To identify factors that contribute to the increased susceptibility and severity of COVID-19 in obese children and adolescents, and its health consequences.
SourcesStudies published between 2000 and 2020 in the PubMed, MEDLINE, Scopus, SciELO, and Cochrane databases.
Summary of findingsObesity is a highly prevalent comorbidity in severe cases of COVID-19 in children and adolescents; social isolation may lead to increase fat accumulation. Excessive adipose tissue, deficit in lean mass, insulin resistance, dyslipidemia, hypertension, high levels of proinflammatory cytokines, and low intake of essential nutrients are factors that compromise the functioning of organs and systems in obese individuals. These factors are associated with damage to immune, cardiovascular, respiratory, and urinary systems, along with modification of the intestinal microbiota (dysbiosis). In severe acute respiratory syndrome coronavirus 2 infection, these organic changes from obesity may increase the need for ventilatory assistance, risk of thromboembolism, reduced glomerular filtration rate, changes in the innate and adaptive immune response, and perpetuation of the chronic inflammatory response.
ConclusionsThe need for social isolation can have the effect of causing or worsening obesity and its comorbidities, and pediatricians need to be aware of this issue. Facing children with suspected or confirmed COVID-19, health professionals should 1) diagnose excess weight; 2) advise on health care in times of isolation; 3) screen for comorbidities, ensuring that treatment is not interrupted; 4) measure levels of immunonutrients; 5) guide the family in understanding the specifics of the situation; and 6) refer to units qualified to care for obese children and adolescents when necessary.
Identificar fatores que contribuem para o aumento da suscetibilidade e gravidade da COVID-19 em crianças e adolescentes obesos e suas consequências para a saúde.
Fontes de dadosEstudos publicados entre 2000 e 2020 nas bases de dados PubMed, Medline, Scopus, SciELO e Cochrane.
Síntese dos dadosA obesidade é uma comorbidade altamente prevalente em casos graves de COVID-19 em crianças e adolescentes e o isolamento social pode levar ao aumento do acúmulo de gordura. Tecido adiposo excessivo, déficit de massa magra, resistência à insulina, dislipidemia, hipertensão, altos níveis de citocinas pró-inflamatórias e baixa ingestão de nutrientes essenciais são fatores que comprometem o funcionamento dos órgãos e sistemas no indivíduo obeso. Esses fatores estão associados a danos nos sistemas imunológico, cardiovascular, respiratório e urinário, juntamente com a modificação da microbiota intestinal (disbiose). Na infecção por SARS-CoV-2, essas alterações orgânicas causadas pela obesidade podem aumentar a necessidade de assistência ventilatória, risco de tromboembolismo, taxa de filtração glomerular reduzida, alterações na resposta imune inata e adaptativa e perpetuação da resposta inflamatória crônica.
ConclusõesA necessidade de isolamento social pode ter o efeito de causar ou agravar a obesidade e suas comorbidades e pediatras precisam estar cientes desse problema. Diante de crianças com suspeita ou confirmação de COVID-19, os profissionais de saúde devem 1) diagnosticar o excesso de peso; 2) aconselhar sobre cuidados de saúde em tempos de isolamento; 3) fazer a triagem de comorbidades, garantindo que o tratamento não seja interrompido; 4) medir os níveis de imunonutrientes; 5) orientar a família respeitando as especificidades da situação; e 6) encaminhamento a unidades qualificadas para cuidar de crianças e adolescentes obesos, quando necessário.
