Windows (Microsoft, WA, USA) and analyzed using the SPSS program, version 22.0 (IBM Corp. Released 2013. IBM SPSS Statistics for Windows, Version 22.0. NY, USA). Quantitative variables were described as means and standard deviations, or medians and interquartile range in asymmetric situations. Categorical variables were described using proportions and percentages. The nonparametric tests used were Fisher's exact test for comparison of categorical data and Student's t-test for comparison of quantitative variables, or the Mann–Whitney test when asymmetry was detected. For adjustments, analysis of covariance (ANCOVA) was used for quantitative data. Asymmetric quantitative data received logarithmic transformation. The adjustment of the categorical data was performed through logistic regression. The level of significance was set at α=0.05. The multivariate analysis used p=0.05 for the probability of inclusion and p=0.1 for the probability of exclusion.
This study was approved by the Institutional Ethics Committee.
ResultsA total of 52 admissions were analyzed, corresponding to 50 patients who were admitted to the PICU of HCC during a 25-month period. Of these, 26 were females and two re-admissions were also female patients; one of the patients had a previous diagnosis of DM1 and the other received the diagnosis at the first hospital admission. For purposes of data analysis, the number displayed corresponds to the hospital admissions and not to the number of patients.
The mean age was 10.2±2.9 years. In 59.6% of the hospital admissions, patients had no previous diagnosis of DM1. The most frequently observed symptoms were nausea and vomiting, followed by weight loss, polyuria, and polydipsia. Patients less frequently reported abdominal pain, asthenia, somnolence, tachypnea, and polyphagia.
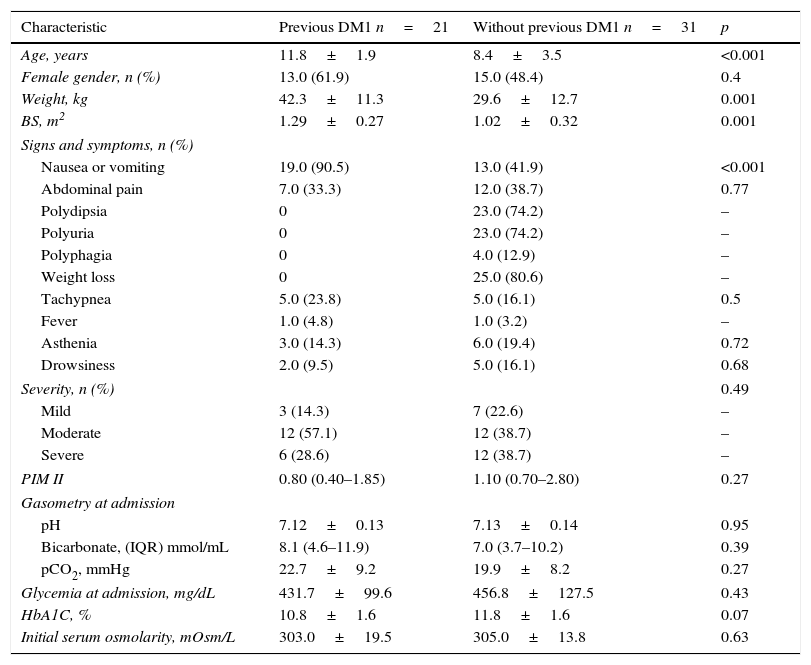
It was observed that children with no prior diagnosis of DM1 were younger at the time of hospital admission and more frequently had polydipsia, polyuria, and weight loss, whereas patients who already had a diagnosis of DM1 more frequently experienced vomiting as the most prevalent symptom. Table 1 shows the comparison between the basal characteristics of the two groups.
