Cystic fibrosis diagnosis is dependent on the chloride ion concentration in the sweat test (≥60mEq/mL – recognized as the gold standard indicator for cystic fibrosis diagnosis). Moreover, the salivary glands express the CFTR protein in the same manner as sweat glands. Given this context, the objective was to verify the correlation of saliva chloride concentration and sweat chloride concentration, and between saliva sodium concentration and sweat sodium concentration, in patients with cystic fibrosis and healthy control subjects, as a tool for cystic fibrosis diagnosis.
MethodsThere were 160 subjects enrolled: 57/160 (35.70%) patients with cystic fibrosis and two known CFTR mutations and 103/160 (64.40%) healthy controls subjects. Saliva ion concentration was analyzed by ABL 835 Radiometer® equipment and, sweat chloride concentration and sweat sodium concentration, respectively, by manual titration using the mercurimetric procedure of Schales & Schales and flame photometry. Statistical analysis was performed by the chi-squared test, the Mann–Whitney test, and Spearman's correlation. Alpha=0.05.
ResultsPatients with cystic fibrosis showed higher values of sweat chloride concentration, sweat sodium concentration, saliva chloride concentration, and saliva sodium concentration than healthy controls subjects (p-value<0.001). The correlation between saliva chloride concentration and sweat chloride concentration showed a positive Spearman's Rho (correlation coefficient)=0.475 (95% CI=0.346 to 0.587). Also, the correlation between saliva sodium concentration and sweat sodium concentration showed a positive Spearman's Rho=0.306 (95% CI=0.158 to 0.440).
ConclusionsSaliva chloride concentration and saliva sodium concentration are candidates to be used in cystic fibrosis diagnosis, mainly in cases where it is difficult to achieve the correct sweat amount, and/or CFTR mutation screening is difficult, and/or reference methods for sweat test are unavailable to implement or are not easily accessible by the general population.
O diagnóstico da fibrose cística depende do valor da concentração de íons de cloreto no teste do suor (≥ 60 mEq/mL – reconhecido como o indicador-padrão para o diagnóstico da doença). Além disso, as glândulas salivares expressam a proteína RTFC igualmente às glândulas sudoríparas. Nesse contexto, nosso objetivo foi verificar a correlação da concentração de cloreto na saliva e a concentração de cloreto no suor e entre a concentração de sódio na saliva e a concentração de sódio no suor em pacientes com fibrose cística e indivíduos controles saudáveis, como uma ferramenta para diagnóstico de fibrose cística.
MétodosContamos com a participação de 160 indivíduos [57/160 (35,70%) com fibrose cística e duas mutações no gene RTFC conhecidas e 103/160 (64,40%) indivíduos controles saudáveis]. A concentração de íons na saliva foi analisada pelo equipamento ABL 835da Radiometer® e a concentração de cloreto no suor e sódio no suor, respectivamente, por titulação manual utilizando o método mercurimétrico de Schales & Schales e fotometria de chama. A análise estatística foi realizada pelo teste qui-quadrado, pelo teste de Mann-Whitney e pela correlação de Spearman. Alpha=0,05.
ResultadosOs pacientes com fibrose cística apresentaram maiores valores na concentração de cloreto no suor, concentração de sódio no suor, concentração de cloreto na saliva e concentração de sódio na saliva do que os indivíduos-controle saudáveis (valor de p<0,001). A correlação entre as concentrações de cloreto na saliva e cloreto no suor mostrou Rho de Spearman (coeficiente de correlação) positivo=0,475 (IC de 95%=0,346 a 0,587). Além disso, a correlação entre concentração de sódio na saliva e concentração de sódio no suor mostrou Rho de Spearman positivo=0,306 (IC de 95%=0,158 a 0,440).
ConclusõesA concentração de cloreto na saliva e a concentração de sódio na saliva candidatas a ser usadas como diagnóstico de fibrose cística, principalmente em casos em que é difícil atingir a quantidade correta de suor, e/ou o exame da mutação RTFC é difícil e/ou o método de referência para o teste do suor não se encontra disponível ou não é de fácil acesso ao público em geral.
