To assess the prevalence of congenital hypothyroidism and the ability of various neonatal thyroid-stimulating hormone (TSHneo) cutoff values to detect this disease.
MethodsThis cohort study was based on the retrospective collection of information available from the Reference Service for Newborn Screening database for all live births from January 1, 2010, to December 31, 2012, assessed using the Newborn Screening Program of a Brazilian state, Brazil. The infants were divided into two groups: I – Control: infants with normal newborn screening tests and II – Study: infants with congenital hypothyroidism. Analysis included comparing the TSHneo levels from both groups. A receiver operating characteristic (ROC) curve was constructed to assess the TSHneo cutoff values.
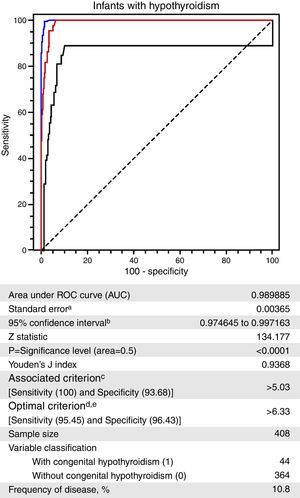
ResultsUsing a TSHneo cutoff value of 5.0μIU/mL, 50 out of 111,705 screened infants had diagnosis of congenital hypothyroidism (prevalence 1:2234 live births). The ROC curve showed that TSHneo value of 5.03μIU/mL had 100% sensitivity and the greatest associated specificity (93.7%). The area under the curve was 0.9898 (p<0.0001).
ConclusionsThe ROC curve confirmed that the TSHneo cutoff value of 5.0μIU/mL adopted by the Newborn Screening Program of a Brazilian state was the most appropriate for detecting congenital hypothyroidism and most likely explains the high prevalence that was found.
Avaliar a prevalência do hipotireoidismo congênito e a capacidade de vários valores de corte do hormônio estimulante da tireoide de neonatos (TSHneo) para detectar essa doença.
MétodosEste estudo de coorte teve como base a coleta retrospectiva de informações disponíveis no banco de dados do Serviço de Referência em Triagem Neonatal de todos os nascidos vivos de 1° de janeiro de 2010 a 31 de dezembro de 2012, avaliados no Programa de Triagem Neonatal de um estado brasileiro. Os neonatos foram divididos em dois grupos: I–Controle: neonatos com testes de triagem neonatal normais e II–Estudo: neonatos com hipotireoidismo congênito. A análise incluiu a comparação entre os níveis de TSHneo dos dois grupos. Uma curva do poder discriminante do teste (ROC) foi criada para avaliar os diferentes valores de corte de TSHneo.
ResultadosUtilizando um valor de corte de TSHneo de 5,0μIU/mL, 50 dos 111.705 neonatos examinados foram diagnosticados com hipotireoidismo congênito (prevalência de 1:2.234 nascidos vivos). A curva ROC mostrou que o valor do TSHneo de 5,03μIU/mL possuía sensibilidade de 100% e a maior especificidade relacionada (93,7%). A área abaixo da curva foi 0,9898 (p<0,0001).
ConclusõesA curva ROC confirmou que o valor de corte de TSHneo de 5,0μIU/mL adotado pelo Programa de Triagem Neonatal de um estado brasileiro foi o mais adequado na detecção do hipotireoidismo congênito e provavelmente explica a alta prevalência constatada.
Congenital hypothyroidism (CH) is a common pediatric endocrine disorder1,2 that can cause mental retardation.3,4 The importance of early diagnosis and treatment, to prevent irreparable brain damage4,5 and growth retardation,3,6 justified the establishment of newborn screening programs (NSPs) for the detection of CH.4,7 These NSPs proved to be significantly cost-effective to society at large.8,9
Measuring the concentration of thyroid-stimulating hormone (TSH) is considered the best screening test strategy for detecting primary CH4 because it exhibits high sensitivity4,10 and accuracy.11 However, CH detection depends directly on the neonatal TSH (TSHneo) cutoff value,4,12 which varies among different NSPs13,14 and may have an influence on the recall rate.13 According to available evidence, many cases of CH would go undetected if the TSHneo cutoff value was increased.9,15 On the other hand, reducing the cutoff value requires prior judicious assessment of the laboratory work quality15 and costs to the screening program.4,9 The criteria used for choosing the TSHneo cutoff for detecting CH should be adapted to the target disease definition.4
Some programs worldwide have lowered the screening test cutoffs5,7 in order to increase the sensitivity of the assay5 and to take changes in its methodology.16 In accordance with that, cutoff values close to 10.0μIU/mL2,8 or as low as 5.0μIU/mL3,17 or 4.5μIU/mL18 are used in several NSPs worldwide to improve the detection of children at risk.
