To analyze the results of the audiological evaluation of children with HIV and AIDS.
Data collectionSystematic review carried out in May 2019 in the Web of Science, PubMed, SciELO, and Scopus databases. Case reports and original articles were included, with no limitationsregarding country or year of publication.
Data synthesis278 articles were identified; 26 were included, in which HIV/AIDS was shown to be a risk factor for hearing loss (OR=5.364; p=0.00). The studies used different audiological exams, with varying methodologies. There was no difference regarding the type of hearing loss (p=0.119).
ConclusionLongitudinal studies using the same type of examination at all stages are suggested, to allow better monitoring of the effects of HIV on the child’s hearing,and studies that provide more methodological details. The knowledge of the influence of HIV on the child’s auditory system may lead to the promotion of measures that minimize the prevalence of hearing loss, allow an early diagnosis and timely rehabilitation, so as not to compromise child development.
Analisar os resultados da avaliação audiológica de crianças com HIV e AIDS.
Coleta dos dadosRevisão sistemática realizada em maio de 2019 nas bases Web of Science, Pubmed, SciELO e Scopus. Relatos de caso e artigos originais foram incluídos, sem limitação quanto ao país ou ano de publicação.
Síntese dos dadosForam identificados 278 artigos, sendo que 26 foram incluídos, nos quais o HIV/AIDS foi mostrado como fator de risco para perda auditiva (OR=5.364; p=0.00). Os estudos utilizaram diferentes exames audiológicos, com diferentes metodologias. Não houve diferença com relação ao tipo de perda auditiva (p=0.119).
ConclusãoSugere-se estudos longitudinais usando o mesmo tipo de exame em todas as fases para possibilitar melhor acompanhamento dos efeitos do HIV na audição da criança e estudos que tragam mais detalhes metodológicos. O conhecimento da influência do HIV no sistema auditivo infantil pode levar à promoção de medidas que minimizem a prevalência da perda auditiva, possibilitem diagnóstico precoce e permita reabilitação em tempo hábil para não comprometer o desenvolvimento infantil.
HIV infection leads to the progressive impairment of the affected individuals’ immunity. With the advent of antiretroviral therapy (ART), the survival of these patients has been prolonged and, consequently, there has been an increase in the spectrum of acute and chronic diseases, especially airway infections such as otitis.1–3
Otitis media can cause hearing loss and be the main cause of impairment in people with HIV/AIDS, especially in childhood, the age group most often affected by upper airway infections.1,2,4 However, hearing loss related to sensory or neural damage (direct damage by opportunistic infections or neuropathy) has also been observed.5,6 It is estimated that 20–50% of people with HIV/AIDS have different degrees of sensorineural hearing loss, and this type is more prevalent among adults than in children.1
The association between HIV/AIDS and hearing loss requires further study, as the literature shows conflicting findings, particularly those related to the type of loss caused by the disease. Nonetheless, HIV/AIDS is recognized as a risk factor for hearing impairment.7 However, there are few studies that consider the association between auditory findings and clinical and laboratory characteristics of the infection (viral load and CD4+ and CD8+Tlymphocyte counts) or the effects of ART.3,8,9
HIV, AIDS, or ART-related hearing sequelae can be better understood by analyzing studies with the pediatric population, as confounding factors could be minimized, such as the effects of chronic exposure to high sound pressure levels, effects of senility, and use of non-ART-related ototoxic medications.10
As the integrity of the peripheral and central auditory system is essential for adequate language and learning development, it is necessary to better understand the effects of HIV on children’s hearing loss to establish preventive measures and implement early diagnosis and rehabilitation measures, to reduce the limitations caused by this impairment.4,11
For this reason, the present study was proposed to analyze the results of the hearing assessment of children with HIV and AIDS available in the literature.
Data collectionIn May 2019, a systematic review of audiological findings in children with HIV and AIDS was performed through a literature search in the Web of Science, PubMed, SciELO, and Scopus databases. Studies were not limited regarding the country or year of publication.
The primary sought outcomes were: (1) the odds ratio (OR) and relative risk (RR) for hearing loss in patients with HIV; (2) the association between hearing loss and HIV status; and (3) the otoscopy performed before audiological examinations.
The keywords used in the research were the MeSH (medical subjective headings) descriptors “HIV” AND “hearing” AND “children OR childhood.” Case reports and prospective or retrospective studies on the association between HIV infection and audiological findings were included in the review. Exclusion criteria were as follows: literature reviews, short communications, abstracts from articles presented at congresses, editorials, studies not written in English or Portuguese, articles including children with HIV and other co-infections, studies on neonatal screening, articles without information regarding which audiological examinations were performed, and abstracts that were not related to the objective of this review.
