to test the clinical utility of an early amplitude-integrated electroencephalography (aEEG) to predict short-term neurological outcome in term newborns at risk of neurology injury.
Methodsthis was a prospective, descriptive study. The inclusion criteria were neonatal encephalopathy, neurologic disturbances, and severe respiratory distress syndrome. Sensitivity, specificity, positive and negative predictive values, and likelihood ratio (LR) were calculated. Clinical and demographic data were analyzed. Neurological outcome was defined as the sum of clinical, electroimaging, and neuroimaging findings.
Resultsten of the 21 monitored infants (48%) presented altered short-term neurologic outcome. The aEEG had 90% sensitivity, 82% specificity, 82% positive predictive value, and 90% negative predictive value. The positive LR was 4.95, and the negative LR was 0.12. In three of 12 (25%) encephalopathic infants, the aEEG allowed for a better definition of the severity of their condition. Seizures were detected in eight infants (38%), all subclinical at baseline, and none had a normal aEEG background pattern. The status of three infants (43%) evolved and required two or more drugs for treatment.
Conclusionsin infants with encephalopathy or other severe illness, aEEG disturbances occur frequently. aEEG provided a better classification of the severity of encephalopathy, detected early subclinical seizures, and allowed for monitoring of the response to treatment. aEEG was a useful tool at the neonatal intensive care unit for predicting poor short-term neurological outcomes for all sick newborn.
testar a utilidade clínica do aEEG precoce em recém-nascidos a termo com risco de lesão neurológica, para prever resultados neurológicos de curto prazo.
Métodosestudo prospectivo e descritivo. Os critérios de inclusão foram encefalopatia neonatal, distúrbios neurológicos e bebês com SARA grave. Sensibilidade, especificidade, valor preditivo positivo e negativo e razão de verossimilhança foram calculados. Dados clínicos e demográficos foram analisados. O resultado neurológico foi definido como a soma de conclusões clínicas, de eletro e de neuroimagem.
Resultadosdentre os 21 neonatos monitorados, dez (48%) apresentaram resultado neurológico de curto prazo alterado. O aEEG apresentou sensibilidade de 90%, especificidade de 82%, valor preditivo positivo de 82% e valor preditivo negativo de 90%. A VR positiva foi de 4,95, e a RV negativa de 0,12. Em três dos 12 (25%) neonatos com encefalopatia foi possível definir melhor a gravidade de sua condição pelo aEEG. Foram detectadas convulsões em oito neonatos (38%), todas subclínicas no início do estudo, e nenhum apresentou um padrão histórico normal no aEEG. O estado de três neonatos (43%) evoluiu e exigiu dois ou mais medicamentos para tratamento.
Conclusõesem neonatos com encefalopatia ou outra doença grave, os distúrbios no aEEG ocorrem com mais frequência. O aEEG forneceu uma classificação melhor da gravidade da encefalopatia, detectou convulsões subclínicas precoces e permitiu que fosse feito o monitoramento da resposta ao tratamento. O aEEG é uma ferramenta útil para prever resultados neurológicos de curto prazo em todos os bebês doentes na UTIN.
Modern neonatal practices involve managing increasingly ill and complex patients. The assessment of neurological function of these patients is limited by the severity of the base illness and the use of sedating medications. It is difficult to determine which patients need specialized neurological follow-up after a neonatal disease.
The introduction of continuous brain monitoring by amplitude-integrated electroencephalography (aEEG) allows for the evaluation of brain function in real time and over long periods. The aEEG wave appears to be the result of the sum of the resting membrane potential, which is influenced primarily by the metabolic status and secondarily by the blood flow to the brain, among other factors. 1–4
While the evolution of aEEG monitoring after hypoxic-ischemic events has proven prognostic value,5–10 its utility for other common neonatal morbidities has not been well-studied. The main limitation of aEEG monitoring is that it provides limited information regarding electrical brain activity. The monitored areas correspond to the limit of irrigation of the three cerebral arteries that are more susceptible to hypoxic-ischemic insults.11 There is disagreement regarding its utility for detecting seizures.12 Another limitation is in distinguishing the true image of artifacts.13
The main objective of this study was to assess the clinical utility of an early aEEG to predict short-term neurological outcomes among term newborns admitted to the neonatal intensive care unit (NICU) at risk of neurological injury.