Baseline characteristics of patients.
| Characteristic | Previous DM1 n=21 | Without previous DM1 n=31 | p |
|---|---|---|---|
| Age, years | 11.8±1.9 | 8.4±3.5 | <0.001 |
| Female gender, n (%) | 13.0 (61.9) | 15.0 (48.4) | 0.4 |
| Weight, kg | 42.3±11.3 | 29.6±12.7 | 0.001 |
| BS, m2 | 1.29±0.27 | 1.02±0.32 | 0.001 |
| Signs and symptoms, n (%) | |||
| Nausea or vomiting | 19.0 (90.5) | 13.0 (41.9) | <0.001 |
| Abdominal pain | 7.0 (33.3) | 12.0 (38.7) | 0.77 |
| Polydipsia | 0 | 23.0 (74.2) | – |
| Polyuria | 0 | 23.0 (74.2) | – |
| Polyphagia | 0 | 4.0 (12.9) | – |
| Weight loss | 0 | 25.0 (80.6) | – |
| Tachypnea | 5.0 (23.8) | 5.0 (16.1) | 0.5 |
| Fever | 1.0 (4.8) | 1.0 (3.2) | – |
| Asthenia | 3.0 (14.3) | 6.0 (19.4) | 0.72 |
| Drowsiness | 2.0 (9.5) | 5.0 (16.1) | 0.68 |
| Severity, n (%) | 0.49 | ||
| Mild | 3 (14.3) | 7 (22.6) | – |
| Moderate | 12 (57.1) | 12 (38.7) | – |
| Severe | 6 (28.6) | 12 (38.7) | – |
| PIM II | 0.80 (0.40–1.85) | 1.10 (0.70–2.80) | 0.27 |
| Gasometry at admission | |||
| pH | 7.12±0.13 | 7.13±0.14 | 0.95 |
| Bicarbonate, (IQR) mmol/mL | 8.1 (4.6–11.9) | 7.0 (3.7–10.2) | 0.39 |
| pCO2, mmHg | 22.7±9.2 | 19.9±8.2 | 0.27 |
| Glycemia at admission, mg/dL | 431.7±99.6 | 456.8±127.5 | 0.43 |
| HbA1C, % | 10.8±1.6 | 11.8±1.6 | 0.07 |
| Initial serum osmolarity, mOsm/L | 303.0±19.5 | 305.0±13.8 | 0.63 |
Quantitative variables are expressed as mean±standard deviation or median and interquartile range.
DM1, type 1 diabetes mellitus; BS, body surface; PIM II, Pediatric Index of Mortality II; IQR, interquartile range; HbA1C%, glycosylated hemoglobin.
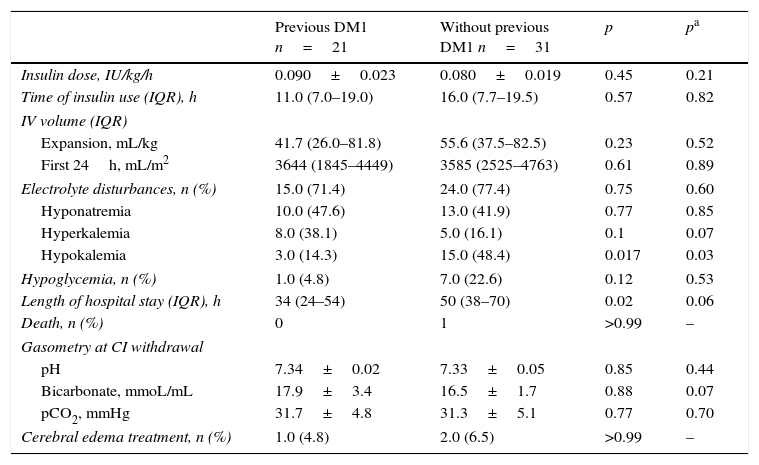
When the groups were compared regarding outcomes, no statistically significant difference were found with regard to insulin dose and time of continuous insulin use, severity at admission, intravenous volume administered in the expansion phase and in the first 24h, and hypoglycemia (Table 2). Also, there was no statistically significant difference in the number of patients who required treatment for cerebral edema. The prevalence of electrolyte disturbances was similar between the two groups; however, when analyzed separately, hypokalemia was more frequent in the group of patients with no previous diagnosis of DM1 (p=0.017). Patients who did not know they were diabetics also had greater length of hospital stay, with a mean of 50h (p=0.02). There was one death in the group of patients with no prior diagnosis of DM1 and the cause of death was cerebral edema.