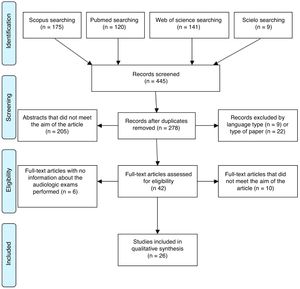
The search resulted in 445 citations, narrowed to 278 articles after removing duplicates. The authors then excluded 205 articles after reading the abstracts, nine articles because of the language, and 22 articles due to the type of study, in that order. Finally, 26 articles were selected according to the established inclusion and exclusion criteria, after an independent analysis by the two authors and the opinion of a third author, in case of divergence (Fig. 1).
Decision-making process of the articles included in this study. Adapted from Moher et al.12.
The articles were assessed regarding the number of studied children with HIV, children’s age, performed audiological exams, otoscopy findings, use of ART, CD4+ T-cell count, and viral load.
The methodological guidelines and checklist established by PRISMA12 were followed. The quality assessment of the elected articles was performed using the modified version of the Quality Assessment Tool for Systematic Reviews of Observational Studies (QATSO).13 The used criteria were as follows: measure objectivity, validation of performed audiological examinations, and probabilistic sample (except for case reports).
The data were exported to STATA 12.0 (Stata Statistical Software: Release 12. College Station, TX, USA) software for analysis. The random effect meta-analysis was performed after the heterogeneity tests were calculated using the Mantel-Haenszel method. Nonexistent values (for studies that did not use a control group) were eliminated to maximize statistical power. Due to the sample discrepancy between studies, square root transformation to OR was used to minimize the risk of statistical bias. OR and RR for hearing loss in children with HIV/AIDS were calculated.
Data synthesisThe quality assessment of the articles found that all selected studies included validated examinations and objective measures of auditory investigation. However, none of them used a probabilistic sample.
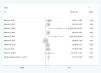
Exams performedSummaries of the exams performed are shown in Table 1. In total, five studies showed that only one type of audiological exam was performed,14–18 19 used conventional or conditioned pure tone audiometry (depending on the child’s age), eight used the auditory brainstem response (ABR) (especially in younger children), and eight used conventional tests. Some authors reported vocal audiometry (mainly hearing discrimination tests).19–21 Seven studies used otoacoustic emissions (OAE) test, of which four were the distortion product type14,22–24 and two were evoked transient tests.25,26 Most studies that included acoustic immittance measurements performed them together with audiometry or ABR to confirm the type of hearing loss (Table 2).
General characteristics of selected articles.
| Authors/year | Country | n | Sample | Audiological exams |
|---|---|---|---|---|
| Bastos et al., 201031 | Brazil | 1 | 7 years | Pure tone audiometry |
| Behavioral tests | ||||
| Buriti et al., 201329 | Brazil | 23 | 2 years–10 years and 11 months | Pure tone audiometry |
| Acoustic immittance measures | ||||
| Buriti et al., 201428 | Brazil | 23 | 2 years–10 years and 11 months | Pure tone audiometry |
| Acoustic immittance measures | ||||
| Chao et al., 20122 | Peru | 139 | 4–19 years | Pure tone audiometry |
| Acoustic immittance measures | ||||
| Chidziva et al., 201617 | Zimbabwe | 380 | 5–17 years | Pure tone audiometry (359) |
| Christopher et al., 20135 | Uganda | 370 | 6–60 months | ABR |
| Tympanogram | ||||
| Christensen et al., 199826 | United States | 1 | Three evaluations: at 21, 34, and 43 months | 21 months: VRA, OAE-TE, ABR |
| 34 months: ABR | ||||
| 43 months: VRA, ABR, tympanogram | ||||
| Govender et al., 201115 | South Africa | 78 | 3 months–12 years | ABR (based on clinical suspicion) |
| Hrapcak et al., 201625 | Malawi | 380 | 4–14 years | Pure tone audiometry (372) |
| VRA (7) | ||||
| Tympanometry | ||||
| OAE-TE | ||||
| Knox et al., 201824 | South Africa | 61 | 4–6 years | OAE-DP |
