To assess the effect of maternal breast milk supplementation on the development of exclusively breast-fed very low birth weight preterm infants at 12 months of corrected age.
MethodsA randomized clinical trial with 53 infants followed-up after discharge from the neonatal unit until a corrected gestational age of 12 months. Newborns in the intervention group were breastfed exclusively with maternal milk and received 2g of a multinutrient supplement (Pré-Nan®, Nestlé, Vevey, Switzerland) added to expressed breast milk twice a day until a corrected age of 4–6 months. The control group was exclusively breastfed without supplementation. After monthly follow-up, developmental assessment was performed using the Bayley III Scale.
ResultsThere was no statistically significant difference on the Bayley III Scale between the intervention and control groups in any of the assessed domains: motor, cognitive, and communication. However, scores in the three domains were always higher in the group that received the supplement. There were a similar number of cases of developmental delay in both groups: seven (28%) in the group that received the supplement and nine (33.3%) in the group that was exclusively breastfed.
ConclusionsThe results failed to show an association between post-discharge multinutrient supplementation and development in the assessed infants.
Avaliar o efeito da suplementação do aleitamento materno exclusivo com aditivo multicomponente no desenvolvimento de lactentes nascidos pré-termo de muito baixo peso aos 12 meses de idade gestacional corrigida.
MétodoEnsaio Clínico Randomizado com 53 lactentes, acompanhados da alta hospitalar na Unidade Neonatal até o 12° mês de idade gestacional corrigida. Aqueles alocados no grupo intervenção permaneciam em aleitamento materno exclusivo e recebiam 02 gramas de suplemento multicomponente em pó (Pré-Nan®, Nestlé, Vevey, Suíça), adicionados ao leite ordenhado duas vezes ao dia, por um período de 4 a 6 meses de idade gestacional corrigida. O grupo controle permanecia em aleitamento materno exclusivo sem suplementação. Após acompanhamento mensal, foi feita avaliação do desenvolvimento por meio da Escala de Bayley III.
ResultadosNa comparação do desenvolvimento pela Escala de Bayley III entre os grupos intervenção e controle, não houve diferença estatística significante em nenhum dos domínios estudados: motor, cognitivo e linguagem. Porém, os valores dos escores foram sempre maiores no grupo intervenção que no grupo controle nos três domínios. O atraso de desenvolvimento se distribuiu de forma similar nos grupos: sete casos (28%) no grupo intervenção e nove (33,3%) no grupo controle.
ConclusõesOs resultados não mostraram associação entre suplementação multicomponente pós-alta e o desenvolvimento dos lactentes analisados pela Escala de Bayley III.
The developing brain is particularly vulnerable to nutritional deficiencies due to several fast neurological process pathways, such as synapse formation and myelination. It is regulated by nutrients such as protein, energy, fat, minerals and growth factors.1–3
A deficiency of these elements can have later consequences, progressing to cerebral dysfunction associated with future alterations in child development.2 Therefore, adequate nutrition, especially in preterm newborns is of great importance to prevent, among other problems, neurodevelopmental delays.4–6
Breast milk is considered the ideal food in the neonatal period, promoting gastrointestinal maturation, generating immunological benefits, and leading to increased levels of docosahexaenoic acid, an important component for cerebral development.5,7–9 Nonetheless, its exclusive use in certain situations can lead to nutrient deficiencies and bone demineralization.10,11
The hospitalization period of preterm newborns, especially those with very low birth weight, is one of the situations where breast milk supplementation is a well-established and current practice during the in-hospital period, promoting better weight gain, increased length and head circumference in the short term, and better development indexes in the medium and long terms.12–14
Even though the benefits of human milk supplementation for hospitalized preterm infants are well documented in the literature, there is no consensus on the effectiveness of this practice after hospital discharge.6,10,15 Recently, the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN Committee on Nutrition) recommended that exclusively breast-feed infants who are underweight for their post-conceptual age when discharged from the hospital should receive supplementation to meet their nutritional needs.16
In recent years, some authors have attempted to clarify this issue by assessing the effectiveness of post-discharge supplementation in studies with experimental design. In a systematic review, Young et al.17 selected only the randomized trials by O’Connor et al.18 and by Zachariassen et al.19 These authors observed good results of supplementation in some specific situations, such as higher length during the study period and higher head circumference in preterm infants with birth weight <1250g. In spite of that, the review authors concluded that there is no consensus on the best way of feeding preterm infants post-discharge in order to provide better growth results.