The prevalence of CH in Brazil is approximately one case per 2500 live births; regional variation has been noted and according to the 2010 Brazilian Health Ministry recommendations, a TSH value higher than 15.0μIU/mL per immunometric assay should be considered positive.19
Because the efficiency of any NSP depends on its ability to cover the largest possible population and to detect the largest number of cases, obtaining reliable TSH values is necessary to maximize the detection of CH cases. However, the lack of agreement on TSH cutoff values in the literature makes this task difficult. Since November 2009, the TSHneo value of 5.0μIU/mL has been the cutoff point adopted by the NSP in Mato Grosso (MT), Brazil. Consequently, the present study aimed to assess the CH detection ability of several TSHneo cutoff values and their effects on the current screening program.
MethodsStudy design and populationThis was a transversal study nested in a cohort study, based on retrospective information collected from the database of the Reference Service for Newborn Screening (RSNS) of MT for all live births, 2010–2012, that were assessed by the NSP-MT. Data were also collected from the clinical records of all individuals with all forms of CH.
The infants included in the study were divided in two groups: Group I – control group (n=220), infants with a normal newborn screening test (NST), and Group II – study group (n=44), infants with confirmed CH, i.e., serum TSH higher than 10.0μIU/mL and normal or low free thyroxine (T4) levels, according to the laboratory reference values. For every child that was included in the study group, there were five newborn infants with normal NSTs included in the control group.
The children in Group I were admitted to the program immediately before the corresponding Group II cases; they were born in the same month and had birth weight ≥2500g. These infants had the NST samples collected when they were 2–6 days old. Potential participants were excluded from Group I when the NST sample remained at the collection site for more than 30 days or the test data were incomplete.
Infants with confirmed CH and birth weight ≥2500g comprised Group II, regardless of the date of newborn screening collection. The age at collection's time of NST was unpaired between the two groups. Infants whose NST samples were collected at 24h after birth or who had comorbidities and/or medication use that were likely to interfere with the laboratory results were excluded.
According to this NSP's guidelines, it is recommended that the collection of the blood sample is taken by puncturing the heel when the infant is 3–5 days old; one drop of blood is collected on filter paper (Schleicher & Schuell, 903) and sent to the laboratory at MT's RSNS for blood spot TSH concentration measurement.
The TSHneo cutoff value was established as 5.0μIU/mL. Values equal to or lower than 5.0μIU/mL were considered normal, values between 5.0 and 15.0μIU/mL were reassessed by collecting a new sample, and values higher than 15.0μIU/mL were referred to confirm the diagnosis. Those with TSHneo concentrations over 5.0μIU/mL on repeat sampling were also referred to the RSNS for further assessment.
The prevalence of CH was calculated as the proportion of live births with a confirmed diagnosis per year relative to the number of live births that underwent a NST that same year. The number of tests performed during the study period was obtained from the RSNS, and the number of live births in the corresponding period was obtained from the State Secretary of Health of MT and the Department of Information Technology of the Brazilian Unified Health System (DATASUS).
There were no requirements for the free and informed consent because there has been no change in medical procedure, no additional blood samples were obtained, and the participants were not identified, as well as because this was a chart review study and the authorization to consult the medical records was provided by the clinical director of the hospital. The study was approved by Human Research Ethics Committees of Júlio Müller University Hospital, Federal University of Mato Grosso, on October 13, 2010, under No. 940/CEP-HUJM/2010 and the Commission for Analysis of Research Projects [Comissão para Análise de Projetos de Pesquisa (CAPPesq)], Clinical Director's Office, Clinical Hospital, School of Medicine, University of São Paulo, on July 19, 2011, under No. 307/11.