Since December 2019, the world has been surprised by the appearance, in China, of a severe pneumonia caused by a new type of coronavirus, an infection that spread rapidly throughout countries, being considered a pandemic three months later; the disease received the name coronavirus disease 2019 (COVID-19).1,2 As in adults, but less frequently, children with comorbidities – chronic kidney and lung diseases, malignancies, diabetes, obesity, sickle cell anemia, immune disorders, chromosomal abnormalities, heart disease, and congenital malformations – are more likely to develop severe conditions from COVID-19.3–6 The present review aims to identify the factors that contribute to the increase in the susceptibility and severity of COVID-19 in obese children and adolescents, and its health consequences, to collaborate for better clinical care of these patients.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and COVID-19Although less frequently, COVID-19 affects the pediatric age group. Some studies indicate that incidence of COVID-19 among children and adolescents can reach 5% of confirmed cases,7 being slightly higher in males.3,8 In addition, it presents with less severity when compared to adults. In the United States, in March 2020, hospitalization rates among individuals under the age of 17 ranged between 0.1 and 0.3/100,000 inhabitants.9 Likewise, mortality among children and adolescents has been shown to be low. An epidemiological study in China with 2135 individuals under 18 years of age described only one death; infants presented, proportionally, a greater number of severe and critical cases.8 In a systematic review10 involving 2228 patients under 16 years of age, two deaths were found, one of which was the same reported in the Chinese study.8
Children and adolescents seem to acquire SARS-CoV-2 mainly through contact with infected family members.4,11–13 However, the role of children and adolescents in transmission remains unclear;12 the presence of SARS-CoV-2 in the oropharynx and stools of asymptomatic and symptomatic individuals has been described14–16 and viral load does not differ from that of adults.17 Vertical transmission seems to be rare, with few cases described.18–20 To date, no viable viral particles have been identified in breast milk, although SARS-CoV-2 RNA has been detected in three samples.21 One study reported the presence of anti-SARS-CoV-2 IgA in breast milk of women who recovered from COVID-19.22 Tests to identify the virus in the umbilical cord, placenta, and amniotic fluid have also been negative.23–26
The incubation period observed in a series of 11 studies involving individuals under the age of 16 was two to 25 days (mean=7).13 Most children and adolescents affected by COVID-19 have mild to moderate symptoms, with a significant percentage of asymptomatic patients;13,27 among those with severe symptoms, a small percentage will require intensive care13 and the highest proportion appears to be concentrated in children under 1year of age.28 A systematic review found that, in 13 newborns infected with SARS-CoV-2 during perinatal period, most remained asymptomatic or had mild symptoms.29 The most common symptoms of SARS-CoV-2 infection among children and adolescents are cough and fever; sore throat, sneezing, myalgia, wheezing, fatigue, rhinorrhea, nasal obstruction, diarrhea, and vomiting; hypoxia and dyspnea are uncommon findings;10,11,13,27,30 in a Chinese study with people under 16 years of age with SARS-CoV-2 infection, 28.7% presented with tachypnea.4 Discrete changes – such as leukocytosis, leukopenia, lymphopenia, and small elevation of acute phase proteins – were the most common laboratory alterations.4,13,30,31 Radiographic changes are generally less pronounced than in adults, with unilateral or bilateral irregular opacification standing out in some case series.3,4,13,31 In a systematic review that analyzed chest computed tomography of under 18 age patients, ‘ground glass’ opacification was observed in 37.8% of examinations, most commonly unilateral in lower lobes, and was considered mild.32
The reasons for the lower severity of COVID-19 in pediatric age group remain unanswered. Some hypotheses have been raised: less exposure to SARS-CoV-2 due to social isolation and closure of schools; lower frequency of comorbidities and exposure to smoking when compared to adults; and greater capacity for pulmonary regeneration.33,34 Children have less angiotensin-converting enzyme 2 (ACE-2) expression than adults, making the process of internalizing the virus less efficient35 and may have more effective trained innate immunity, which is an innate memory response of medium duration, due to increased exposure to viruses and vaccines.33,34,36 This phenomenon has been used to explain the lower death rates by COVID-19 in countries that carry out universal BCG vaccination, compared to those that do not adopt such strategy.33,34,37 Furthermore, children and adolescents do not have the immunosenescence observed in older individuals, a phenomenon characterized by, among other features, a chronic inflammatory state.33,34,38 Finally, particularities of the expression of ACE-2 in younger individuals, as observed in animal models,39 could limit consequences of the decreased expression of that enzyme caused by the invasion of pneumocytes by SARS-CoV-2,34 especially those related to the elevation of angiotensin-II.40