Cystic fibrosis (CF) (Online Mendelian Inheritance in Man [OMIM]: #219700) diagnosis is based on the chloride concentration achieved from the sweat test (ST); ≥60mEq/mL is recognized as the gold standard indicator for CF diagnosis.1 However, there is variability in the ST, mainly in sweat chloride values, which is a reflection of many factors and their interaction, for example: sweat weight, age, sex, ethnicity, cystic fibrosis transmembrane conductance regulator (CFTR) mutations, body mass index and body composition, modifier genes, and the influence of other ions channels, e.g. potassium, sodium, and alternative chloride channels.2–4 Historically, the ST is the gold standard for CF diagnosis; however, many tools are being studied to improve the diagnosis, including: evaporimeter, rectal biopsy, nasal potential difference, chloride concentration from saliva, and CFTR mutation screening.5–14 In the literature, the use of saliva as a diagnosis test is not well elucidated and should be better analyzed.9,15
Curiously, several systemic diseases can impair the functioning of salivary glands and saliva production, which can affect physical and chemical properties from saliva being a useful tool to diagnosis.9,14–17 In this context, the objective was to verify the correlation between saliva chloride concentration (SaCl) and sweat chloride concentration (SwCl), and between saliva sodium concentration (SaNa) and sweat sodium concentration (SwNa), in patients with CF and healthy control subjects, as a tool to perform the CF diagnosis. Moreover, it was hypothesized that SwCl and SaCl showed a positive correlation between them, and that the same should occur between SwNa and SaNa. In this case, the saliva ion concentrations could be a useful tool to CF diagnosis, mainly in cases where it is difficult to achieve the correct sweat amount (sweat weight≥75mEq/L) and/or CFTR mutation screening was difficult and/or reference method for sweat test are unavailable to implement or are not easily accessible by the general population.
MethodsPatients with CF and two known CFTR mutations and healthy controls subjects were enrolled.
Determination of saliva ion concentration9Saliva collection was performed after rinsing the mouth with water for one minute to eliminate contamination and stimulate the salivary glands. The collection took place at the same time of the day (afternoon) for both groups – patients with CF and healthy control subjects – to avoid possible physiological and environmental interferences.
The saliva samples were collected with a Salivette® (Sardest, Germany – http://www.sarstedt.com) by chewing sterile cotton rolls for one minute in the mouth. The samples were immediately centrifuged at 1800rpm for 15minutes after collection, and the saliva volume was measured (μL-scale).
SaNa and SaCl were determined with an ABL 500 gas analyzer (model 835, Radiometer®, Denmark) using 400μL of saliva by the direct ion-selective electrode technique (http://www.radiometer.com).
The procedures performed in the article to determine saliva ion concentration were based on a previous study published by the same group.9
Determination of sweat ion concentrations1The method of Gibson and Cooke was performed in two stages of the ST: induction and collection. Sweat induction was done using pilocarpine and a proprietary device constructed in our reference center. Determinations of chloride and sodium ions was conducted, respectively, by manual titration using the Schales & Schales mercuric nitrate procedure, and flame photometry.
The distance of two cm and five cm between the electrodes was adopted for newborns and the other participants, respectively. To minimize the risk of burn, the gauze was kept completely moist with pilocarpine, and the electrode was attached onto the gauze with an elastic band to prevent electrode displacement on the arm.
During the ST, the times of 10minutes and 30minutes were used for sweat induction and collection. Utilizing the ST, the following data were evaluated: sweat weight (mg), indicating that a sufficient amount of sweat was obtained during the induced sweat (weight greater than 75mg), and diagnostic parameters of CF by chloride concentration (mEq/L): healthy control individuals<30mEq/L; borderline subjects ≥30mEq/L to <60mEq/L; patients with CF≥60mEq/L.1
CFTR genotype screeningCFTR mutations were analyzed by polymerase chain reaction techniques for F508del, and enzymatic digestion for G542X, R1162X, R553X, G551D, and N1303K. Other mutations in CFTR were also identified by sequencing or by using the multiplex ligation – dependent probe amplification (SALSA MLPA) technique Kit P091-C1 CFTR-MRC-Holland with the MegaBACE 1000® (GE Healthcare Biosciences, Pittsburgh, USA) and the ABI 3500 (Applied Biosystems – Thermo Fisher Scientific, São Paulo, Brazil).18 The CFTR mutation classification was performed according to literature.19 In addition, the clinical and functional translation of CFTR (CFTR2) database was used to determine if some mutations were CF-causing.20
Ethical disclosureAll procedures performed in this study that involved human participants were in accordance with the ethical standards of the institutional and/or national research committees and with the 1964 Helsinki declaration and its later amendments, or comparable ethical standards. Moreover, this institution's Research Ethics Committee approved the study, and written informed consent was obtained from all individuals included in the study and or, when the participants were children, from a parent or guardian.