Biochemical methodsThe blood spot TSH concentration was measured using a time-resolved immunofluorometric assay with AutoDELFIA® (Perkin Elmer®, Turku, Finland). For cases with values above the established cutoff of 5.0μIU/mL, a second assay was performed, and the reported results correspond to the mean of both measurements. According to the method's sensitivity, the highest detectable concentration was 250.00μIU/mL; higher values were reported as >250.00μIU/mL and considered as 251.00μIU/mL for analysis.
To ensure the uniformity and equivalence of the results, the milli-international units per liter (mIU/L) were expressed as micro-international units per milliliter (μIU/mL). A chemiluminescent assay (ADVIA Centaur® XP Immunoassay System, Siemens, Germany) for measurement of serum TSH concentrations (μIU/mL) was used and the results were interpreted according to the reference values per age, i.e., up to 25.000μIU/mL at the first week of life and, from 0.800 to 6.000μIU/mL from 2nd week until 11th months of life. The serum free T4 concentration (ng/dL) was measured using the chemiluminescence method (ADVIA Centaur® XP Immunoassay System – Siemens), and the reference value varied from 0.70 to 1.80ng/dL for adults and children according to the reference values given by the supporting laboratory.
Statistical analysisBased on disease prevalence data provided by the NSP-MT, α=0.05 and β=0.20 (80% power); assuming an area under the ROC curve of 90% and a confidence interval (CI) of 70–100%, the sample size was initially calculated as 45 confirmed cases of CH. This number was reached in the period chosen for this study and, after application of the exclusion criteria, 44 children were analyzed with a confirmed diagnosis of CH (study group). There were 220 infants with normal NST included in control group.