Materials and methodsPatientsTerm newborns from the period of September of 2005 to August of 2006 were selected for aEEG monitoring if they were considered as evolving with a neurological risk. Thus, any babies with a neonatal encephalopathy, neurologic disturbance, or with severe respiratory distress syndrome (RDS) were included. Diagnoses were made by the attending physicians based on clinical and laboratory criteria. In hypoxic ischemic encephalopatic (HIE) neonates, the Sarnat classification I-III was used in order of increasing severity.9 Entry criteria for hypothermia protocol were term infants, less than 6hours old, with acute fetal distress (prolonged resuscitation need, and/or cord pH < 7.0; and/or Apgar score at 5minutes < 5), evolving encephalopathy, and an altered aEEG record after the first hour of life. RDS patients were monitored when they reached an oxygenation index (OI) > 18. Extracorporeal membrane oxygenation (ECMO) treatment criteria were OI > 40 after maximum respiratory management, and reversible lung disease. Newborns with a known prenatal brain lesion or malformation were excluded, as well as any simultaneous patients, since only one brain monitor was available. The study was approved by the ethics committee of this institution, and an informed consent was obtained from the parents.
MonitoringElectrodes were installed on patients’ shaved scalps, and were monitored with a device (BRM2 Brainz Instruments) that provided information regarding both brain hemispheres, in locations equivalent to C3-P3 and C4-P4 of a standard EEG. This device amplifies the signal obtained after filtration between 2 to 15Hz, integrates information from the amplitude of the waves obtained, and then scans to display patterns on the screen at 6cm/h and raw EEG in real time. A physician (not blinded), assisted by medical supervisors trained in this technique, interpreted the tracings. The information was stored on a computer hard disk and extracted for further analysis using two software (Analyze and Chart Analyzer). The patterns obtained were classified according to Hellström-Westas classification, where type 1 is normal, and types 2, 3, 4, and 5 are altered in increasing order of severity.14 Monitoring was installed as soon as possible and continued until patients were stable. EEG was requested for selected episodes as a standard clinical need. The presence and management of seizures were registered, as well as adverse events related to the aEEG technique. The use of drugs that affect the central nervous system was recorded.15–18
Data collection and outcome measuresDemographic and clinical data were prospectively collected. The main outcome was short term neurological evolution, classified as normal or abnormal.
The abnormal neurological outcome was defined by standard clinical practices: the presence of an altered physical exam (disturbances in consciousness, hyper- or hypotonia, absent visual fixation and/or absent gag, suck, and feeding autonomy) documented by the attending physician after the acute evolution was resolved, combined with an altered brain imaging (presence of gray or white matter injury, basal ganglia compromise, and hemorrhagic and/or stroke lesions) and/or altered pre-discharge EEG (altered background pattern, persistent depressed voltage, and/or seizures). Neuroimaging was requested based on clinical need (head ultrasound, transmission computed tomography, or magnetic resonance imaging), and performed by an independent neuroradiologist blinded to the clinical and aEEG findings.
AnalysisDescriptive data were presented as mean±SD or median (range), as appropriate. Two- sided Student's t-test, with 95% of confidence interval, was used for parametric variables, and the Mann-Whitney test was used for non-parametric variables. Categorical variables were compared through the mid-p exact test.
Sensitivity, specificity, positive and negative predictive values, and positive and negative likelihood ratios (LR) were calculated with 95% confidence interval, in order to evaluate whether aEEG is a predictive tool for short-term neurological evolution for all patients, regardless of the diagnosis.