Statistical analysisA descriptive analysis was used with number of observations, mean value, standard deviation, median, minimum and maximum values, and 95% confidence interval for the mean for continuous variables. For categorical variables, the data are presented as frequencies and percentages.
The statistical analysis was performed by Mann–Whitney test (to compare the difference between patients with CF and healthy control subjects, taking into account the values of SwCl, SaCl, SwNa, SaNa, and age) and Spearman's Rho (to show the correlation between SwCl and SaCl or SwNa and SaNa, taking into account the groups of patients with CF and healthy control subjects). Additionally, the chi-squared test was used to compare the association between groups and sex. Statistical analysis was conducted with SPSS version 23.0 (SPSS, Inc., Chicago, IL). The value of alpha was 0.05. Also, the sample power was estimated with the Australia and New Zealand Melanoma Trials Group (ANZMTG) Statistical Decision Tree program using Spearman's Rho correlation and in G*Power version 3.1.9.2 software using the Mann–Whitney test. The calculation indicated a sample power of 0.978 considering the minor positive correlation achieved (Rho=0.306; alpha=0.05; sample=160). In addition to Mann–Whitney test, the authors stipulate, as an ideal sample size, the value of 150 (allocation ratio: N1=50 patients with CF; N2=150 control health subjects) using the two-tailed test, with an effect size (d) of 0.5; alpha error of 0.05; and power of 0.80.
ResultsThere were 160 subjects enrolled in the study; 57/160 (35.70%) patients with CF and two known CFTR mutations. In addition, there were ∼50% females in both groups, and the patients with CF were younger when compared with the healthy controls subjects (p=0.005) (Table 1). The known CFTR mutation genotypes found in patients with CF are shown in Table 2. In the table it can be observed that all patients with CF had two known CFTR mutations identified and that the mutations were considered as CF-causing mutations by CFTR2, except for the 622-2A>C (c.490-2A>G, rs397508735) mutation that was not included in the CFTR2 list and was considered by this study as a class I mutation – being a splice acceptor variant.
Descriptive data from the patients with cystic fibrosis and healthy controls subjects enrolled in the study.
CFTR genotype and classes of identified mutations.
| Distribution of patients according to genotype | Frequency | Percentage (%) | CFTR class |
|---|---|---|---|
| F508del/F508del | 26 | 45.6 | II/II |
| F508del/G542X | 8 | 14 | II/I |
| F508del/2183AA>G | 2 | 3.5 | II/I |
| F508del/R1162X | 2 | 3.5 | II/I |
| F508del/1812-1G>A | 1 | 1.8 | II/I |
| F508del/3120+1G>A | 1 | 1.8 | II/I |
| F508del/N1303K | 1 | 1.8 | II/II |
| F508del/P205S | 1 | 1.8 | II/IV |
| F508del/Q809X | 1 | 1.8 | II/I |
| F508del/R1066C | 1 | 1.8 | II/II |
| F508del/R347P | 1 | 1.8 | II/IV |
| F508del/R553X | 1 | 1.8 | II/I |
| F508del/S549R (T>G) | 1 | 1.8 | II/III |
| F508del/Y1092X | 1 | 1.8 | II/I |
| F508del/1717-1G>A | 1 | 1.8 | II/I |
| F508del/S4X | 1 | 1.8 | II/I |
| F508del/621+1G>T | 1 | 1.8 | II/I |
| G542X/R1162X | 1 | 1.8 | I/I |
| G542X/I618T | 1 | 1.8 | I/IV |
| G542X/R334W | 1 | 1.8 | I/IV |
| R1162X/R1162X | 1 | 1.8 | I/I |
| 622-2A>C/711+1G>T | 1 | 1.8 | Uncertaina/I |
| 2183AA>G/2183AA>G | 1 | 1.8 | I/I |
CFTR, cystic fibrosis transmembrane conductance regulator.