In addition to the infants included in Group I and Group II, to construct the ROC curve, infants with false-positive results were also included (n=24), as well as five infants with a normal NST for each false-positive result (n=120).
The sensitivity, specificity, positive and negative predictive value, and likelihood ratio corresponding to various TSHneo values were then calculated using Microsoft® Excel (Microsoft®, version 2007, USA) and Stata® (StataCorp. 2011. Stata Statistical Software: version 12, USA). Continuous data are shown as measures of central tendency (mean and median) and dispersion (standard deviation – SD, minimum and maximum). To analyze the relationship between concentrations of both serum TSH and TSHneo, the Spearman correlation test was used. The binomial z-test was used to compare multiple proportions. To compare the TSHneo between groups, the non-parametric Mann–Whitney test was used. The significance level was established as p<0.05.
ResultsA total of 151,245 infants were born in MT within the study period, and 111,705 infants (73.9%) underwent a NST. CH was confirmed in 50 infants, 44 of whom were included in the present study. The calculated CH prevalence was 1:2234.
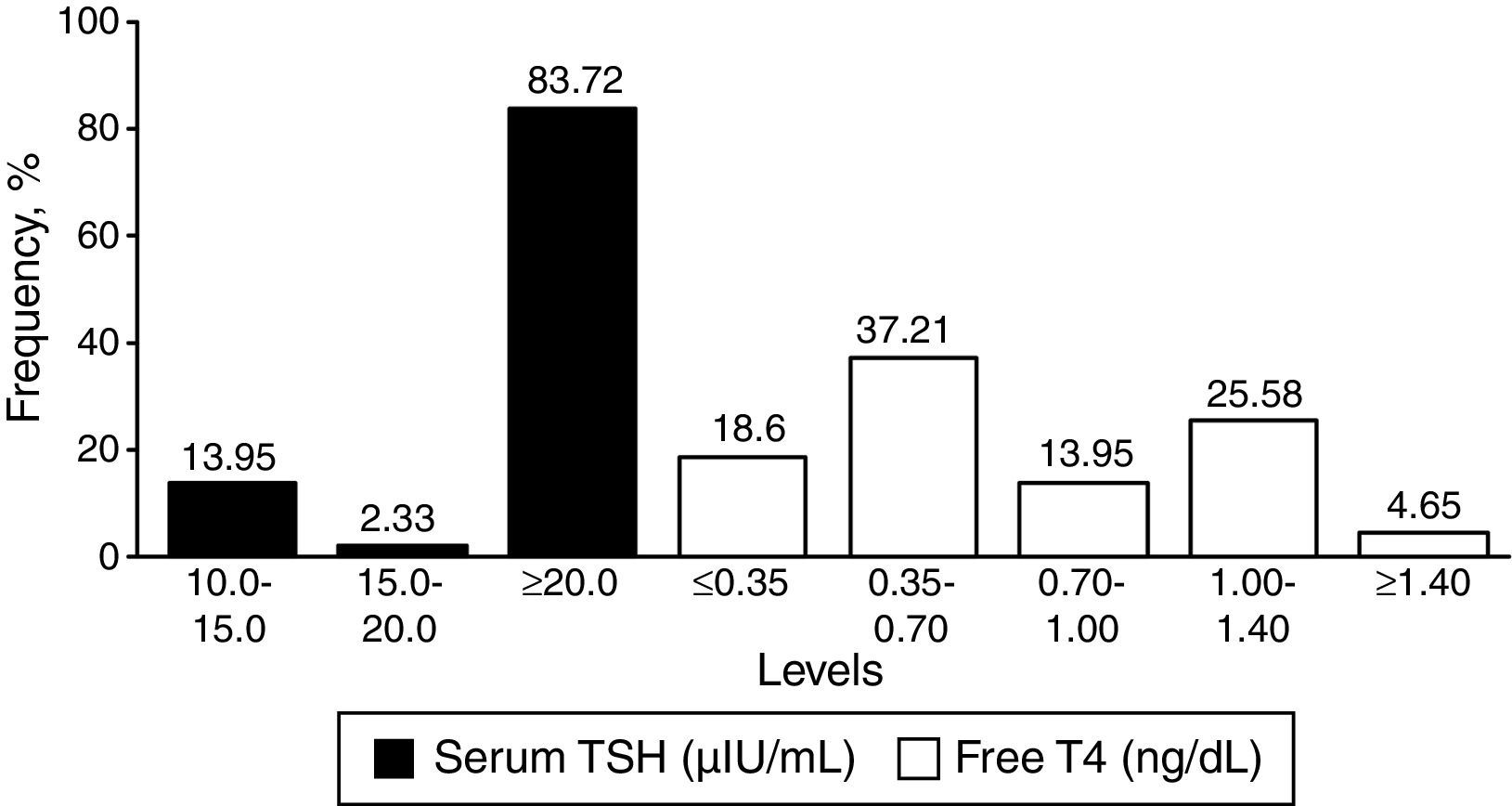
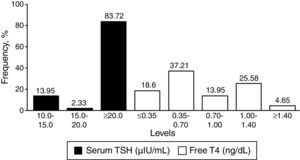
Among the infants with CH, the mean TSHneo value was 93.97μIU/mL (SD=98.15; minimum of 5.10 and maximum of >250.00μIU/mL). The median serum TSH value was in turn 101.00μIU/mL, with a mean of 86.72μIU/mL (SD=52.34) and it ranged from 10.07 to >150.00μIU/mL. The mean (SD) and median of serum free T4 value were 0.70ng/dL (SD=0.39) and 0.62ng/dL, respectively, ranging from 0.11 to 1.44ng/dL.
