to assess whether 25hydroxivitaminD or 25(OH)vitD deficiency has a high prevalence at pediatric intensive care unit (PICU) admission, and whether it is associated with increased prediction of mortality risk scores.
Methodprospective observational study comparing 25(OH)vitD levels measured in 156 patients during the 12hours after critical care admission with the 25(OH)vitD levels of 289 healthy children. 25(OH)vitD levels were also compared between PICU patients with pediatric risk of mortality III (PRISM III) or pediatric index of mortality 2 (PIM 2) > p75 [(group A; n=33) vs. the others (group B; n=123)]. Vitamin D deficiency was defined as < 20ng/mL levels.
Resultsmedian (p25‐p75) 25(OH)vitD level was 26.0ng/mL (19.2‐35.8) in PICU patients vs. 30.5ng/mL (23.2‐38.6) in healthy children (p=0.007). The prevalence of 25(OH)vitD < 20ng/mL was 29.5% (95% CI: 22.0‐37.0) vs. 15.6% (95% CI: 12.2‐20.0) (p=0.01). Pediatric intensive care patients presented an odds ratio (OR) for hypovitaminosis D of 2.26 (CI 95%: 1.41‐3.61). 25(OH)vitD levels were 25.4ng/mL (CI 95%: 15.5‐36.0) in group A vs. 26.6ng/mL (CI 95%: 19.3‐35.5) in group B (p=0.800).
Conclusionshypovitaminosis D incidence was high in PICU patients. Hypovitaminosis D was not associated with higher prediction of risk mortality scores.
avaliar se a deficiência da 25‐hidroxivitamina D, ou 25 (OH) vitD, tem prevalência elevada em internações na unidade de terapia intensiva pediátrica, e se estaria relacionada à previsão de escores de risco de mortalidade.
Métodoestudo observacional prospectivo comparando níveis de 25 (OH) vitD de 156 pacientes, mensurados nas primeiras 12 horas da internação em terapia intensiva, com níveis de 25 (OH) vitD de 289 crianças saudáveis. Os níveis de 25 (OH) vitD também foram comparados entre pacientes na UTIP com escore PRISM III ou PIM 2 > p75 (Grupo A; n=33), e o restante, (Grupo B; n=123). A deficiência de vitamina D foi definida como níveis < 20ng/mL.
Resultadoso nível médio (p25‐p75) de 25 (OH) vitD foi 26,0ng/mL (19,2‐35,8) em pacientes internados na UTIP, em comparação a 30,5ng/mL (23,2‐38,6) em crianças saudáveis (p=0,007). A prevalência de 25 (OH) vitD < 20ng/mL foi de 29,5% (IC 95%, 22,0‐37,0), em comparação a 15,6% (IC 95%,12,2‐20,0) (p=0,01). Os pacientes em terapia intensiva pediátrica apresentaram uma razão de chance (RC) para hipovitaminose D de 2,26 (IC 95%, 1,41‐3,61). Os níveis de 25 (OH) vitD foram 25,4ng/mL (IC 95%, 15,5‐36,0) no grupo A, em comparação a 26,6ng/mL (IC 95%, 19,3‐35,5) no grupo B (p=0,800).
Conclusõesa incidência de hipovitaminose D foi elevada em pacientes em terapia intensiva pediátrica, mas não foi associada à maior previsão de escores de risco de mortalidade.