In addition, no CFTR mutations or chloride concentrations higher than 30mEq/L were found in the healthy controls subjects.
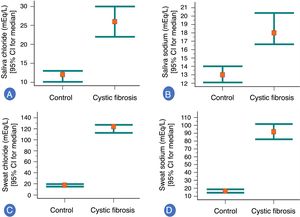
In the association analysis, patients with CF showed higher values of SwCl, SwNa, SaCl, and SaNa than healthy controls subjects (p<0.001) (Fig. 1). Briefly, the analysis was performed in four experiments (the data are shown as mean±standard deviation; median [minimum and maximum]; 95% confidence interval for the mean): (i) healthy control subjects vs. patients with cystic fibrosis, measuring SaCl: (healthy control subjects) 12.87±5.85; 12 (6 to 35); 11.73 to 14.02 vs. (patients with cystic fibrosis) 28.98±14.18; 26 (12 to 89); 25.22 to 32.75; (ii) healthy control subjects vs. patients with cystic fibrosis, measuring SaNa: (healthy control subjects) 14.37±6.50; 13 (6 to 52); 13.10 to 15.64 (patients with cystic fibrosis) 21.09±9.29; 18 (8 to 48); 18.62 to 23.55; (iii) healthy control subjects vs. patients with cystic fibrosis, measuring SwCl: (healthy control subjects) 17.57±6.15; 17.54 (7.35 to 28.99); 16.37 to 18.77 (patients with cystic fibrosis) 120.85±24.89; 123.89 (63.70 to 185.71); 114.25 to 127.45; (iv) healthy control subjects vs. patients with cystic fibrosis, measuring SwNa: (healthy control subjects) 17.67±7.58; 16.37 (5.03 to 36); 16.18 to 19.15 (patients with cystic fibrosis) 95.62±28.38; 91.73 (32.2 to 172.81); 88.09 to 103.15. Also, curiously, eight patients with cystic fibrosis showed SwCl values greater than 160mEq/L, as follow: 161.29, 164.51, 167.97, 168.47, 173.20, and 185.71.
Association between healthy control group (103 subjects) and patients with cystic fibrosis (57 subjects) to saliva ion concentrations and sweat ion concentrations (mEq/L). (A) Association between healthy control group and patients with cystic fibrosis to saliva chloride ion concentration. (Control group) 12.87±5.85; 12 (6 to 35); 11.73 to 14.02. (Patients with cystic fibrosis) 28.98±14.18; 26 (12 to 89); 25.22 to 32.75. (B) Association between healthy control group and patients with cystic fibrosis to saliva sodium ion concentration. (Control group) 14.37±6.50; 13 (6 to 52); 13.10 to 15.64. (Patients with cystic fibrosis) 21.09±9.29; 18 (8 to 48); 18.62 to 23.55. (C) Association between healthy control group and patients with cystic fibrosis to sweat chloride ion concentration. (Control group) 17.57±6.15; 17.54 (7.35 to 28.99); 16.37 to 18.77. (Patients with cystic fibrosis) 120.85±24.89; 123.89 (63.70 to 185.71); 114.25 to 127.45. (D) Association between healthy control group and patients with cystic fibrosis to sweat sodium ion concentration. (Control group) 17.67±7.58; 16.37 (5.03 to 36); 16.18 to 19.15. (Patients with cystic fibrosis) 95.62±28.38; 91.73 (32.20 to 172.81); 88.09 to 103.15. All p-values were <0.001. The statistical analysis was performed using Mann–Whitney test. Alpha=0.05. The medians and the 95% confidence intervals are represented in the graphs. Also, in the legends the mean±standard deviation; median (minimum and maximum); 95% confidence interval for the mean. Eight patients with cystic fibrosis showed sweat chloride value greater than 160mEq/L, as follows: 161.29, 164.51, 167.97, 168.47, 173.20 and 185.71.