| Makar et al., 201220 | India | 67 | 4–16 years | Pure tone audiometry |
| Acoustic immittance measures | ||||
| Maro et al., 201622 | Tanzania | 131 | 1.3–18 years | Tympanometry |
| 113 HIV-negative controls | Pure tone audiometry (75) | |||
| OAE-DP (97) | ||||
| Gap detection test (48) | ||||
| ABR (90) | ||||
| Martins et al., 200134 | Brazil | 22 | 8 months–12 years | Pure tone audiometry |
| Behavioral tests | ||||
| Acoustic immittance measures | ||||
| Matas et al., 200021 | Brazil | 18 | 18 months–2 years and 6 months | Behavioral tests |
| Controls: | VRA | |||
| - Exposed: 34 | Acoustic immittance measures | |||
| - Sero-reverted: 91 | ||||
| Matas et al., 20064 | Brazil | 51 | 3–10 years | Pure tone audiometry |
| 50 HIV-negativecontrols | Acoustic immittance measures | |||
| ABR | ||||
| Matas et al., 20086 | Brazil | 18 | 1–30 months | Behavioral audiometry |
| Controls: | VRA | |||
| - Exposed: 34 (<18 months) | Acoustic immittance measures | |||
| - Sero-reverted: 91 | ||||
| Matas et al., 201027 | Brazil | 51 | 3 years–10 years and 11 months | Pure tone audiometry |
| 50 HIV-negative controls | Vocal audiometry | |||
| Acoustic immittance measures | ||||
| ABR | ||||
| Nakku et al., 201716 | Uganda | 148 | 6–12 years | Pure tone audiometry |
| 79 HIV-negative controls | ||||
| Palacios et al., 200819 | Mexico | 23 | 5 months–17 years | ABR |
| Pure tone audiometry (> 4 years: 12) | ||||
| Speech discrimination test | ||||
| Rezende et al., 200436 | Brazil | 1 | 10 years | Pure tone audiometry |
| Vocal audiometry | ||||
| Acoustic immittance measures | ||||
| Romero et al., 201732 | Brazil | 15 | 8–9 years | Pure tone audiometry |
| Vocal audiometry | ||||
| Acoustic immittance measures | ||||
| Behavioral tests | ||||
| Smith et al., 201718 | Ethiopia | 107 | 7–20 years | Pure tone audiometry |
| Taipale et al., 201133 | Angola | 78 | 9–178 months | Pure tone audiometry |
| 78 HIV-negative controls | ABR | |||
| Torre et al., 201230 | United States and Puerto Rico | 145 | 7–17 years | Pure tone audiometry |
| 86 HIV-negative controls | Tympanometry | |||
| Torre et al., 201523 | South Africa | 37 | 4–14 years | Tympanometry |
| 24 HIV-negative controls | OAE-DP | |||
| Pure tone audiometry | ||||
| Torre et al., 201514 | United States and Puerto Rico | 89 | 7–16 years | OAE-DP |
| 83 HIV-negative controls |
ABR, auditory brainstem response; OAE, otoacoustic emissions; DP, distortion product; TE, transient evoked; VRA, visual reinforced audiometry.
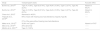
Results of acoustic immittance measurements in the selected studies.
| Study | Tympanogram (ears) | Acoustic reflex |
|---|---|---|
| Buriti et al., 201329 | Type A (10.9%), Type B (67.4%), Type As/Ar (10.9%), Type C (8.7%), Type Ad (2.2%) | Absent in 65.2% |
| Buriti et al., 201427 | Type A (10.9%), Type B (67.4%), Type As/Ar (10.9%), Type C (8.7%), Type Ad (2.2%) | Absent in 65.2% |
| Chao et al., 20122 | Abnormal in 46.3%. | ----- |
| Hrapcak et al., 201625 | 84% of ears with hearing loss had alterations (majority Type B) | ----- |
| 21% of the ears without hearing loss had alterations | ||
| Makar et al., 201220 | 32.8% not Type A | Absent in 47/67 |
| Maro et al., 201622 | 25% abnormal | ------- |
| Martins et al., 200134 | Type C (13.6%) | ------- |
Most procedures (audiometry, ABR, and OAE) were performed similarly in several studies, although some authors lack methodological details.5,15,16,20,24 Some showed small differences, such as the tested frequencies or the thresholds that defined hearing loss.4,25–27 For analysis purposes, the definition of hearing loss reported in each article was followed.
Physical examination: otoscopyTo provide more reliable information about the audiological results, 14 studies mentioned that they performed otoscopic evaluation (Table 3). However, some did not report details of this examination.6,22,23,28 Hrapcack et al.25 and Smith et al.18 found more than one abnormal finding in the same child.