Pathophysiology of obesity and its relationship with COVID-19The relationship between obesity and viral diseases has been studied for several years. During the H1N1 epidemic, this area gained particular interest, as it was observed that obese patients had a higher risk of developing the disease, longer intensive care unit (ICU) stay, and higher mortality.41 This fact was demonstrated even for children, with impairment in immune response, especially cellular, to influenza virus, and also inadequate vaccine response when they were obese.42 Recently, during the COVID-19 epidemic in Canada, obesity was the third most prevalent demographic factor among children admitted to the ICU, behind only those with serious associated diseases, immunosuppression, and cancer.6 In New York, obesity was the most prevalent comorbidity among 50 severe cases of COVID-19 affecting children and adolescents.43
COVID-19 and risks related to obesity among adultsRegarding COVID-19, observations at the beginning of the pandemic demonstrated the existence of risk factors, such as arterial hypertension, cardiovascular diseases, diabetes, chronic respiratory conditions, and cancer;44 however, obesity was later included in this list.45 In March 2020, Wu et al.,46 describing the characteristics of 280 Chinese patients, found a statistically significant difference between the BMI of patients with mild and moderate conditions (23.6kg/m2) and severe ones (25.8kg/m2), but this fact did not attract the attention of these researchers, because they were unable to demonstrate the independence of BMI as a risk factor. Simonet et al.,47 in April 2020, showed a high prevalence of obesity among patients with COVID-19 exposed to mechanical ventilation. In addition, the proportion of people who needed this intervention increased according to BMI, reaching 85.7% when BMI was over 35kg/m2.47 Finally, they found that obesity was a risk factor regardless of age, gender, or presence of diabetes or hypertension, and the risk of requiring invasive mechanical ventilation was 7.36 times higher when patients with a BMI greater than 35kg/m2 were compared to those with BMI less than 25kg/m2.47 Other studies confirmed these findings: Bhatraju et al.,44 reporting the first cases in the Seattle region (United States), found a mean BMI of 33.2 among 24 critically ill patients admitted to the ICU. Among Italians hospitalized for COVID-19, Busetto et al.48 found that those with overweight and obesity, even if younger, needed assisted ventilation and intensive care more frequently than patients with normal weight. Data from New York, regarding 770 patients, showed that obese people were more likely to have fever, cough, and dyspnea, in addition to significantly higher rates of ICU admission or death.49 More recently, with accumulated data from three different populations, a systematic review confirmed obesity as an independent risk factor for greater severity of COVID-19, including admission to the ICU. Finally, a very relevant finding was the demonstration, by Yates et al.,50 that the risk of acquiring the disease is greater among obese people.
COVID-19 and risks related to obesity among children and adolescentsIt is still not possible to explain why the number of children affected and their manifestations vary among different regions.51 The effects of pediatric obesity on COVID-19 are not yet adequately studied and some data are inferences due to the lack of considerable number of studies published on this subject in this age group. The three main risk factors that link obesity to COVID-19 demonstrated for adults52 are also present among children and adolescents: chronic subclinical inflammation, impaired immune response, and underlying cardiorespiratory diseases. Virtually all comorbidities found in adults can be observed during childhood and adolescence,53 and obese children have inadequate immune responses to other infections, such as bacterial pneumonia,54 a common severe complications of COVID-19. Studies in animal models show that rats fed a high-fat diet have increased expression of ACE-2 in lungs, which may help explain the greater severity of the disease among obese individuals.55 Zhang et al. showed that obesity predisposes to high mortality due to COVID-19 even in young patients, aged 14 years and older56 and it is considered that it is precisely the high prevalence of obesity among young people that can shift the age curve of mortality in countries where the prevalence of overweight is higher in this group.57 The mechanisms involved include numerous aspects related to obesity itself and also to its comorbidities,6 and it should be emphasized that the risks may be present even in mildest cases of obesity.58 Below, each will be covered in detail.
Insulin resistance and dyslipidemiaIn childhood and adolescence, even in the presence of obesity, type 2 diabetes mellitus is relatively uncommon.59 The high pancreatic capacity of insulin production, characteristic of younger individuals, allows compensation to occur as a result of hyperinsulinism, which has a high prevalence associated with obesity.60 Although glycemia frequently remains at normal levels, the entire pathophysiological process is present, leading to several health repercussions, such as dyslipidemia, arterial hypertension, non-alcoholic steatohepatitis, micronutrients deficiencies, increased oxidative stress, and hyperuricemia. In situations of intense metabolic activity, such as during immune response to coronavirus infection, beta cells are required to produce a high amount of insulin, which may not be achieved when they are already working at their limit;61 SARS-CoV-2 can also lead to rupture of beta cells, through interaction with ACE-2, further aggravating this process.61 In addition, insulin resistance leads to a reduction in phosphoinositidyl 3-kinase, impairing the vasoprotective and anti-inflammatory effects of nitric oxide.62