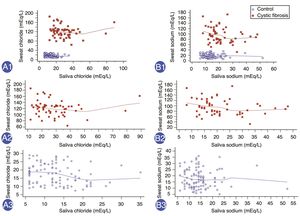
Finally, there was a positive correlation between SwCl and SaCl, with Spearman's Rho (correlation coefficient) of 0.475 (95% CI: 0.346 to 0.587) and another positive correlation between SwNa and SaNa, with Spearman's Rho of 0.306 (95% CI: 0.158 to 0.440) (Fig. 2). But, no high significant Spearman's Rho was observed for: (i) SwCl and SaCl in patients with cystic fibrosis – Spearman's Rho=−0.185; 95% CI: −0.425 to 0.079 (p=0.168); (ii) SwCl and SaCl in healthy control subjects – Spearman's Rho=−0.226; 95% CI: −0.402 to −0.034 (p=0.022); (iii) SwNa and SaNa in patients with cystic fibrosis – Spearman's Rho=−0.251; 95% CI: −0.480 to 0.012 (p=0.060); (iv) SwNa and SaNa in healthy control subjects – Spearman's Rho=−0.062; 95% CI: −0.252 to 0.133 (p=0.534).
Correlation between the values of saliva ion and sweat ion concentrations (mEq/L) in healthy control subjects and patients with cystic fibrosis. (A1) Correlation between sweat chloride concentration (SwCl) and saliva chloride concentration (SaCl) in all subjects at the same time. n=160 subjects. Spearman's Rho=0.475; 95% confidence interval (95% CI)=0.346 to 0.587; p<0.001. (A2) Correlation between SwCl and SaCl in patients with cystic fibrosis. n=57 subjects. Spearman's Rho=−0.185; 95% CI=−0.425 to 0.079; p=0.168. (A3) Correlation between SwCl and SaCl in healthy control subjects. n=103 subjects. Spearman's Rho=−0.226; 95% CI=−0.402 to −0.034; p=0.022. (B1) Correlation between sweat sodium concentration (SwNa) and saliva sodium concentration (SaNa) in all subjects at the same time. n=160 subjects. Spearman's Rho=0.306; 95% CI=0.158 to 0.440; p=0.0001. (B2) Correlation between SwNa and SaNa in patients with cystic fibrosis. n=57 subjects. Spearman's Rho=−0.251; 95% CI=−0.480 to 0.012; p=0.060. (B3) Correlation between SwNa and SaNa in healthy control subjects. n=103 subjects. Spearman's Rho=−0.062; 95% CI=−0.252 to 0.133; p=0.534. Red, patients with cystic fibrosis; blue, healthy controls subjects. Statistical analysis was performed by Spearman's correlation test. Alpha=0.05.
Sex did not influence the values of SwCl, SwNa, SaCl and SaNa independently of the group (patients with cystic fibrosis or health control subjects).
DiscussionAfter decades of utilizing ST for CF diagnosis, there are still some problems related with diagnosing CF.3,4,21 In this context, other techniques have been included, but have shown some limitations, such as costs, level of evidence, the need for many procedures, and the need for a multidisciplinary team with a high level of qualification to carry out a single procedure. However, in some conditions, saliva offers an easy, low-cost tool that should be studied as a new method to CF diagnosis. The present study is in accordance with the study by Camargo et al.,22 evidencing that some “simple aspects” could promote a greater benefit as the indication to perform the ST and reduce costs associated with other tools.22
Taking into account saliva as a diagnostic tool for CF disease, the principles behind the salivary process must be known. In this context, salivary secretion is a process that involves two steps: the first step occurs in the acini and the second in the salivary duct. The acini produce a secretion that contains ptyalin primary and/or mucin in an ionic solution with concentrations close to typical extracellular fluid. Considering that the primary secretion flows through the ducts, there are two major active transport processes that modify the saliva ionic composition. In all salivary ducts, sodium ions are actively reabsorbed, while potassium ions are secreted. Therefore, the sodium ion concentration in the saliva is low, while the potassium ion concentration is increased. During the salivation, the ion concentration in the saliva undergoes drastic changes, since the formation of primary salivary secretion by the acini can increase by up to 20 times. Consequently, the fast passage of secreted by the acinar ducts reduces the ion absorption from the secretion in the ducts. Therefore, when the greater amount of saliva is secreted, the SaCl and SaNa raise by about one-half to two-thirds in the plasma concentration, whereas the potassium concentration drops by four-fold.21–26 In this context, it can be visualized that the saliva ion concentration is dependent on CFTR protein activity expressed in the salivary ducts. Also, CFTR mutations lead to abnormal salivary ion concentrations, equal to sweat ion concentrations, mainly acting on the values of chloride and sodium. Moreover, the authors believe that other studies are needed to verify the use of saliva as a diagnostic method for CF; however, these ideas are supported by a physiological concept of equality between SwCl and SaCl (or SwNa and SaNa).