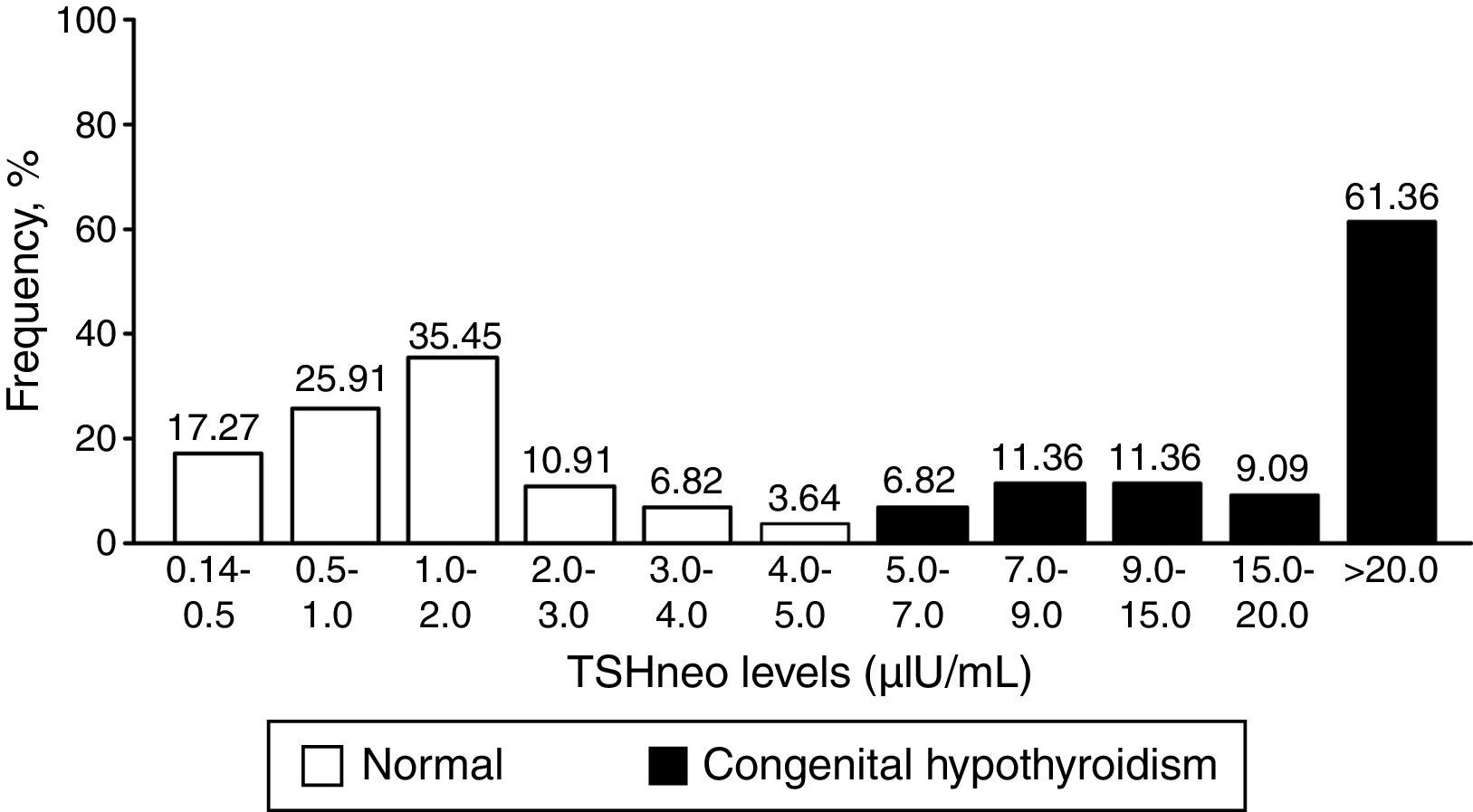
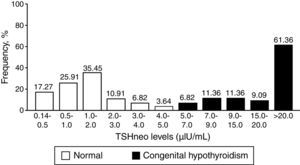
Fig. 1 depicts the distribution of the TSHneo values in Groups I and II. The results show that the TSHneo values of 78.63% of the infants with a normal NST ranged from 0.14 to 2.0μIU/mL, while the values of the remaining 21.37% ranged from 2.0 to 5.0μIU/mL. Among the infants with CH, the initial TSHneo values were higher than 9.0μIU/mL in 81.81% of the cases; the vast majority of the infants had values higher than 20.0μIU/mL. The proportion of false-positive cases detected with the TSHneo cutoff of 5.0μIU/mL corresponded to a proportion of 0.02%.
The mean TSHneo value of the infants with a normal NST was 1.40μIU/mL (SD=1.2; ranging from 0.14 to 4.70μIU/mL). The median TSHneo values differed significantly between Groups I (1.15μIU/mL) and II (26.55μIU/mL) (p<0.001).
Fig. 2 depicts the results of the confirmatory tests (serum TSH and free T4).