Low levels of vitamin D are common in adult and pediatric populations.1 Vitamin D deficiency has been classically related with osseous illness, such as rickets. Currently, vitamin D deficiency is considered to be related with overall mortality,2 prevention of infections, innate immunity,3 hypertension,4 hypertriglyceridemia, type 1 and 2 diabetes mellitus,5 neoplasms,6 and autoimmune disorders.7 In children, it has been related to severe asthma, bronchiolitis episodes, and lower response to corticoids.8
The most important source of vitamin D is the skin, through the action of ultraviolet B radiation on 7dehydrocholesterol. Vitamin D must be metabolized to 25 hydroxivitamin D (25(OH)vitD) in the liver, which is an inactive precursor with a half‐life of approximately two to three weeks. The half‐life of the active form (1,25(OH)vitD) is only four to 24hours.9 For that reason, 25 (OH)vitD has been the most common form of vitamin D measured in previous studies10 In healthy children, age, skin pigmentation, season of the year, sun exposure, and dietary calcium intake influence 25(OH)vitD concentrations. Most studies have adopted the definition of vitamin D insufficiency as 25(OH)vitD concentrations lower than 30ng/mL, and vitamin D deficiency as concentrations below 20ng/mL.10
Recently, vitamin D deficiency has been associated with higher illness severity upon admission, mortality, and worse short and long term outcomes in adult intensive care units (ICU) patients.11–13 Several studies14–16 provided new information regarding the relationship between vitamin D status and critical illnesses in children admitted to pediatric ICUs (PICUs). It was observed that hypovitaminosis D is a common finding in critically‐ill children. McNally et al.16 also reported that vitamin D deficiency was associated with greater severity of critical illness. However, Rippel et al.14 did not find an association between hypovitaminosis D and length of stay or hospital survival. Vitamin D status may play an important role in acute stress and critical illness, but its pleiotropic effects in acute illness are not completely understood. Many confounding factors (hemodilution, interstitial extravasation, decreased synthesis of binding proteins, renal wasting of 25(OH)vitD, pH, underlying disease, season of the year, age, and dietary supplementation, among others) influence vitamin D status during critical illness.17 To date, there is no consensus regarding the optimal definitions of vitamin D deficiency, nor the threshold levels to define health benefits.17,18
Therefore, this study aimed to investigate whether vitamin D deficiency is highly prevalent in patients admitted to a PICU. The secondary objective was to verify whether vitamin D deficiency would be associated with increased mortality risk scores and illness severity at PICU admission.
Patients and methodsThis study was a secondary analysis of data and biological samples collected as part of the new prognosis biomarkers investigation, a prospective observational study set in the eight‐bed PICU of the Hospital Universitario Central de Asturias, in Oviedo, Spain. The study protocol was approved by the Hospital Ethics Committee. The study was conducted in 156 patients admitted to the PICU and aged less than 16 years. The exclusion criteria were no blood extraction during the first 12hours after admission; lack of consent to participate by parents or by children older than 12 years; known or suspected adrenal, pituitary, or hypothalamic disease; and use of systemic steroids for > ten days in the previous month, or more than one dose of systemic steroids within 24hours of admission (except for dexamethasone).
On every blood test sampled in the first 12hours after admission, the following variables were recorded: age, weight, underlying disease, and diagnosis. Respiratory rate, heart rate, blood pressure, O2 saturation, urine rate, and administration of vasopressor agents were recorded hourly. Radiographic and microbiologic diagnostics were performed when indicated. Blood cultures were performed when there was clinical suspicion of infection or when the patient's temperature was > 38°C. The pediatric index of mortality 2 (PIM 2) value was calculated at admission, and the pediatric risk of mortality III (PRISM III) value was calculated during the first 12h after admission, as it was the normal clinical practice. Routine biochemical assays, including C‐reactive protein (CRP) and procalcitonin (PCT), were performed during the first 12hours after admission. Venous blood samples were collected in tubes containing ethylene‐diamine‐tetra‐acetic acid (EDTA). A plasma aliquot was frozen and stored at ‐80°C for further determination of 25(OH)vitD, mid‐regional pro‐adrenomedullin (MR‐proADM), and carboxy‐terminal pro‐endothelin‐1 (CT‐proET‐1).
Healthy childrenThe 25(OH)vitD levels of the PICU patients were compared with the 25(OH)vitD levels that were obtained as part of a study on vitamin D status that is currently under development in a population of healthy children from the city of Oviedo (Asturias, Spain). Data from 289 healthy children were obtained.
Definition of hypovitaminosis DVitamin D deficiency was defined as < 20ng/mL 25(OH)vitD levels.10
Mortality risk groupsPatients were divided in two groups according to mortality risk scores. Higher risk mortality group (group A) included patients with a PIM 2 or PRISM III score > p75 (n=33); lower risk mortality group (group B) comprised the remaining patients (n=123).
Measurement of 25(OH)vitD, CRP, PCT, MR‐proADM, and CT‐proET‐1Serum 25(OH)vitD was measured using a direct competitive chemiluminescence immunoassay (LIAISON® Analyzer). The assay range is 4.0 to 150ng/mL. Plasma CRP was measured on a Modular Analytics Cobacs 6000 (Roche Diagnostics ‐ IN, USA) using an immunoturbidimetric technique. The analytical detection limit was 0.07mg/dL.