Besides of the present findings, many limitations should be discussed in this study, including: (i) low number of patients with CF and CFTR mutations from class IV, V, or VI to perform a statistical analysis among different CFTR mutations classes and the two techniques (sweat vs. saliva); (ii) the authors did not intend to associate the clinical markers with saliva and sweat, but, in the future, the ionic values could be compared using these data; (iii) the parents of patients with CF could be included to provide information about the subjects with a copy of the CFTR mutated allele and the influence in the final amount of ions in saliva and sweat; (iv) other diagnostic tools could be compared between the groups and correlated with the saliva sample; (v) a greater number of subjects should be included to analyze the influence of age on SaCl and SaNa, also in each age range to determine a follow-up curve; (vi) SwCl is recognized as the gold standard for CF diagnosis. However, in saliva, altered values were noticed when patients with CF and healthy control subjects were compared. But, no reference value for SaCl has been published until this moment.
In conclusion, the saliva ion concentration can be an alternative method for CF diagnosis. Moreover, the saliva ion concentration could validate the borderline STs and improve the analysis of response to precision/personalized medicine – particularly when considering the individual response to drugs.27 Genetic screening is not currently available to all patients with CF, and the saliva ion concentration could help in CF diagnosis. Also, saliva is easily accessed and has lower cost of analysis than ST. Finally, this study achieved similar values for the difference in chloride ion concentration and sodium ion concentration between patients with CF and healthy control subjects regarding saliva and sweat. New studies should be performed with a larger population of patients with CF and healthy controls to create a normal curve for saliva ion concentration. Moreover, saliva could be used in cases when the ST is not easy to conduct or could be used to confirm newborn screening, representing an easy-to-perform second test. In brief, SaCl and SaNa are candidates to be used for CF diagnosis, mainly in cases where it is difficult to achieve the correct sweat amount, and/or CFTR mutation screening is difficult, and/or when the reference methods for ST are unavailable or are not easily accessible by the general population.
FundingFundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) for supporting (#2015/12858-5) to FALM and (#2014/00611-2) ALG.
Conflicts of interestThe authors declare no conflicts of interest.
The authors thank the Multi-user Laboratory of Medical Genetics for contributing to CFTR genotype screening (http://www.laboratoriomultiusuario.com.br/); Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) for supporting (#2015/12858-5) to FALM and (#2014/00611-2) ALG. They also thank Stéphanie Villa-Nova Pereira, Priscila Dentini, Luciana Montes Rezende, Luciana Cardoso Bonadia, Maria Ângela Gonçalves de Oliveira Ribeiro, Maria de Fátima Corrêa Pimenta Servidoni, Andressa Oliveira Peixoto, Adyléia Aparecida Contrera Dalbo Toro, Renan Marrichi Mauch, Roberto José Negrão Nogueira, Eulália Sakano, Natasha Matsunaga, Alfonso Eduardo Alvarez, Carla Cristina de Souza Gomez, Elizete Aparecida Lomazi, Paloma Lopes Francisco Parazzi, Larissa Lazzarini Furlan, Emília Cristina Gonçalves, Milena Baptistella Grotta Silva, André Moreno Morcillo, Jaqueline Mores, Mariana Zorrón Mei Hsia Pu, Arthur Henrique Pezzo Kmit, Adriana Mendes Vinagre, Maura Mikie Fukujima Goto, Gabriel Hessel, and Aléthea Guimarães Faria, who contributed to the studies on CF in this reference center.
Please cite this article as: Gonçalves AC, Marson FA, Mendonça RM, Bertuzzo CS, Paschoal IA, Ribeiro JD, et al. Chloride and sodium ion concentrations in saliva and sweat as a method to diagnose cystic fibrosis. J Pediatr (Rio J). 2019;95:443–50.