Otoscopy findings.
| Study | Otoscopy (% ears) |
|---|---|
| Chao et al., 20122 | Abnormal otoscopy (59.7%): tympanic perforation (13.7%), cerumen (17.3%) and tympanic inflammation |
| Chidziva et al., 201617 | Abnormal otoscopy (61%): cerumen (37.2%), chronic suppurative otitis media (10.4%) inactive chronic otitis media (5.2%), otitis media with effusion (16%), acute otitis media (3%), tympanic retraction (2.2%) |
| Hrapcak et al., 201625 | Cerumen (25.5%), otorrhea (5.4%), tympanic perforation (6.3%), fungal otitis (1.3%), tympanic inflammation (2.9%), changes in the appearance of the tympanic membrane (31.3%), tympanum not evaluated (10.8%) |
| Makar et al., 201220 | Cerumen and fungal otitis (56.7%), chronic suppurative otitis media (16.4%) |
| Martins et al., 200134 | Thickening of ear drum (31.8%), red/bulgingear drum (9.1%), perforated ear drum (4.5%) |
| Matas et al., 200021 | Tympanic retraction and thickening (31.8%), red/bulging eardrum (9.1%), perforated eardrum (4.5%), neomembrane (4.5%) |
| Palacios et al., 200819 | Tympanic perforation (4.3%) |
| Rezende et al., 200436 | Tympanic perforation |
| Smith et al., 201718 | Tympanic perforation (17.75%), otorrhea (8.41%), other abnormal findings (16.82%) |
| Taipale et al., 201133 | Acute otitis media (10%), chronic otitis media (27%), tympanic perforation (9%), cerumen (21%), retraction (2%) |
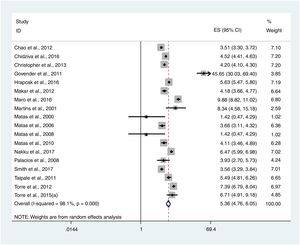
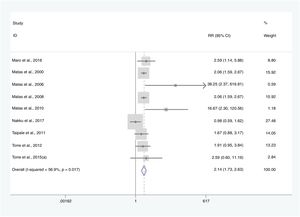
For the definition of hearing loss, the results of behavioral tests were not considered. HIV infection or AIDS were considered risk factors for hearing loss (OR=5,364, p=0.00) (Fig. 2). Eleven studies compared the findings of children with HIV/AIDS with control groups (HIV-negative, perinatally exposed but not infected, or unknown status for HIV). In these, there was a higher rate of hearing loss among children with HIV/AIDS when compared to the control groups (RR=2,135, 95% CI=1,733–2,631, p=0.00; Fig. 3).
Mixed hearing loss was the type of loss less frequently found in children with HIV/AIDS, and no statistical difference was found between conductive and sensorineural loss (p=0.119) between the studies.
Influence of age and genderNo study reported a statistically significant influence of gender on hearing loss in children with HIV/AIDS. Some studies have shown that older children with HIV/AIDS tend to have higher hearing loss rates,4,5,14,17 but only Nakku et al.16 and Buriti et al.28 showed a statistically significant difference.
Hearing loss, CD4+ T lymphocyte count, and viral load countDifferent ways of assessing HIV infection status were identified in different studies (Centers for Disease Control [CDC] classification, presence of opportunistic infections, clinical history, duration of HIV infection, and laboratory findings such as CD4+ T lymphocyte count and viral load, with different cut-off values for these). Consequently, due to the lack of standardization of these classifications, it was not possible to perform the meta-analysis.
Palacios et al.19 concluded that patients with hearing loss had earlier manifestations of HIV-related symptoms, higher viral load, and lower absolute values of CD4+ T-cell counts at the time of hearing evaluation and also at the time of ART start. A viral load greater than 400 copies/mL was associated to abnormalities in the distortion product OAE test.14 The association between hearing impairment and duration of HIV infection was also emphasized by Buriti et al.28 and Makar et al.20
Buritiet al.29 did not find a correlation between audiological findings and viral load. Since all patients evaluated in the study by Torre et al.23 had viral load values below the detection limit, this parameter was not discussed.
On the other hand, Chao et al.2 suggested that CD4+ T lymphocyte counts below 500 cells/mm3 would be risk factors for hearing loss in children with HIV (OR=3.53; p=0.02). Chidziva et al.17 reported that the reference value for this risk would be a count below 350 cells/mm3 (OR=2.1; p<0.037). Also, Torre et al.30 demonstrated an indeterminate association between hearing loss and CD4+T-cell count below 20%.
Effects of antiretroviral therapyNine studies did not provide any information on ART use among the studied children and five others reported that the evaluated children were using ART,but did not describe the adopted regimens.15,30–33
Some treatment regimens reported in other studies were based on nucleoside/non-nucleoside reverse transcriptase inhibitors. Protease inhibitors were mentioned,2,8,23,29,34 as well as immunoglobulins.34 Only two studies reported dual-drug regimens.26,34 Hearing loss was not correlated with ART use or its different treatment regimens22,25 but the findings were conflicting in the literature.2,29 ART duration was significantly associated with hearing loss in three studies.16,17,28
Due to the heterogeneity of these data, no meta-analysis could be performed regarding the effects of ART on the hearing status of children with HIV in the literature.