MR‐proADM, CT‐proET‐1, and PCT were measured in EDTA plasma using a sandwich immunoassay (TRACE technology; Brahms ‐ Hennigsdorf, Germany). Analytical detection limits were 0.08 nmol/L for pro‐ADM, 0.4pmol/L for CT‐proET‐1, and 0.02ng/mL for PCT.
Statistical analysisPatients’ clinical and biological parameters were described using frequencies, percentages, means, medians, and ranges (p25‐p75). Groups of patients were compared using the Mann–Whitney U‐test for continuous variables, and cross tables and exact chi‐squared test were used for categorical data. Adjusted odds ratios (OR) were estimated by multivariate logistic regression analysis (step‐forward criteria including all relevant likelihood ratio based variables). A p‐value < 0.05 was considered as statistically significant.
ResultsBaseline characteristicsThis study comprised 156 PICU patients. Baseline demographic, clinical, and laboratory characteristics of the PICU patients are shown in Table 1. The main reasons for PICU admission were postoperative and respiratory and infectious disease. Seventy‐six patients (48.7%) were younger than 4 years.
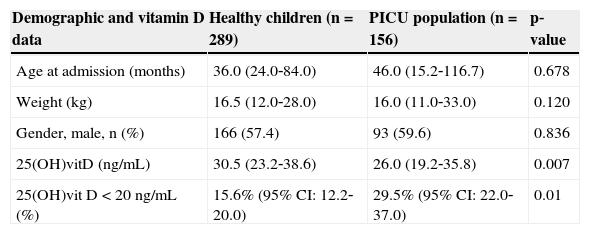
Demographic and vitamin D data in healthy children and in PICU population (median, p25‐p75).
| Demographic and vitamin D data | Healthy children (n=289) | PICU population (n=156) | p‐value |
|---|---|---|---|
| Age at admission (months) | 36.0 (24.0‐84.0) | 46.0 (15.2‐116.7) | 0.678 |
| Weight (kg) | 16.5 (12.0‐28.0) | 16.0 (11.0‐33.0) | 0.120 |
| Gender, male, n (%) | 166 (57.4) | 93 (59.6) | 0.836 |
| 25(OH)vitD (ng/mL) | 30.5 (23.2‐38.6) | 26.0 (19.2‐35.8) | 0.007 |
| 25(OH)vit D < 20ng/mL (%) | 15.6% (95% CI: 12.2‐20.0) | 29.5% (95% CI: 22.0‐37.0) | 0.01 |
CI, confidence interval; PICU, pediatric intensive care unit; 25 (OH)vitD, 25 hydroxivitamin D.
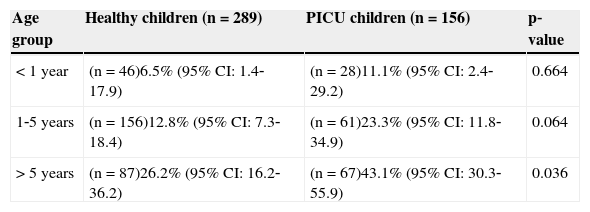
Demographic and vitamin D data in the PICU population and healthy children are reported in Table 1. 25(OH)vitD levels were lower and the incidence of hypovitaminosis D was higher in the PICU population. Vitamin D values in critically ill children had a negative correlation with patient's age (Spearman's correlation coefficient: ‐0.421; p < 0.001). Therefore, vitamin D deficiency was compared between healthy and PICU children in different age groups (Table 2). The incidence of vitamin D deficiency increased with age in both groups of patients. PICU patients had a crude OR for hypovitaminosis D of 2.26 (CI 95%: 1.41‐3.61). The age, weight, and gender‐adjusted OR was 1.83 (CI 95%: 1.10‐3.06).