Dyslipidemias are highly prevalent among obese children and adolescents,60 and low concentrations of HDL-cholesterol and increased LDL-cholesterol are proven risk factors for progression of endothelial dysfunction and atherosclerosis.63
Respiratory systemIn New York, obesity was the most important risk factor for necessity of respiratory support among 50 pediatric cases of COVID-19.43 Normal respiratory physiology is usually impaired in obese patients, including children and adolescents.64 As the lung is one of the main targets and leads to greater risks for patients with COVID-19, this aspect must always be considered. In fact, hematosis is impaired in obesity, which becomes even more relevant when the exchange areas are reduced due to coronavirus action.65 The pressure exerted by abdominal adiposity on the lungs, through the diaphragm, also acts to limit the movement of respiratory muscles, with less oxygen saturation66 and worsening clinical presentation due to the lower lung volume of obese patients.67 In addition, some comorbidities linked to obesity may contribute to a higher risk of pulmonary infections, such as the presence of asthma, which is highly prevalent among obese children,68 and obstructive sleep apnea.69 Regarding asthma, the same inflammatory mechanisms linked to leptin and IL-2, which explain the high prevalence and severity of this disease in obese children,70 are also involved in the severity of COVID-19. Finally, in addition to aspects related to impaired lung function, obese children have low exercise tolerance, which closes a vicious circle.71
Cardiovascular systemCardiac anatomy changes linked to obesity is recognized even in very young children, in whom hypertrophy of left ventricle is observed, related to the degree of obesity and blood pressure, among other structural changes.72 Obese children and adolescents have higher blood pressure, which increases potential endothelial injury, one of the bases of COVID-19 pathophysiology.72 Children, especially obese ones, treated with antihypertensive drugs that inhibit angiotensin-converting enzyme or block angiotensin receptors, have increased expression of ACE-2, increasing their susceptibility to coronavirus.73 Childhood obesity increases risk of cardiovascular disease later in adulthood, and the explanation for this phenomenon lies in the fact that endothelial dysfunctions, in association with insulin resistance, effectively start during childhood.74 The intima layer of arteries is thickened in obese children, foreshadowing the onset of atherosclerosis, which occurs very early.75 Endothelial dysfunction occurs even in the mildest cases of obesity.76 Hardening of the arteries, associated with impaired nitrogen performance and chronic oxidative stress, has been implicated in changes linked to the severity of COVID-19, such as inflammation of endothelium, myocarditis, multiple organ failure, severe acute respiratory syndrome, and venous thromboembolism.62 Recent data from post mortem anatomopathological studies shows inclusion of coronavirus structures in endothelial cells, possibly through the use of ACE-2 receptors in the endothelium by the virus; in these cases, accumulation of inflammatory cells, venous congestion in small pulmonary veins, and inflammation of the endothelium in the intestinal circulation have been found.77 Leptin, which is usually elevated among obese people, damages endothelium leading to less nitric oxide production and increased expression of monocyte chemoattractant protein-1, contributing to the inflammatory infiltrate in vascular cells.62 Perivascular adipose tissue contributes to vasoconstriction and endothelial dysfunction through the production of inflammatory mediators, oxidative stress, and reduction in nitric oxide production.62
Subclinical inflammationOne of the most relevant aspects for understanding the severity of COVID-19 among obese patients is related to inflammatory issues.78 After coronavirus contamination, most patients develop immune defense mechanisms, which include processes related to inflammation, and this happens in a modulated way, so that the host organism is not harmed. However, some patients trigger an uncontrolled process, known as a cytokine storm, which causes tissue damage and intense homeostatic dysregulation, leading to damage of several organic functions, especially regarding the respiratory area.79 Obese patients are known to have chronic subclinical inflammation, characterized by a permanent inflammatory state, albeit of mild intensity.80 High serum concentrations of C-reactive protein and IL-6 prove this process61 which can start early. It is believed that, at least in part, this process is due to cytokines, particularly adipokines with inflammatory properties, produced by adipose tissue61 and also the drop in adiponectin, which has anti-inflammatory properties.81
CoagulationObese people, including children and adolescents, with COVID-19 are at increased risk of developing coagulopathy associated with poor clinical outcomes. Chronic inflammation leads to negative regulation of anticoagulant proteins (tissue factor pathway inhibitor, antithrombin, and the protein C anticoagulation system). However, it leads to positive regulation of procoagulant factors (tissue factor pathway inhibitor) and adhesion molecules (P-selectin), in addition to increases in thrombin generation and enhanced platelet activation, increasing the risk of thrombosis.62 In severe SARS-CoV-2 infections, risk of venous thromboembolism is important, as a significant percentage of patients show elevated levels of D-dimers, while others meet clinical criteria for disseminated intravascular coagulation (DIC).62 Severe infections and sepsis are a leading cause of DIC, and pro-inflammatory and immune activation observed in severe COVID-19 is likely sufficient to trigger DIC.82