DiscussionIt was observed that HIV and AIDS can influence the hearing health of children, according to this meta-analysis carried out without temporal limitation. No differentiation was made between the influence of HIV infection or AIDS, separately, because the assessed studies did not perform it either. The inclusion criteria adopted by most studies were only age and a positive test result for the virus.
After the neonatal hearing screening, the authors did not observe, among the selected studies, a standardization of hearing assessment in the children regarding the type of exam used or the moment at which they should be performed. In some of these studies, the same subject underwent different examinations, with different findings, turning harder the analysis.19,20,22,26,33 It was not possible to make comparisons even when the same exam was used, because the studies used different methodologies,23,25,27 as previously highlighted in the review article by Ensink et al.35
Even the hearing loss classification was not standardized between studies. The ABR results were considered to classify hearing losses as conductive, sensorineural, or mixed, but also as central or peripheral hearing loss.4 Peripheral hearing loss can be considered an impairment of the outer ear, middle ear, and even the cochlea, but this definition was not clear.
Also, four studies considered the number of ears instead of the number of affected children28-30,32 and were excluded in some meta-analyses. It seems more appropriate to consider the number of affected children, since the involvement of one or both ears does not necessarily reflect the severity of HIV infection in human hearing. However, it is possible that the same child may present different types of hearing loss in either ear.36
The collaboration of children and their parents or caregivers could limit the reliability of the findings between the exams, especially the subjective ones, but no study has discussed this possible bias. The most reliable exam in this regard would be the ABR, but it is a more difficult exam to perform because of its longer duration, higher cost, and sometimes the need for the child’s sedation.
Another criticism is that the presence of cerumen in the external ear canal, acute otitis media, and even fungal otitis externa were considered as causes of hearing loss in some studies, instead of being treated before the hearing evaluation, which clearly influenced the obtained result.
Little is known about the effects of ART on the hearing health of children and adolescents with HIV.30,37 As shown, different drug regimens, time of use, age at onset, and even incomplete information led to inconsistent findings.2,22,27 Some researchers have pointed out that some drugs may cause mitochondrial DNA damage.1,38 The ototoxic effects of some medications commonly administered to patients with HIV (such as antibiotics) may also be considered confounding factors.16,33,34 No selected studies have evaluated hearing thresholds at higher frequencies, which are most commonly compromised in cases of ototoxicity.11
Few studies have evaluated the correlation between the laboratory status of HIV infection (mainly viral load and CD4+ T lymphocyte count) and hearing loss. Once again, conflicting findings in the literature were observed, especially related to the different cut-offs for CD4+ T lymphocytes. In turn, it should be noted that all studies used cross-sectional measures, which may not reflect the actual status of HIV infection, especially regarding viral load. Furthermore, some treatment regimens were started immediately after birth, and viral replication itself may not reached levels high enough to lead to direct damage.22
ConclusionHearing loss in childhood can lead to important language, social, educational, and psychological limitations. It is known that HIV is a risk factor for hearing loss, but the literature shows conflicting findings in this association. Also, there is no standardization regarding the best hearing tests to be employed or the age of onset for evaluation after the neonatal hearing screening.
Further studies are needed to explain the effects of HIV/AIDS on children’s hearing, as well as its management. It is suggested that prospective studies be performed using the same audiological exams in all phases, with greater methodological detail. Therefore, the evolution of human hearing in people with HIV could be observed longitudinally. This knowledge may contribute to preventive measures for childhood hearing loss.
As HIV/AIDS affects children worldwide, it is also suggested that protocols for periodic auditory assessment of these children after neonatal screening be developed. Early diagnosis leads to timely rehabilitation, aiming to prevent the limitations caused by hearing loss.
Conflicts of interestThe authors declare no conflicts of interest.
The authors would like to give special thanks to Professors Monica Gama and Vanda Simões for their contributions to this work.
The authors are grateful for the support of Fundação de Amparo à Pesquisa e Desenvolvimento Científico e Tecnológico do Maranhão (FAPEMA).
Please cite this article as: Bentivi JO, Azevedo CM, Lopes MK, Rocha SC, Silva PC, Costa VM, et al. Audiological assessment of children with HIV/AIDS: a meta-analysis. J Pediatr (Rio J). 2020;96:537–45.
Study conducted at Federal University of Maranhão, São Luís, MA, Brazil.