ResultsDuring the study period; 2,196 patients were born at this center and 118 were transferred from other centers. Of these, 225 term neonates were hospitalized in the NICU. Twenty-one newborns were monitored, of whom 13 were outborns. The diagnoses of the patients included were: neonatal encephalopathy in 12 newborns (five began hypothermia protocol), pulmonary hypertension secondary to RDS in eight (four began ECMO), and one with suspected neonatal seizures. The gestational age was 38.6±1.4 weeks (mean±SD). Monitoring started at 10 (4-20) hours [median (PC25-PC75)] of life, and the mean duration of monitoring was 54 (27-120) hours; 1,626hours of monitoring were obtained. None of the patients died during the study period.
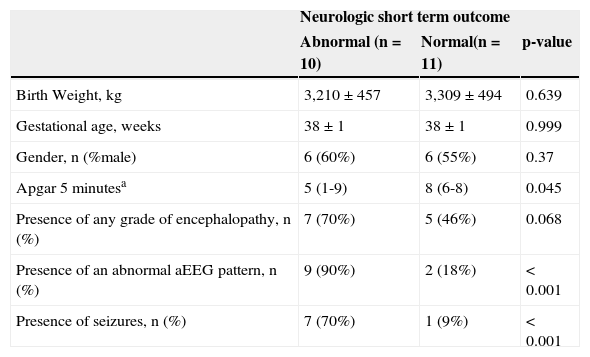
Patients’ demographic and clinical characteristics are described on Table 1. Of the 21 patients studied, ten presented altered short-term neurological outcome as defined through clinical, EEG, and/or altered neuroimaging criteria. The neurological abnormal babies had lower Apgar scores at five minutes than the normal group, and were more likely to develop seizures during evolution. The group of abnormal neurological outcome was mostly represented by encephalopathy patients, but the difference was not statistically significant. The three patients who were not encephalopathic at this group, also had a complicated neurological course, but this did not appear to be attributable to an ischemic perinatal hypoxic event. aEEG was abnormal in nine of these ten newborns (sensitivity of 90%, 95% CI: 59.6-98.2).Of the 11 neurologically normal patients, nine also had a completely normal aEEG (specificity of 82%, 95% CI: 52.3-94.9). The positive predictive value was 82% (95% CI: 52.3-94.9) and the negative predictive value was 90% (95% CI: 59.6-98.2). The positive LR was 4.95 (95% CI: 1.81-13.51) and the negative LR was 0.12 (95% CI: 0.02-0.91).
Demographic and clinical characteristics of the study group.
| Neurologic short term outcome | |||
|---|---|---|---|
| Abnormal (n=10) | Normal(n=11) | p-value | |
| Birth Weight, kg | 3,210±457 | 3,309±494 | 0.639 |
| Gestational age, weeks | 38±1 | 38±1 | 0.999 |
| Gender, n (%male) | 6 (60%) | 6 (55%) | 0.37 |
| Apgar 5minutesa | 5 (1-9) | 8 (6-8) | 0.045 |
| Presence of any grade of encephalopathy, n (%) | 7 (70%) | 5 (46%) | 0.068 |
| Presence of an abnormal aEEG pattern, n (%) | 9 (90%) | 2 (18%) | < 0.001 |
| Presence of seizures, n (%) | 7 (70%) | 1 (9%) | < 0.001 |
Data presented as mean±SD, except where noted (a). a median (range).
aEEG, amplitude-integrated electroencephalography; SD, standard deviation.
48% of the newborns started with a normal aEEG pattern and progressed normally until the end of the study. None of them had seizures. The recordings showed that abnormal type 2, 3, 4, and 5 aEEG patterns were more likely to continue to be altered.
38% of the children had seizures during monitoring. All detections were initially performed through monitoring since there were about three hours after clinical correlation (Fig. 1). All children who had seizures had an abnormal aEEG pattern at the start of monitoring. 75% of the newborns that evolved to a convulsive status required two or more drugs for the treatment of seizures (Fig. 2).