Renal systemObesity causes several structural, metabolic, and hemodynamic changes in the kidneys, leading to a lesser functional reserve of this organ.83 Ectopic deposition of fat in renal sinus is responsible for increasing its weight and volume. Hemodynamic changes lead to increased renal plasma flow and glomerular filtration rate, greater absorption of water and sodium by proximal tubules, glomerular stress, tubular hypertrophy, and glomerulomegaly, which in turn cause proteinuria and secondary glomerular sclerosis, culminating in chronic kidney disease.84 The increase in body weight and consequent reduction in urinary pH predispose to urinary lithiasis due to increased excretion of urinary oxalate, sodium, phosphate, and uric acid; obesity can also favor the appearance of some types of neoplasia in renal tissue.85 The dysregulation of lipid metabolism and hormonal responses also play a role in deterioration of renal function. Oxidative stress caused by increased fat deposition promotes inflammation, cellular hypertrophy, increased mesangial matrix, apoptosis, endothelial dysfunction, and renal fibrosis.86 Fatty acids released by adipocytes stimulate secretion of tumor necrosis factor (TNF)-alpha by macrophages, resulting in increased secretion of IL-6 in adipocytes, amplifying inflammation in renal tissue. While TNF-alpha plays a fundamental role in progression of renal fibrosis, the increase in intracellular lipids has a nephrotoxic effect (culminating in glomerulosclerosis), compromising the structure and functioning of mitochondria, which contributes to progression of kidney disease.87 Increased insulin production and insulin resistance contribute to mesangial expansion and renal fibrosis, and the observed activation of the renin-angiotensin-aldosterone system, since the vasoconstrictor effect of angiotensin II on renal arterioles leads to increased production of endothelin-1, stimulating proliferation of mesangial matrix, sodium retention, and vasoconstriction of renal arterioles.88 Coronavirus can cause acute kidney damage in up to 15% of cases, which contributes to mortality. Aggression is due to direct cytotropic effect induced by the virus through ACE-2, which is highly expressed in the kidney,89 and inflammatory response caused by cytokines due to activation of the renin-angiotensin-aldosterone system.90,91 In addition, acute tubular necrosis and thrombotic effects secondary to endothelial dysfunction are also observed in COVID-19.92
Gut microbiotaIntestinal microbiota is a complex ecosystem with thousands of bacterial phyla and several species distributed throughout digestive tract. It is mostly composed of anaerobic microorganisms and about 90% of fecal bacteria belong to two phylogenetic lineages: Firmicutes and Bacteroidetes.93 Colonization occurs from birth and is individually different, changes over time, and can be influenced by eating habits or diseases, such as obesity and metabolic syndrome. Several studies have demonstrated a correlation between Firmicutes/Bacteroidetes ratio in both obese children and adults, suggesting intestinal dysbiosis.94 Regarding patients with COVID-19, there are reports of intestinal dysbiosis and a decrease in intestinal Lactobacillus and Bifidobacterium populations, and some hospitalized patients were treated with probiotics in order to regulate microbiota balance and reduce risk of infection secondary to bacterial translocation.95
Immune systemObesity in childhood and adolescence alters entire immune system, changing concentrations of cytokines and proteins and the number and function of immune cells. This imbalance leads to a pro-inflammatory state, resulting in the onset or exacerbation of several diseases such as asthma, allergy, atopic dermatitis, and sleep apnea.96 In relation to COVID-19, whose severe conditions evolve with an intense and severe systemic inflammatory reaction (cytokine storms), the imbalance of immune system observed in obesity may contribute to a worse clinical outcome.79 Adipocytokines, especially leptin, play an important role in this process, as they influence number and function of immune cells through directly effects on cell metabolism. In this context, there may be an increase in cytotoxic and effector T-cells (Th1 and Th7) and M1 macrophages and, in parallel, a reduction in Treg cells and M2 macrophages. However, other molecules that are influenced by nutritional status also act on immunity, which may be increased (IL-1β, IL-6, IL-8, IL-10, IL-1RA, resistin, visfatin, TNF-α, MCP-1, MIF, MIP1 alpha and beta) or reduced (adiponectin, IL-33) in obese patients.62 In addition, imbalance between actions of lymphocytes Treg and CD17+ also contributes to the pro-inflammatory state observed in obesity.97 Furthermore, in obesity, macrophages cells constitute about 40% to 60% of cells of immune system derived from visceral adipose tissue; these macrophages are mostly activated (M1) and secrete high amounts of TNF-alpha, IL-6, IL-12, Il-1b, MCP-1, and nitric oxide.97