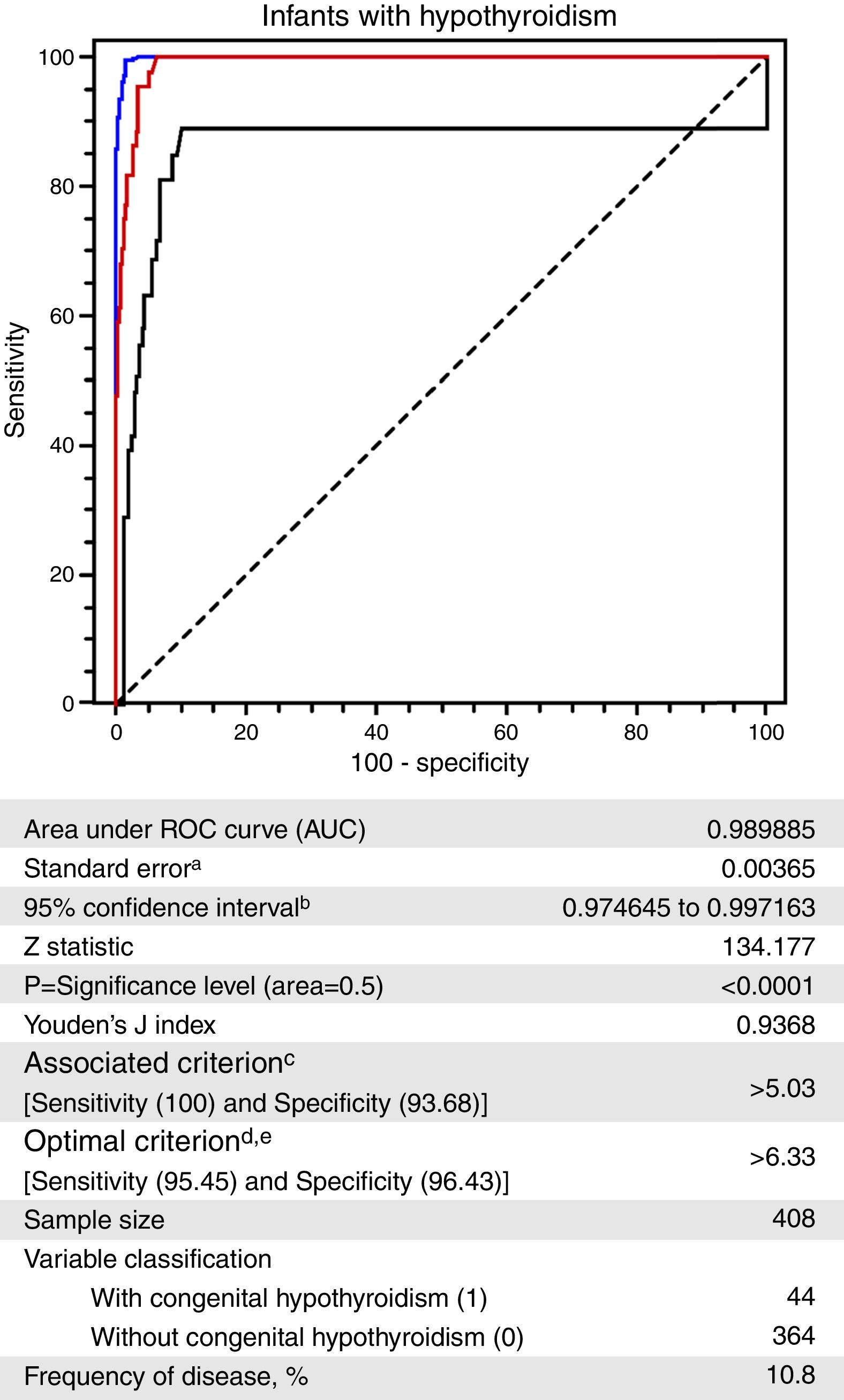
The ROC curve constructed with the TSHneo values of Groups I and II is depicted in Fig. 3.20 A TSHneo value of 5.03μIU/mL exhibited the highest specificity (96.68%; 95% CI=90.7–96.0) for a sensitivity of 100% (95% CI=92.0–100.0). A cutoff value of 6.33μIU/mL exhibited the best combination of sensitivity (95.45%; 95% CI=84.5–99.4) and specificity (96.45%; 95% CI=94.0–98.1).