Vitamin D deficiency (25(OH)vit D < 20ng/mL) in healthy and PICU children in different age groups.
| Age group | Healthy children (n=289) | PICU children (n=156) | p‐value |
|---|---|---|---|
| < 1 year | (n=46)6.5% (95% CI: 1.4‐17.9) | (n=28)11.1% (95% CI: 2.4‐29.2) | 0.664 |
| 1‐5 years | (n=156)12.8% (95% CI: 7.3‐18.4) | (n=61)23.3% (95% CI: 11.8‐34.9) | 0.064 |
| > 5 years | (n=87)26.2% (95% CI: 16.2‐36.2) | (n=67)43.1% (95% CI: 30.3‐55.9) | 0.036 |
CI, confidence interval; PICU, pediatric intensive care unit; 25 (OH)vitD, 25 hydroxivitamin D.
Median (p25‐p75) 25(OH)vitD levels during the different seasons of the year in PICU patients were: spring, 30.1ng/mL (18.2‐36.5); summer, 28.1ng/mL (20.5‐33.3); fall, 24.9ng/mL (19.6‐39.0); and winter, 23.0ng/mL (15.4‐38.0), p = 0.761.
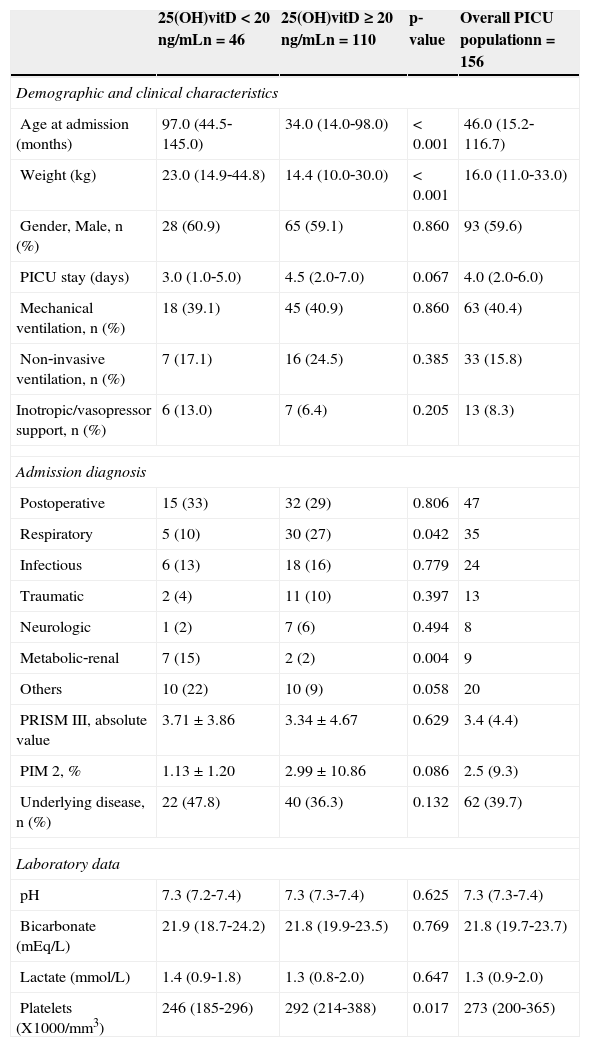
Baseline demographic, clinical, and laboratory characteristics of the PICU vitamin D deficient group vs. the remainder of the PICU sample are shown in Table 3. Vitamin D deficient patients were older and heavier. PICU stay, inotropic support, and need for mechanical ventilation and non‐invasive ventilation showed no difference between both groups. Respiratory diagnosis at admission was less frequent in vitamin D deficient patients, whereas metabolic‐renal diagnosis was more common. Underlying disease incidence was higher in hypovitaminosis D patients (47.8% vs. 36.3%; p = 0.132 (Table 3). Median (p25‐p75) 25(OH)vitD levels were 23.4ng/m (18.6‐33‐3) in patients with underlying disease (n=62) vs. 30.1ng/mL (20.0‐39.3) in patients without underlying disease (n=94); (p = 0.039).