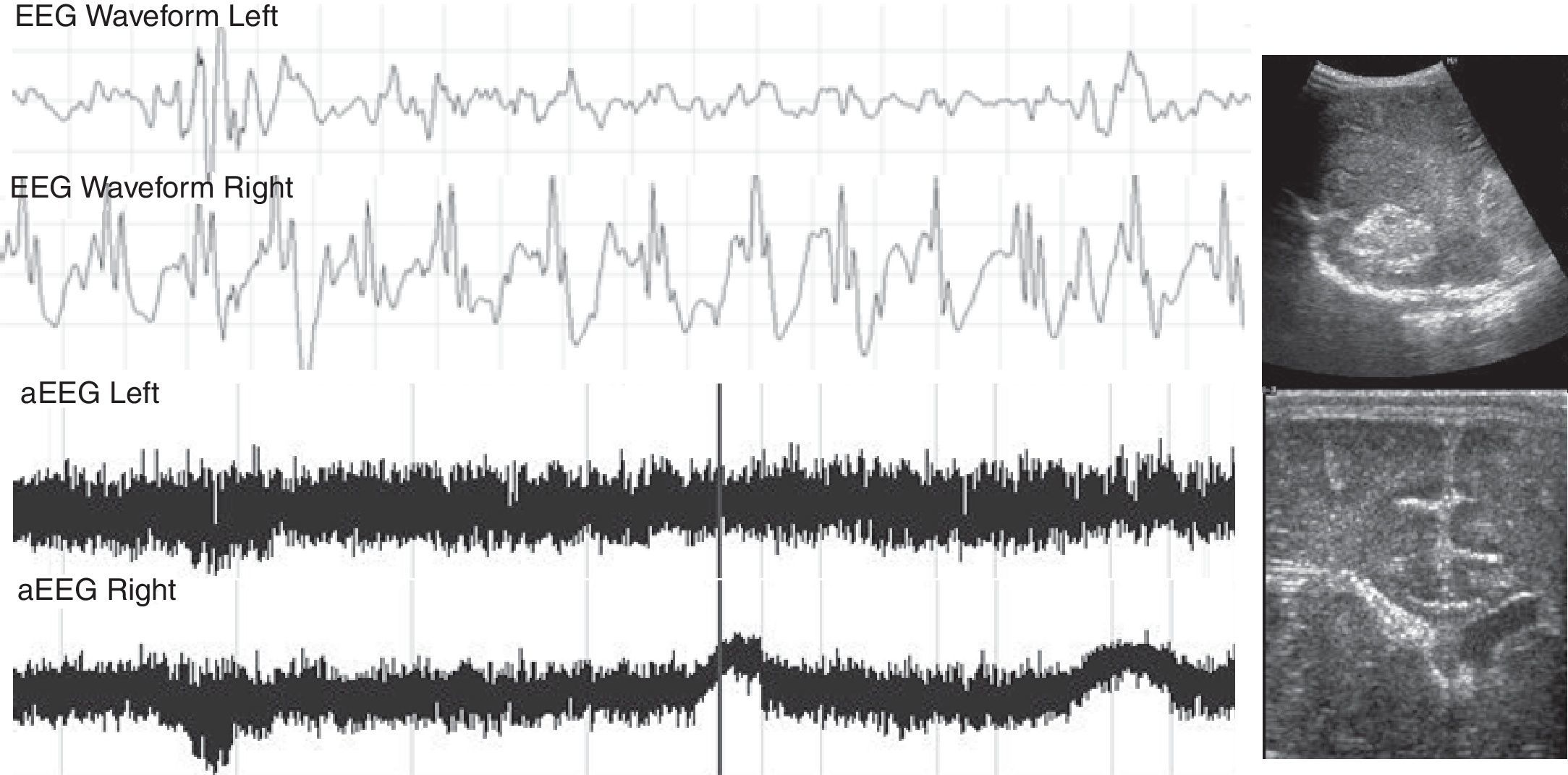
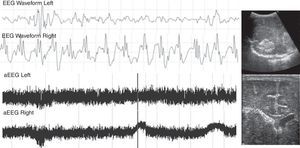
Patient with severe pulmonary hypertension secondary to a dilated cardiomiopathy with heart failure. On the third day of life, the aEEG showed seizures in the right hemisphere. Due to clinical suspicion, a head ultrasound was performed, showing intraventricular hemorrhage in the right side of the brain. aEEG, amplitude-integrated electroencephalography.