Receiver operating characteristic (ROC) curve for the neonatal thyroid-stimulating hormone (TSHneo) levels of infants without and with a confirmed diagnosis of congenital hypothyroidism, Newborn Screening Program, state of Mato Grosso, Brazil, 2010–2012.
a Hanley & McNeil,20 1982.
b Binomial exact.
c Greater specificity for a sensitivity of 100%.
d Higher sensitivity and specificity associated and above 95%.
e Taking into account disease prevalence and estimated costs: cost false positive: 1; cost false negative: 1; cost true positive: 0; cost true negative: 0.
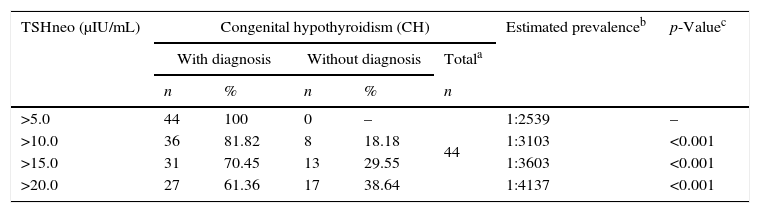
Table 1 describes the CH prevalence according to different TSHneo cutoff values. The prevalence decreased consistently as the TSHneo cutoff value increased (p<0.001).
Percentage distribution according to the neonatal thyroid-stimulating hormone (TSHneo) cutoff value and the estimated CH prevalence of the 44 infants in Group II, Newborn Screening Program, state of Mato Grosso, Brazil, 2010–2012.
The present study assessed the ability of various TSHneo cutoff values to detect CH cases and evaluated the effects of these levels on neonatal screening for CH in a population assessed using the NSP-MT. The results showed that the prevalence of CH increased over time as the adopted TSHneo cutoff value decreased. The TSHneo cutoff value of 5.0μIU/mL proved to be the most effective for detecting CH in the investigated population.
A total of 50 cases of CH were detected during the study period. The corresponding prevalence (1:2234) was four times higher than the prevalence found by Stranieri and Takano21 at the same NSP in 2003–2004, which was 1:9448 live births using the TSHneo cutoff value of 15.0μIU/mL by enzymatic-colorimetric assay. The difference in CH prevalence among neonatal screening services that use different laboratory methods for detection is present in the literature.22 Using a similar laboratory method, Mengreli et al.8 found an increased prevalence of permanent CH (1:1749 vs. 1:3384) when different TSHneo cutoff values were used.
Studies conducted in the United States showed that changes in the TSHneo cutoff value13 could result in an increase in CH prevalence. Therefore, the data reported here strongly indicate that the change in the TSHneo cutoff value is likely the main factor that caused the increase in CH prevalence in the NSPs of MT.
The average of TSHneo concentration in Brazilian infants from the state of Sergipe at age 2 to 6 days old was 1.33±1.08μIU/mL,23i.e., similar to the TSHneo concentration in the present study (1.40±1.02μIU/mL). Despite the high average temperature in the state of MT, seasonal variations and effects associated with sample storage at the collection site do not seem to have influenced the TSH values in the present study, as samples that were stored for more than 30 days were not included in the analysis.
The TSHneo value was higher than 20.0μIU/mL in 61.36% of the infants with CH in the present study, with a mean of 93.97±98.15μIU/mL. These findings agree with those reported by Ramalho et al.,23 who found values higher than 19.70μIU/mL in 62.5% of infants with CH.
The efficiency of any NSP depends of the choice of the cutoff values, which should have a high sensitivity while maintaining specificity. Increasing the cutoff value increases its sensitivity, but there will also be an increase in false-positive results.24
In the present study, the TSHneo cutoff value of 5.0μIU/mL detected 50 cases of CH from which many would not have been detected using higher TSH cutoff values. The better ability to detect CH cases might indicate the diagnostic power of lower TSH cutoff levels and reflect the structuring of the local NSP. Botler et al.25 analyzed various TSH cutoff values for detecting CH and found 42 cases using a cutoff value of ≥20.0μIU/mL in 2005, but 165 cases were found in 2007 when the cutoff value was reduced to ≥10.0μIU/mL. Korada et al.6 found 120 infants using a TSHneo cutoff value of 6.0μIU/mL, unlike the study by Ramalho et al.,23 where eight cases of CH were detected with cutoff of 5.2μIU/mL.