Demographic, clinical, and laboratory data in the overall PICU population and in patients with and without vitamin D deficiency.
| 25(OH)vitD < 20ng/mLn=46 | 25(OH)vitD ≥ 20ng/mLn=110 | p‐value | Overall PICU populationn=156 | |
|---|---|---|---|---|
| Demographic and clinical characteristics | ||||
| Age at admission (months) | 97.0 (44.5‐145.0) | 34.0 (14.0‐98.0) | < 0.001 | 46.0 (15.2‐116.7) |
| Weight (kg) | 23.0 (14.9‐44.8) | 14.4 (10.0‐30.0) | < 0.001 | 16.0 (11.0‐33.0) |
| Gender, Male, n (%) | 28 (60.9) | 65 (59.1) | 0.860 | 93 (59.6) |
| PICU stay (days) | 3.0 (1.0‐5.0) | 4.5 (2.0‐7.0) | 0.067 | 4.0 (2.0‐6.0) |
| Mechanical ventilation, n (%) | 18 (39.1) | 45 (40.9) | 0.860 | 63 (40.4) |
| Non‐invasive ventilation, n (%) | 7 (17.1) | 16 (24.5) | 0.385 | 33 (15.8) |
| Inotropic/vasopressor support, n (%) | 6 (13.0) | 7 (6.4) | 0.205 | 13 (8.3) |
| Admission diagnosis | ||||
| Postoperative | 15 (33) | 32 (29) | 0.806 | 47 |
| Respiratory | 5 (10) | 30 (27) | 0.042 | 35 |
| Infectious | 6 (13) | 18 (16) | 0.779 | 24 |
| Traumatic | 2 (4) | 11 (10) | 0.397 | 13 |
| Neurologic | 1 (2) | 7 (6) | 0.494 | 8 |
| Metabolic‐renal | 7 (15) | 2 (2) | 0.004 | 9 |
| Others | 10 (22) | 10 (9) | 0.058 | 20 |
| PRISM III, absolute value | 3.71 ± 3.86 | 3.34 ± 4.67 | 0.629 | 3.4 (4.4) |
| PIM 2, % | 1.13 ± 1.20 | 2.99 ± 10.86 | 0.086 | 2.5 (9.3) |
| Underlying disease, n (%) | 22 (47.8) | 40 (36.3) | 0.132 | 62 (39.7) |
| Laboratory data | ||||
| pH | 7.3 (7.2‐7.4) | 7.3 (7.3‐7.4) | 0.625 | 7.3 (7.3‐7.4) |
| Bicarbonate (mEq/L) | 21.9 (18.7‐24.2) | 21.8 (19.9‐23.5) | 0.769 | 21.8 (19.7‐23.7) |
| Lactate (mmol/L) | 1.4 (0.9‐1.8) | 1.3 (0.8‐2.0) | 0.647 | 1.3 (0.9‐2.0) |
| Platelets (X1000/mm3) | 246 (185‐296) | 292 (214‐388) | 0.017 | 273 (200‐365) |
CI, confidence interval; PICU, pediatric intensive care unit; PIM 2, pediatric index of mortality 2; PRISM III, pediatric risk of mortality III; 25 (OH)vitD, 25 hydroxi vitamin D.
PRISM III and PIM 2 are expressed as mean ± standard deviation, admission diagnosis as absolute value and %, and the remaining variables as median (P25‐P75).
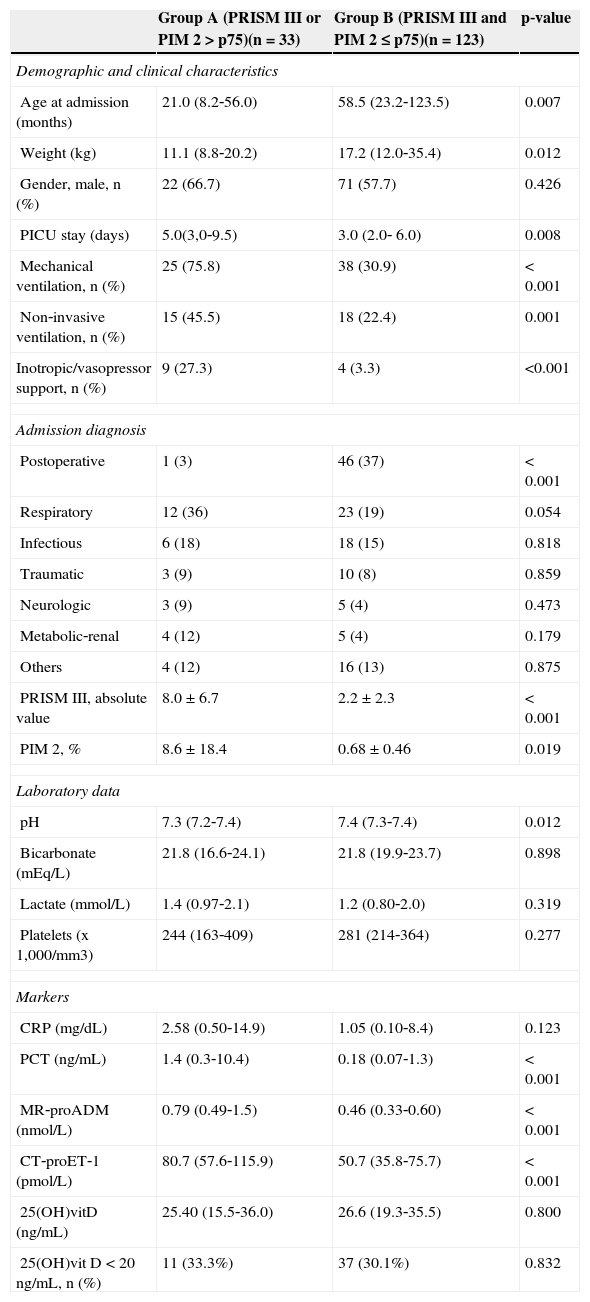
Baseline demographic, clinical, and laboratory characteristics of the patients with higher risk of mortality (group A) vs. the rest of the sample are shown in Table 4. Group A patients were younger and lighter. PICU stay, inotropic support, and need for mechanical ventilation and non‐invasive ventilation were higher in group A. Postoperative diagnosis at admission was less frequent in group A, whereas respiratory diagnosis was more frequent. PCT, MR‐proADM, and CT‐proET‐1 plasma levels were significantly higher in patients with higher prediction of mortality risk scores, whereas CRP and 25OH(vitD) levels were no different between groups A and B.