Outcomes.
| Previous DM1 n=21 | Without previous DM1 n=31 | p | pa | |
|---|---|---|---|---|
| Insulin dose, IU/kg/h | 0.090±0.023 | 0.080±0.019 | 0.45 | 0.21 |
| Time of insulin use (IQR), h | 11.0 (7.0–19.0) | 16.0 (7.7–19.5) | 0.57 | 0.82 |
| IV volume (IQR) | ||||
| Expansion, mL/kg | 41.7 (26.0–81.8) | 55.6 (37.5–82.5) | 0.23 | 0.52 |
| First 24h, mL/m2 | 3644 (1845–4449) | 3585 (2525–4763) | 0.61 | 0.89 |
| Electrolyte disturbances, n (%) | 15.0 (71.4) | 24.0 (77.4) | 0.75 | 0.60 |
| Hyponatremia | 10.0 (47.6) | 13.0 (41.9) | 0.77 | 0.85 |
| Hyperkalemia | 8.0 (38.1) | 5.0 (16.1) | 0.1 | 0.07 |
| Hypokalemia | 3.0 (14.3) | 15.0 (48.4) | 0.017 | 0.03 |
| Hypoglycemia, n (%) | 1.0 (4.8) | 7.0 (22.6) | 0.12 | 0.53 |
| Length of hospital stay (IQR), h | 34 (24–54) | 50 (38–70) | 0.02 | 0.06 |
| Death, n (%) | 0 | 1 | >0.99 | – |
| Gasometry at CI withdrawal | ||||
| pH | 7.34±0.02 | 7.33±0.05 | 0.85 | 0.44 |
| Bicarbonate, mmoL/mL | 17.9±3.4 | 16.5±1.7 | 0.88 | 0.07 |
| pCO2, mmHg | 31.7±4.8 | 31.3±5.1 | 0.77 | 0.70 |
| Cerebral edema treatment, n (%) | 1.0 (4.8) | 2.0 (6.5) | >0.99 | – |
Quantitative variables are expressed as mean±standard deviation or median and interquartile range.
IU, international units; IV, intravenous; IQR, interquartile range; PIM II, Pediatric Index of Mortality II; CI, continuous insulin.
Hospital admissions for DKA account for approximately 5% of hospitalizations each year in this service. The worldwide incidence of DM1 ranges from 0.1 to 36.8 cases per 100,000 inhabitants per year and, despite the educational programs and the numerous studies on the subject, mortality from DKA has remained around 1–2% since 1970.2
The present study included 52 admissions, corresponding to 50 patients. Of these, 40.4% had a previous diagnosis of DM1. The mean age of admission was 11.8 years, which demonstrates the importance of parental involvement in the treatment of their children and in educational programs.
As for the symptoms at admission, the most frequent were nausea and vomiting. Patients who did not know they were diabetics more often had records in their medical files of the classic symptoms of DM1, such as polydipsia, polyuria, and weight loss. These findings were not observed in other studies.3,6 This may have occurred because the patients who were already using insulin had more acute clinical decompensation, due to the inappropriate use of medication or due to the more objective medical approach when a diagnosis of DM1 had already been established.7
The most frequently used continuous insulin dose in this sample was 0.1IU/kg/min, the same dose recommended in the last consensus on DKA treatment.3,8–10 It is considered a low dose, but one that achieves high physiological concentrations, with complete inhibition of lipolysis and ketogenesis, complete suppression of liver glucose production, and almost total tissue use of glucose, except in situations of insulin resistance.11,12 Some recent research has compared the normally recommended dose with insulin infusion of 0.05IU/kg/h. An observational study suggested that the dose of 0.05IU/kg/h was as effective as the dose of 0.1IU/kg/h in the treatment of 93 DKA episodes, at least in the initial 6h of treatment.13 Another retrospective study comparing 33 children who used the dose of 0.05IU/kg/h with 34 children who received insulin at a dose of 0.1IU/kg/h suggested that treatment with lower doses of insulin can be safe, promoting a more gradual reduction in serum osmolality and, thus, reducing the risk of cerebral edema.11 Analysis of the present study showed no difference between the two groups regarding the administered insulin dose.