Nutrition and immunonutrientsNutrition plays an important role in immune and inflammatory response, since some nutrients modulate cellular and humoral defense systems, either by modifying formation of inflammatory mediators or interfering with cellular signal transduction pathways. Nutrients have an immunomodulatory action by stimulating the cell-mediated response, modifying the balance between pro-inflammatory and anti-inflammatory cytokines, and attenuating depletion of tissue nutrients.98 Immune response depends on the sufficient supply of nutrients and adequate nutritional status has been considered an important element for coronavirus capacity response. Zhang & Liu,99 in a systematic review, showed that some nutrients are fundamental for adequate response to coronavirus: vitamins A, C, D, and E; omega 3 fatty acids; and the minerals zinc and iron. A suitable qualitative and quantitative supply allows to maintain and repair defense systems, which require adequate energy and structural support.100 Obesity has peculiarities that may impair immune response, because diet often has characteristics that can lead to “hidden hunger.” This is because, despite eating above energy needs, quality is not adequate; numerous studies point to vitamin and mineral deficiencies in those with excess weight.101
Among the most common nutritional deficiencies, hypovitaminosis D stands out, not only linked to insufficient intake, but also, and mainly, to the displacement of part of the organic pool to adipose tissue due to the lipid affinity of this vitamin.102 Additionally, it is known that a sedentary lifestyle is characteristic of obese children, causing the practice of physical activities outdoors – which would increase exposure to sun and skin formation of vitamin D – to be reduced in this group.102 Several studies have linked hypovitaminosis D to an increased risk of severe COVID-19,78 which makes this issue particularly relevant in the pediatric obese population, where the prevalence of this deficiency is high.103 Vitamin D (VD) has immunomodulatory effects, and activated T-lymphocytes and antigen presenting cells, such as macrophages and dendritic cells, express VD receptor in their membranes, leading to anti-proliferative and immunosuppressive effects on immune system cells. It inhibits secretion of IL-12 by macrophages, a fundamental cytokine in differentiation of CD4+ T-lymphocytes in Th1 cells.104 By decreasing activation of Th1 response and production of pro-inflammatory cytokines (IL-2, interferon-γ, TNF-α), VD contributes to the targeting and activation of Th2 response, favoring greater secretion of anti-inflammatory cytokines, such as IL-4.104 This immunosuppression mechanism is important to minimize deleterious effects in transplants, and in autoimmune and inflammatory diseases. Although VD induces Th2 response, it also induces expression of antimicrobial peptides in neutrophils and monocytes, as well as promotes an increase in phagocytic capacity and rapid release of reactive oxygen species.104 Induction of cathelicidins and defensins, caused by VD, reduces viral replication and concentration of proinflammatory cytokines that have potential for lung injury in cases of COVID-19.105 Additionally, hypovitaminosis D has repercussions for disorders with potential impact on COVID-19, such as arterial hypertension, hepatic steatosis, and hyperuricemia.106
Omega-3 fatty acids are considered essential lipids for humans. Eicosapentaenoic (EPA) and docosahexaenoic (DHA) fatty acids are biologically more potent immunomodulators than alpha-linolenic acid. EPA and DHA decrease activity of nuclear transcription factors that promote transcription of genes that encode proteins with pro-inflammatory action, such as TNF-α and IL-1β.107 In addition, EPA and DHA compete with arachidonic acid (omega-6) in the constitution of plasma membrane phospholipids and, consequently, for the metabolism of cyclooxygenase in cell membrane, modulating the production of prostaglandins and leukotrienes.108 Higher concentrations of EPA and DHA favor synthesis of series-3 prostaglandins and series-5 leukotrienes, which attenuate inflammatory response; and inhibit production of series-2 prostaglandins and series-4 leukotrienes, which depress thecytotoxic activity of macrophages, lymphocytes, and natural killer cells,109 with a consequent reduction in synthesis of pro-inflammatory cytokines such as IL-1, IL-6, and TNF-α.107 Immunomodulation exerted is dependent on omega-3/omega-6 ratio: a 1:5 ratio does not impair immune response; however, Western diets provide a ratio of 1:15 to 1:50, with pro-inflammatory effects.110 Consumption of omega-3 fatty acids, especially DHA, is low in most countries in the world, including Brazil.111 Although omega-3 precursors are present in vegetable oils, their conversion into DHA is low and the very excess of omega-6 intake diverts the converting enzymes, further impairing the formation of DHA and contributing to a high deficiency prevalence. Inadequate proportion of omega-3/omega-6, common in obese children,112 leads to loss of modulation of immune response, which may contribute to exacerbation of inflammatory reactions, in addition to aggravating cardiometabolic risks.113