Demographic, clinical, laboratory data, and marker levels in patients with higher vs. lower prediction of risk mortality scores.
| Group A (PRISM III or PIM 2 > p75)(n=33) | Group B (PRISM III and PIM 2 ≤ p75)(n=123) | p‐value | |
|---|---|---|---|
| Demographic and clinical characteristics | |||
| Age at admission (months) | 21.0 (8.2‐56.0) | 58.5 (23.2‐123.5) | 0.007 |
| Weight (kg) | 11.1 (8.8‐20.2) | 17.2 (12.0‐35.4) | 0.012 |
| Gender, male, n (%) | 22 (66.7) | 71 (57.7) | 0.426 |
| PICU stay (days) | 5.0(3,0‐9.5) | 3.0 (2.0‐ 6.0) | 0.008 |
| Mechanical ventilation, n (%) | 25 (75.8) | 38 (30.9) | < 0.001 |
| Non‐invasive ventilation, n (%) | 15 (45.5) | 18 (22.4) | 0.001 |
| Inotropic/vasopressor support, n (%) | 9 (27.3) | 4 (3.3) | <0.001 |
| Admission diagnosis | |||
| Postoperative | 1 (3) | 46 (37) | < 0.001 |
| Respiratory | 12 (36) | 23 (19) | 0.054 |
| Infectious | 6 (18) | 18 (15) | 0.818 |
| Traumatic | 3 (9) | 10 (8) | 0.859 |
| Neurologic | 3 (9) | 5 (4) | 0.473 |
| Metabolic‐renal | 4 (12) | 5 (4) | 0.179 |
| Others | 4 (12) | 16 (13) | 0.875 |
| PRISM III, absolute value | 8.0 ± 6.7 | 2.2 ± 2.3 | < 0.001 |
| PIM 2, % | 8.6 ± 18.4 | 0.68 ± 0.46 | 0.019 |
| Laboratory data | |||
| pH | 7.3 (7.2‐7.4) | 7.4 (7.3‐7.4) | 0.012 |
| Bicarbonate (mEq/L) | 21.8 (16.6‐24.1) | 21.8 (19.9‐23.7) | 0.898 |
| Lactate (mmol/L) | 1.4 (0.97‐2.1) | 1.2 (0.80‐2.0) | 0.319 |
| Platelets (x 1,000/mm3) | 244 (163‐409) | 281 (214‐364) | 0.277 |
| Markers | |||
| CRP (mg/dL) | 2.58 (0.50‐14.9) | 1.05 (0.10‐8.4) | 0.123 |
| PCT (ng/mL) | 1.4 (0.3‐10.4) | 0.18 (0.07‐1.3) | < 0.001 |
| MR‐proADM (nmol/L) | 0.79 (0.49‐1.5) | 0.46 (0.33‐0.60) | < 0.001 |
| CT‐proET‐1 (pmol/L) | 80.7 (57.6‐115.9) | 50.7 (35.8‐75.7) | < 0.001 |
| 25(OH)vitD (ng/mL) | 25.40 (15.5‐36.0) | 26.6 (19.3‐35.5) | 0.800 |
| 25(OH)vit D < 20ng/mL, n (%) | 11 (33.3%) | 37 (30.1%) | 0.832 |
CRP, C‐reactive protein; CT‐proET‐1, carboxy‐terminal pro‐endothelin‐1; MR‐proADM, mid‐regional pro‐adrenomedullin; PCT, procalcitonin; PICU, pediatric intensive care units; PIM 2, pediatric index of mortality 2; PRISM III, pediatric risk of mortality III; 25 (OH)vitD, 25 hydroxivitamin D.