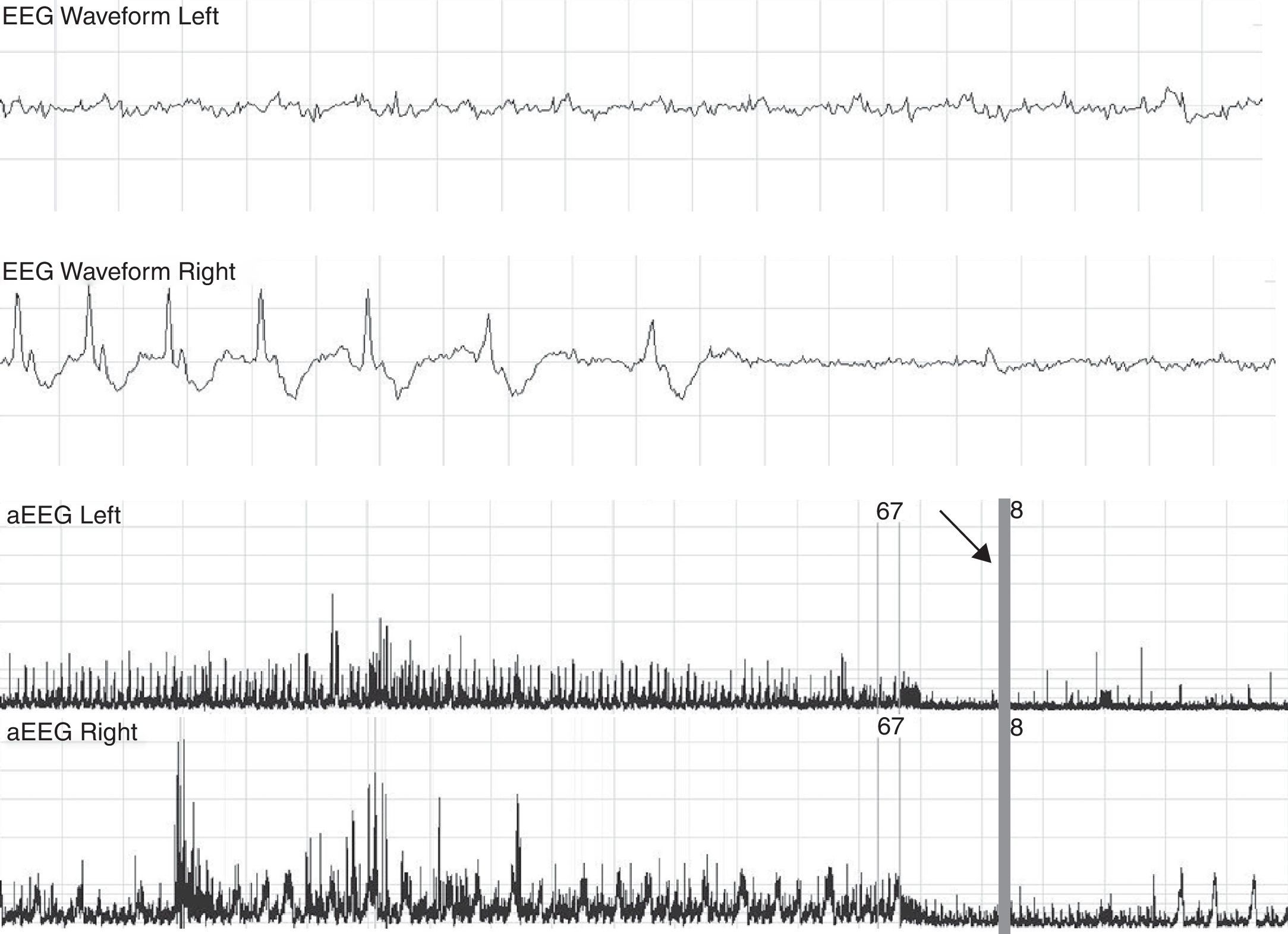
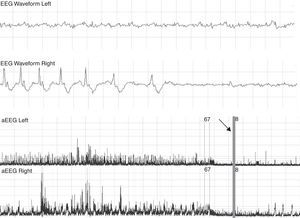
Patient with a suppressed pattern of aEEG evolves to status epilepticus. He was treated with 60mg/kg of phenobarbital without effects on seizures. The arrow shows how seizures are controlled with phenitoin, and base line becomes more suppressed. aEEG, amplitude-integrated electroencephalography.