Vitamins A, E, and C are fundamentally found in fruits and vegetables, usually little consumed by children and adolescents, including obese ones.114 Impaired nutritional status of these vitamins may impact immune response.115 Immune cells are at constant risk of oxidative damage by free radicals, which can permanently impair their ability to respond to pathogens. Increased amounts of free radicals in activated macrophages are part of normal response. Vitamin E (VE) acts as an antioxidant and protects the cell membrane against reactive oxygen species.114 Animal studies have shown that VE supplementation increases resistance to infections, including influenza viruses.116 Vitamin A (VA) guarantees the regeneration of the mucosal barriers damaged by infection and supports protective function of macrophages, neutrophils, and natural killer cells.117 It is also necessary for adaptive immunity and plays a role in development of T- and B-cells. Like VD, VA can prevent production of IL-12 cytokines by macrophages, decreasing activation of the Th1 response and increasing Th2.117 VA deficiency impairs Th2 response, which culminates in a lack of IL-4 and fails to induce IgA, impairing salivary IgA response to influenza virus infection, and intestinal response to cholera toxin.117 Vitamin C (VC) contributes to the integrity of the epithelial barrier and accumulates in phagocytic cells, such as neutrophils, which improves chemotaxis; phagocytosis; production of reactive oxygen species; and induces microbial death.118 It also contributes to apoptosis and removal of neutrophils, which have suffered cell death, from infection sites, reducing necrosis and possible tissue damage.118 VC seems to promote differentiation and proliferation of B- and T-cells, probably due to its genetic regulatory effect.118,119 Deficiency also impairs cytotoxic capacity of neutrophils and T-lymphocytes.114 VC supplementation appears to be able to prevent and treat respiratory and systemic infections,118,119 and a recent review highlights VC, as well as zinc and VD, as micronutrients with stronger evidence regarding their role in immunity.120
In relation to iron, obese children are at risk for iron deficiency anemia121 due to the low nutritional quality and low iron bioavailability of the diet122 and anemia has been widely demonstrated in this group.123 In addition to inadequate intake, hepcidin, the main controller of iron absorption, has increased expression in obese individuals, contributing to the condition by reducing absorption of iron by enterocytes and their export by macrophages and hepatocytes, increasing splenic and hepatic sequestrations.124
Due to the fact that they have similar nutritional sources, zinc deficiency is also believed to be present among obese children in the same way as iron deficiency, and this has been demonstrated in studies in pediatric age group.125 It should be noted that, in addition to its immunological role, zinc also participates in insulin and leptin metabolism, which can aggravate metabolic dysregulations in obese children, contributing to inadequate inflammatory response.126 Zinc acts as a cofactor for the enzyme superoxide dismutase, which reduces cellular oxidative stress and decreases activation of signaling pathways that promote inflammatory response.127 It induces monocytes to produce IL-1 and IL-6, and to inhibit the production of TNF-α, and is also involved in regulation of peroxisome proliferator-activated receptors (PPARs), whose activation is positively correlated with decrease in inflammatory response. The direct influence of zinc on immune system is due to its ability to stimulate activity of enzymes involved in mitosis.128 Zinc deficiency is related to decreased production of cytokines and interferon-α by leukocytes, atrophy of the thymus and other lymphoid organs, and changes in the proportion of lymphocytes.128
Psychosocial repercussions of COVID-19 on obesityIf, on the one hand, obese patients exhibit COVID-19 with particular characteristics, on the other hand, the pandemic has also led to socioeconomic changes that may impact childhood obesity,129 especially among the poorest.130 In this sense, an important study projected the impact of COVID-19 pandemic on the prevalence of childhood obesity in United States, according to different scenarios:131 closing schools for two months; school closures for two months associated with a 10% drop in physical activity in two subsequent months of summer; adding two more subsequent months of closed schools; adding two more subsequent months of school closures. The increase in prevalence of obesity would be 0.640, 0.972, 1.676, and 2.373 percentage points, respectively. In Italy, Pietrobelli et al.132 followed 41 obese children and adolescents during three weeks of lockdown in Verona and found no changes in vegetables consumption, but observed increased consumption of fruits, chips, red meat, and sugary drinks; the time spent in sports activities was reduced by two and a half hours per week and, in contrast, sleep period increased by 0.65h/day; and the most impactful data refers to screen time, which increased by 4.85h/day. An interesting aspect is the idea often disseminated in lay texts that physical activity should be avoided to protect immunity and supposedly that exercise could reduce body’s defenses, a fact that has not been scientifically demonstrated, even among athletes.133 On the contrary, physical activity is important for the proper formation of VD when performed outdoors.102 In fact, with regard to situations related to sedentary lifestyle, such as watching TV or playing video games, changes occur that are related to higher risk of obesity, such as high consumption of fast food and sugar-sweetened beverages,134 in addition to sleep disorders.135 In relation to this last aspect, the COVID-19 pandemic brings high risks for health, as several factors can contribute to quality and duration of sleep being unsatisfactory, such as stress in face of illness, interruption of activities in the morning, time available for screen use, etc.132 In this sense, in addition to predisposing to weight gain and abdominal adiposity, sleep disorders have other health repercussions, such as insulin resistance, deterioration in food quality, poor school performance, and sedentary lifestyle.136