PRISM III and PIM 2 were expressed as mean ± standard deviation, admission diagnosis as absolute value; the other variables were expressed as median (P75‐P25).
Additional evaluation using a multivariate logistic regression analysis found an adjusted OR by age, season, and underlying disease of 2.42 (95% CI: 0.86‐6.84) for vitamin D deficiency and prediction of mortality risk scores (p = 0.09).
DiscussionThis study demonstrated that, in a sample of critically ill children from the north of Spain, the prevalence of hypovitaminosis D was high at PICU admission. The present study supports recent investigations14–16 showing that hypovitaminosis D is common in critically ill children. It was observed that 29.5% of the present PICU patients had 25(OH)vitD < 20ng/mL, similar to the rate of 34.5% from the study by Rippel et al.14 in a cohort of critically ill Australian children, and lower than the 40.1% and 69% reported by Madden et al.15 and by McNally et al.16 in North American and Canadian children, respectively.
The25(OH)vitD levels from the present PICU patients were compared with the 25(OH)vitD levels that were obtained as part of a study on vitamin D status that is currently under development in a population of healthy children from the city of Oviedo (Asturias, Spain). The prevalence of vitamin D deficiency in Oviedo's population of healthy children was similar to the reported prevalence of 18% in Mansbach's population‐based study of healthy North American children,19 but lower than the published prevalence of vitamin D deficiency in North American and Australian adolescents, which ranged from 29% to 68%.20,21 The explanation for these differences is the age. A previously described inverse correlation between 25(OH)vitD levels and age was confirmed by the present study. The median age of the present healthy children cohort was 3 years, much younger than the adolescent sample from the United States and Australia. The incidence of vitamin D deficiency was compared between healthy and PICU children in different age groups (Table 2). As expected, incidence of vitamin D deficiency increased with age in both group of patients. PICU patients had double incidence of hypovitaminosis D in all age groups, but the differences were clearly statistically significant in the older age group, and were almost significant in the medium age group. The probable reason is that the fragmentation of the sample produced a small sample size in the younger age group. Regarding the season of the year, there were no significant differences in 25(OH)vitD levels in the present study, although values tended to be lower in fall and winter, which agree with previous studies performed in the North of Spain18 and in other countries.20 In healthy children, factors consistently associated with 25(OH)vitD levels were age, season of the year, and dietary calcium intake.22 Unfortunately, data regarding children's calcium intake was not available.
Regarding admission diagnosis, a lower respiratory diagnosis rate was observed in patients with vitamin D deficiency. In the few published pediatric studies,23 an association was found between vitamin D deficiency and lung function, as well as with the risk for upper respiratory tract infections. A higher metabolic‐renal diagnosis rate in patients with vitamin D deficiency was also observed. Metabolic‐renal diseases can negatively influence the vitamin D metabolic pathways, affecting 25(OH)vitD levels.17
In the present sample, patients with underlying disease had lower levels of 25(OH)vitD. These patients are at a higher risk for reduced vitamin D levels through abnormal diets, altered metabolism, or reduced environmental exposure.