In the present study, the TSHneo cutoff value of 10.2μIU/mL, with sensitivity of 81.82% (95% CI: 67.3–91.8) and specificity of 98.08% (95% CI: 96.1–99.2), would have failed to detect approximately 18% of the screened infants who required further diagnostic investigation. In the study by Tu et al.,26 the sensitivity of the TSHneo cutoff point of 10.0μIU/mL using the DELFIA method (immunoassay system for routine screening and diagnostic programs) was better (98.93%) than the sensitivity found in the present study; however, the specificity (99.48%) was similar in both studies. Here, with a TSH cutoff value of 15.5μIU/mL, almost 30.0% of the CH cases would be missed because of the low sensitivity of the test (68.18%; 95% CI: 52.4–81.4). However, healthy infants with values below 10.2μIU/mL or 15.5μIU/mL would be correctly identified as healthy in 98.0% of the cases.
The odds of finding healthy infants with TSHneo values of 30.8μIU/mL were zero because of the high specificity (100%; 95% CI: 99.0–100) and very low sensitivity (47.73%, 95% CI: 32.5–63.3) of that cutoff value, which would have failed to detect more than 50% of the infants at actual risk of disease.
That shortcoming notwithstanding, because of its positive predictive value of 100%, TSHneo values above 30.8μIU/mL reliably indicated the need for treatment before the confirmatory test results were available, as disease was confirmed in all of the infants who exhibited values above that level. According to Léger et al.,4 when the TSHneo values are above ≥40.0μIU/mL, it is recommended to start treatment after a good venous sample is obtained, without waiting for the confirmatory test result.
In the present study, TSHneo cutoff values of 15.0 and 20.0μIU/mL would have failed to detect 30% and 40%, respectively, of the screened infants who required confirmation of disease. Barone et al.18 found that in 24.4% of infants with CH and TSHneo values at the lower end of the range, their values varied from 4.5 to 5.4μIU/mL. In the present study, 6.82% of the infants with CH exhibited TSHneo values from 5.0 to 7.0μIU/mL.
This analysis was based on a TSH cutoff value of 5.0μIU/mL, the accuracy of which is shown by the ROC curve (area under the curve=98.98%) and p<0.0001. In addition, that cutoff value exhibited 100% sensitivity and 93.68% associated specificity. Considering the equivalent result for these two measures, the Youden index of 0.9368 has indicated that the choice of TSHneo cutoff value is most likely to be correct, as it was associated with the lowest proportion of misclassification.
One of the limitations of this study is the non-inclusion of children who underwent screening by private and contracted health care system, and even a small proportion who did not perform the exam. However, it must be stressed that all live births in the public health care system who underwent NST were included. The other limitation is related to the fact that some infants have not yet been reassessed for identifying the transient form of CH because they are under 3 years old.
The results obtained suggest that in the population of infants screened for CH using the NSP-MT, the low TSHneo cutoff value adopted may have contributed to the high prevalence of the disease found. This study shows that the current threshold of TSHneo at the NSP-MT in terms of cost-effectiveness is the most adequate cutoff point for the detection of CH; it was able to detect all cases of CH, including those milder forms of the disease. However, this protocol increased the occurrence of false-positive tests that were associated with increased psychological effects on parental anxiety. This proportion of false-positives could be a limitation to the TSHneo cutoff of 5.0μIU/mL, but as it corresponded to such a small proportion (0.02%), it did not contribute to the increased costs of the program. The possibility of detecting the largest possible number of cases of the disease justified the adoption of this cutoff.
Unfortunately, whether or not the additional mild cases of CH detected in this way are associated with a decrease in cognitive performance is not known and, therefore, whether or not they need to be screened, ensuring significant increase in the cost of recall, is controversial at the present. According to Léger et al.,4 the aim of a neonatal screening should be to detect all forms of primary CH, especially the more severe. Besides that, further studies are needed to assess the cost-effectiveness of adopting lower cutoff of TSHneo for the state program.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Silvestrin SM, Leone C, Leone CR. Detecting congenital hypothyroidism with newborn screening: the relevance of thyroid-stimulating hormone cutoff values. J Pediatr (Rio J). 2017;93:274–80.