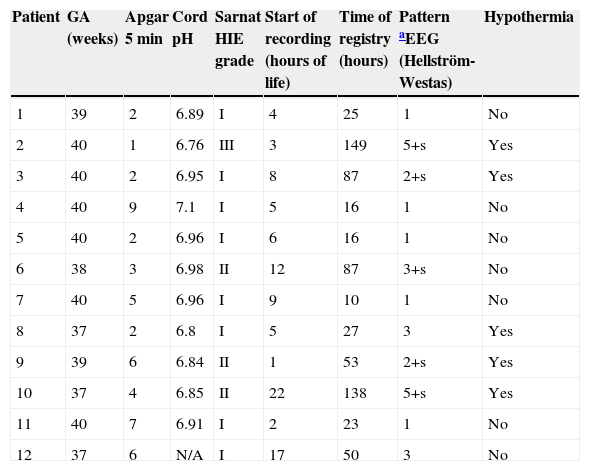
Of the 12 encephalopathic infants (Table 2), the normal recordings of five helped clinicians to decide that they did not require hypothermia protocol. Two encephalopathic newborns had altered aEEG patterns, but came late to hypothermia treatment (which is recommended to start before 6hours of life). Patients No. 3, 8, and 12 had symptoms of HIE grade I at the time of evaluation, but the aEEG registration showed an abnormal path; one of the infants subsequently developed seizures, so all three patients actually evolved into a HIE grade II instead of a HIE grade I, as was the first clinical approach.
Clinical and aEEG characteristics of encephalopathy patients.
| Patient | GA (weeks) | Apgar 5 min | Cord pH | Sarnat HIE grade | Start of recording (hours of life) | Time of registry (hours) | Pattern aEEG (Hellström-Westas) | Hypothermia |
|---|---|---|---|---|---|---|---|---|
| 1 | 39 | 2 | 6.89 | I | 4 | 25 | 1 | No |
| 2 | 40 | 1 | 6.76 | III | 3 | 149 | 5+s | Yes |
| 3 | 40 | 2 | 6.95 | I | 8 | 87 | 2+s | Yes |
| 4 | 40 | 9 | 7.1 | I | 5 | 16 | 1 | No |
| 5 | 40 | 2 | 6.96 | I | 6 | 16 | 1 | No |
| 6 | 38 | 3 | 6.98 | II | 12 | 87 | 3+s | No |
| 7 | 40 | 5 | 6.96 | I | 9 | 10 | 1 | No |
| 8 | 37 | 2 | 6.8 | I | 5 | 27 | 3 | Yes |
| 9 | 39 | 6 | 6.84 | II | 1 | 53 | 2+s | Yes |
| 10 | 37 | 4 | 6.85 | II | 22 | 138 | 5+s | Yes |
| 11 | 40 | 7 | 6.91 | I | 2 | 23 | 1 | No |
| 12 | 37 | 6 | N/A | I | 17 | 50 | 3 | No |
Standard EEGs were performed in 11 of the newborns (57%), and the results were consistent with those of the aEEG.