A study comparing 117 children hospitalized for DKA in a hospital in Pakistan showed a longer continuous time of insulin use in patients that had no diagnosis of DM1 at admission. However, these patients were categorized as being more severe.14 A study in Campinas, state of Sao Paulo, Brazil, showed that the time of continuous insulin use was directly proportional to laboratory abnormalities and, thus, to DKA severity.2 The time of continuous insulin use in the present study was slightly longer in patients who had no diagnosis of DM1 prior to the DKA episode, but there was no statistically significant difference.
When the outcome electrolyte disturbance was evaluated, the groups showed no difference; however, when each disorder was analyzed separately, hypokalemia was significantly more frequent in patients without a prior diagnosis of DM1. An international study showed no difference between groups when hypokalemia was evaluated as a complication of treatment.14 In another study, in which most of the children had DKA as the first manifestation of DM1, hypokalemia was detected in 41% of cases during treatment.15 The children with DKA have a total body deficit of potassium of around 3–6mmol/kg.8 Potassium is usually lost as a result of vomiting, osmotic diuresis, and hyperaldosteronism secondary to volume depletion, which also promotes urinary excretion of potassium. Insulin administration and acidosis correction cause potassium to be diverted to the intracellular environment, further decreasing serum levels of this ion, which may predispose to arrhythmias.4,8
For the authors, a possible reason why the children with no diagnosis of DM1 had higher hypokalemia might have been the fact that they were exposed to the abovementioned pathophysiological mechanisms for a longer period of time, with a more depleted total body potassium in relation to children already undergoing treatment for DM1. More studies are needed to confirm this finding.
Hypoglycemia as a complication of treatment occurred in 15% of hospitalizations, which is similar to other studies.14,15 When the two groups were compared, children without DM1 had more hypoglycemia, but this finding was not statistically significant and variations in glucose input used in this service can probably explain this fact.
Regarding length of hospital stay, this study showed that patients without a previous diagnosis of DM1 remained in the PICU for a longer period of time, as was also observed in a study carried out in Pakistan.14 This can be explained by the fact that these patients often require more time for insulin dose adjustment and for the family to get used to the treatment.
As greater severity was not observed in patients without a previous diagnosis of DM1, nor a statistically significant difference in time of insulin use, these factors do not seem to have influenced the length of hospital stay.
In this study, three patients received treatment for cerebral edema (hypertonic saline solution or mannitol), which corresponds to 5.7% of the study population. The literature describes an incidence of 0.5–1% of cerebral edema due to DKA; however, changes in the mental status with Glasgow Coma Scale score <14 occur in approximately 15% of children treated for DKA and are associated with evidence of cerebral edema in imaging studies.3,8 Taking these data into account, it can be considered that this sample follows the patterns reported in other studies.
It is possible that more patients showed some degree of cerebral edema, characterized by symptoms such as headache, drowsiness, vomiting, and lethargy. As this study was based on chart review, the data may have been lost, because clinical symptoms are frequently not recorded in the medical files.
There was no statistically significant difference in the incidence of cerebral edema between the two groups, which was expected, since ketoacidosis severity at admission and the amount of administered volume were similar. There is evidence that more severe dehydration and refractory shock are risk factors for development of cerebral edema.16
The present study shows the high prevalence of hospitalizations due to DKA in this service; this fact indicates the need for public awareness about the symptoms, as well as the importance of family involvement in the management of these patients.
This PICU does not have a treatment protocol for DKA cases, which makes the management heterogeneous, possibly influencing the observed clinical outcomes. This was a retrospective study, no trigger factors were identified, and the sample size was small, a limitation.
It was concluded that there were no differences in severity between the groups. Patients with a prior DM1 diagnosis were younger at admission, had more hypokalemia during treatment, and had greater length of hospital stay. Further studies are needed to perform a better epidemiological assessment of these groups.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Lopes CL, Pinheiro PP, Barberena LS, Eckert GU. Diabetic ketoacidosis in a pediatric intensive care unit. J Pediatr (Rio J). 2017;93:179–84.