Risk of mortalityVitamin D deficiency has recently been shown to be associated with mortality in critically ill adults.11–13,24,25 Other recent investigations have not observed this relationship.26,27 Considering that the present study was not sufficiently powered to observe differences in survival, other surrogate markers of PICU outcome were used, such as mortality scores. In accordance with Rippel et al.,14 no associations were observed between vitamin D status and predicted PRISM III and PIM 2 mortality. However, Madden et al.15 and McNally et al.16 demonstrated that 25(OH)vit D levels at admission were inversely associated with PRISM III in North American children. Furthermore, duration of mechanical or non‐invasive ventilation and length of PICU stay did not show differences between low and normal 25(OH)vitD groups in the present sample, in agreement with the data observed by Rippel et al.14 in Australian children. However, McNally et al.16 found an association of vitamin D deficiency with longer length of stay. Geographic and ethnic differences, as well as different causes of PICU admissions, could explain the similar results in the present study and in the Australian study, and the differences with both North American studies. Variations in patient responses to acute stress and critical illness may depend on the degree of vitamin D insufficiency and the patient's tissue requirements.17
Other prognostic markers, such PCT, MR‐proADM. and CT‐proET‐1 were associated with risk of mortality (Table 2). Therefore, these biomarkers would have more utility than 25(OH)vitD to establish the risk of mortality in critically ill children.
By regulating the expression of more than 200 genes, including those influencing cell growth, 1,25‐dihydroxyvitamin D3 plays an important role in the proliferation, maturation, and death of cells. The identification of modifiable risk factors could help to guide new preventative or therapeutic strategies for pediatric critical illness. However, recent evidence17 suggests that the interpretation of vitamin D status based on 25(OH)vitD levels in acute illness should be performed with caution. Significant variation in 25(OH)vitD levels may occur from hour to hour in acutely ill patients, and single point assessment may be inaccurate in certain cases. Moreover, vitamin D deficiency would not only be dependent on the severity of vitamin D depletion, but would also be related to tissue requirement.28 Therefore new studies are necessary in order to determine reliable markers of vitamin D status in the acute care setting, as well as strategies to confirm whether vitamin D supplementation is useful for hypovitaminosis D in critically ill children.
The present study has limitations. Firstly, parathyroid hormone (PTH) was not measured. The diagnosis of vitamin D deficiency usually requires the association of serum 25(OH)vitD levels lower than 20ng/mL and elevated serum PTH concentrations.29 Secondly, the relatively small sample size and the low mortality limited the capacity to analyze specific subgroup of patients. Thirdly, the original study was not intended to estimate the prevalence of vitamin D deficiency; therefore, a specific questionnaire about dietary habits, vitamin D supplementation, or sun exposure was not performed. Finally, 25(OH)vitD levels were analyzed during the first 12hours after PICU admission; evolution of 25(OH)vitD levels during the first days of admission would have higher accuracy.
In conclusion, in a population of children from the North of Spain, hypovitaminosis D incidence was high at PICU admission. To the authors’ knowledge, this is the first prospective study comparing 25(OH)vitD levels in critically ill patients with healthy children population from the same area. Hypovitaminosis D was not associated with higher prediction of mortality risk scores, length of stay, and inotropic or respiratory support. Further studies are required to identify reliable markers of vitamin D status in the acute care setting, as well as strategies to confirm whether vitamin D supplementation could be useful in critically ill children with hypovitaminosis D.
FundingThis study was partly funded by a grant from “Fundación Ernesto Sánchez Villares”. Pablo Martínez‐Camblor was supported by the Grant MTM2011‐23204 of the Spanish Ministry of Science and Innovation (FEDER support included).
Conflicts of interestCorsino Rey had received speaker honoraria from Brahms Company to attend meetings related to sepsis biomarkers. The remaining authors declare no conflicts of interest. Fundación Ernesto Sánchez Villares, Spanish Ministry of Science and Innovation, and Brahms Company had no participation in the development of the manuscript, including study design, collection analysis, interpretation of data, writing of the report, nor the decision to submit the paper for publication.
The authors would like to thank the children and parents who participated in this study. The authors also acknowledge the assistance of the PICU medical and nursing staff of Hospital Universitario Central de Asturias.
Please cite this article as: Rey C, Sánchez‐Arango D, López‐Herce J, Martínez‐Camblor P, García‐Hernández I, Prieto B, et al. Vitamin D deficiency at pediatric intensive care admission. J Pediatr (Rio J). 2014;90:135–42.