Living with stress during COVID-19 pandemic, in addition to bringing risks of deterioration of immunity,137 may have consequences for pediatric health, in particular for nutritional and emotional areas.138 One such consequence refers to the worsening of sleep quality, discussed above. Additionally, stress increases food consumption, activates brain reward centers that raise interest in highly palatable foods (sugar, salt, and lipids), increases emotional instability, and worsens quality of life.138 Due to the prolonged time of social isolation, another aspect demonstrated in the United States was a drop in adherence to immunization programs, due to the fear of taking children to vaccinate.139 Finally, a Brazilian study showed a high incidence of peri-obstetric mortality due to COVID-19140 and this fact, in cases where delivery is feasible, may lead to a large number of infants being deprived of breastfeeding, known as a protective factor against obesity.141
Final considerations and complications for treatmentThe present review has two important limitations. The first is that it is not possible, currently, to carry out a systematic review of the literature with the expected rigor in study classification, since most of published data is based on observations made less than a year ago and, generally, refer to observational and cross-sectional studies. The second is that there are few publications referring to the pediatric age group, which means that some information is, in fact, inferences about the approach to the disease in children and adolescents, based on what is observed in adults.
When the patient presents with mild COVID-19 symptoms, the treatment is only symptomatic and recovery is usually complete,142 ranging from supportive measures for mild cases (isolation, hygiene, rest, hydration, and attention to food intake) to invasive hospital procedures, such as mechanical ventilation. The convalescence period may range from one to three weeks in mild case, or up to six weeks for the most severe.143
Non-obese children are usually asymptomatic and even less susceptible to the infection.144 In these cases, telemedicine alternatives have been proposed in order to help families receive adequate guidance without the need to travel.129 However, for those who develop the most severe forms, the coexistence of obesity can hinder therapy and worsen prognosis, since the inflammatory condition is as severe as in adults.145 Also, extensive involvement of the cardiopulmonary system is frequent146 and respiratory disorders such as Pickwick syndrome, obstructive apnea, and surfactant dysfunctions may be present.147 There are also technical issues, such as the fact that many pieces of equipment may not adequately adapt to the obese patient148 and the greater difficulty of intubation of obese patients, which may lead to the occurrence of lesions and longer periods of hypoxia while the procedure is being completed.148 Nursing care is always more difficult: the possibility of more precarious hygiene and secondary contamination, as well as harder venipuncture and more likelihood to cause injury; control procedures such as blood pressure measurement and cardiac auscultation are more difficult and more error-prone; and the risk of bedsores during intensive care unit stay is always higher the higher the patient’s weight.149
For families, supportive measures that provide high quality information and guidance to help them make decisions on medications, the need to go to the emergency room, and how to conduct emergency care are essential.129 For adolescents, adequate information can often aid to minimize extreme behaviors, both in the aspect of excessive fear, for some, and in the sense of invulnerability, for others.150
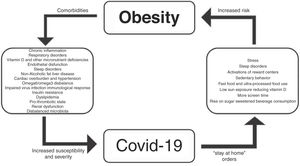
Fig. 1 shows a brief synopsis regarding the relationships between obesity and COVID-19.
In conclusion, obesity in childhood and adolescence can be considered a risk factor for greater susceptibility and severity of COVID-19 and is associated with nutritional, cardiac, respiratory, renal, and immunological alterations, which may potentiate the complications of SARS-CoV-2 infection. The need for social isolation can have the effect of causing or worsening obesity and its comorbidities, and pediatricians need to be aware of this issue. It is necessary that health professionals, when faced with the care of children with suspected or confirmation of COVID-19, carry out the assessment of nutritional status in order to diagnose overweight; be concerned with guidance on care, in periods of isolation, with the general state of health, including the areas of nutrition, immunization, and psychosocial aspects; trace comorbidities associated with obesity, ensuring that their treatment is not interrupted; screen immunonutrients levels to assess the need for supplementation; provide information to the family respecting the specificities of the condition; and determine, when necessary, referral to hospital units suitable for the care of obese children and adolescents.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Nogueira-de-Almeida CA, Ciampo LA, Ferraz IS, Ciampo IR, Contini AA, Ued FV. COVID-19 and obesity in childhood and adolescence: a clinical review. J Pediatr (Rio J). 2020;96:546–58.
Study conducted at Universidade Federal de São Carlos, Departamento de Medicina, São Carlos, SP, Brazil.