There were no adverse local or general events related to the use of this technique. Artifacts were reported in 29% of the patients, associated with some difficulties with the aEEG sensing signal, especially in patients with scalp edema and in three hypothermic patients. Intermittent signals were due to sensors coming unstuck and in the case of one newborn, due to high-frequency ventilator interference.
DiscussionPrior and Maynard created the aEEG in the 1960s to monitor adult cardiac patients.19 Since the late 1970s, it has been used with newborns, but initially only in Europe.20–23 The need to identify encephalopathic newborns with high neurological risk led to the development of this technique in parallel with multicenter studies of hypothermia,24 motivating its use in the rest of the world.25 There is now increasingly sophisticated and less invasive equipment. Thus, this study was designed to test the emerging technology in order to better understand the clinical approach.
At the time of the study, approximately 10% of the term infants were very sick and qualified for aEEG monitoring. With the inclusive criteria used to monitor patients at this NICU, early aEEG performed as a good diagnostic test to predict poor short-term neurological outcomes. It was less precise in predicting normal outcomes, where the common diagnostic tools were helpful.
Among the most notable findings was that over half of the patients showed alterations in aEEG recordings. It was also found that patients with an altered aEEG pattern were more likely to develop seizures, and this was not only observed with encephalopathy patients. Seizures were initially all subclinical, as described in the literature.26 However; there was also a notable latency period between the onset of the electrical disturbance and clinical emergence at this setting. In status epilepticus infants, two or more drugs were needed to control seizures, and the aEEG helped to manage the anticonvulsivant therapy.
A theme of discussion is whether aEEG is needed to decide if an encephalopathic newborn is a candidate for cooling therapy.27 aEEG has been used for the inclusion criteria in some cooling protocols,25,28 but not in others. In the present study, it was observed that early aEEG monitoring, especially in infants with apparent grade I HIE, 24 was more effective than clinical assessment alone in identifying which encephalopathic patients would develop serious neurological conditions. This finding becomes more important when considering that the time to decide whether a child is a candidate for hypothermia therapy is limited, and that is important to correctly initiate this crucial therapy.
There is a recent prospective study of use of early aEEG (< 9hours) in moderate and severe encephalopathic patients, which didn’t present any advantage compared with only staging to predict long term neurological outcome.29 That study differs in both the patients included and main outcome; thus, a comparison with the present study is not feasible.
An unexpected finding was the inability of the clinical examination to distinguish patients evolving with a severe neurological condition in real-time and how the use of aEEG influenced the adoption of more agile therapies. This was most striking in RDS patients.
The main limitations of the study were the small number of patients, and the absence of a strict protocol for total monitoring time or for EEG or neuroimaging request. However, this scenario is similar to the actual practice with infants in the NICUs.
The long-term benefits of the use of aEEG have not been evaluated. There is one report that, among patients with seizures in the neonatal period, the subsequent incidence of epilepsy was much lower with the use of this technique than without (9.4% vs. 56%).30 Thus, aEEG appears to be an interesting complementary tool.
In conclusion, early aEEG at the NICU allowed for more accurate diagnoses, better selection of patients for hypothermia therapy, appropriate detection and early treatment of seizures, and selection of patients for neurological follow-up.
Brain monitoring at the NICU offers many possibilities; it would be constructive to better study its application with respiratory, cardiologic, and ECMO patients, as well as extremely preterm infants.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Toso PA, González AJ, Pérez ME, Kattan J, Fabres JG, Tapia JL, et al. Clinical utility of early amplitude integrated EEG in monitoring term newborns at risk of neurological injury. J Pediatr (Rio J). 2014;90:143–8.
Neonatal Intensive Care Unit, Hospital Clínico, Pontificia Universidad Católica de Chile, Santiago, Chile.