Regarding the effects of breast milk supplementation on the post-discharge development, Aimone et al.,20 studying the same population as O’Connor et al.,19 observed a trend to better weight gain and higher head circumferences in children from the supplemented group in comparison with those from the control group after 12 weeks. When evaluated at 18 months of corrected gestational age (CGA), the infants from the intervention group had better scores on the Bayley II Scale in the language and motor domains, but without statistical significance.
Given the lack of evidence in this area, the present study aimed to investigate whether children discharged from the neonatal unit (NICU), born preterm, with very low birth weight, and who were fed breast milk and a multinutrient supplement had better psychomotor development scores when compared to those who were fed non-supplemented breast milk.
MethodsThis was a randomized clinical trial conducted from December of 2010 to October of 2013 with 53 preterm infants with birth weight <1500g, admitted at the NICU of Hospital Universitário Unidade Materno-Infantil of Universidade Federal do Maranhão, and who were discharged on exclusive breastfeeding (EBF). After discharge, all children that met these criteria were followed-up until 12 months of CGA. Those with conditions that could interfere with neuropsychomotor development (NPMD): major malformations; hydrocephalus; chromosomal abnormalities; fetal hydrops; congenital infections; maternal use of illicit drugs, tobacco, alcohol, and continuous use of corticosteroids; twin pregnancy; necrotizing enterocolitis sequelae; and cerebral palsy were excluded from the study.
Before discharge, the mothers were instructed to offer the breast to their children, on demand, prioritizing the hindmilk. Seven to ten days after discharge on EBF, the dyads returned to the first assessment. If they continued on EBF, the randomization was performed. This is justified because of the large number of mothers who give up on EBF soon after discharge, when faced with real-life situations at home.
Random numbers were generated for children allocation in the control group or intervention group. For randomization, an opaque and sealed envelope was used. For ethical reasons, placebo could not be used, and it was not possible to blind the mothers and professionals.
To calculate the sample size, the values obtained in a pilot study were used. For that purpose, a mean value (90 points) was assumed for the language domain of the Bayley Scale III of the control group at 12 months of CGA, whereas the score in the intervention group was 10 points higher, with a standard deviation of 12 points in both groups. A power of 80 and the probability of type 1 error of 0.05 were also assumed, thus calculating 23 infants per group as the necessary number.
The intervention consisted of breast milk supplementation for a period of four to six months and was conducted as follows.
After randomization, the mothers from the intervention group were instructed on how to add the supplement to breast milk: 10mL of breast milk were expressed by manual milking and 2g of PRE-NAN® powder formula (Nestlé) were added. A 50mL graduated plastic cup was used. After homogenization, the mixture was offered to the child. The procedure was conducted before two feedings during the day, one in the morning and another in the afternoon. The addition of the supplement corresponded to an increase of 20kcal/day. On average, this increase was enough to raise the caloric intake of the child at discharge (considering 1.8–2.0kg of weight at discharge) to approximately 140kcal/kg/day.21 Furthermore, it also corresponded to a daily increase of 2.12g of carbohydrates, 1.04g of fats, 0.56g of proteins, 1.04g of total fat, 0.44g of saturated fats, 0.164g of linoleic acid, 19.6mg of alpha linoleic acid, 10.76g of sodium, 30.4mg of calcium, 0.4mg of iron, 24.2mg of potassium, and 1.6mg of taurine and vitamins.
The infants were followed-up monthly to assess their general and nutritional status, as well as development in a breastfeeding follow-up and motivation outpatient clinic. The Bayley III Scale was applied to evaluate the NPMD up to 12 months of CGA. The evaluation was performed by an occupational therapist who had special training with an authorized team in properly equipped room to apply the test. The evaluator was not informed about the group to which the child belonged.
To verify the effectiveness of randomization, characteristics of the assessed infants up to randomization were compared in both groups. The compared variables were: weight, gender, gestational age, head circumference (HC), length at birth, weight at randomization, weight for gestational age (SGA/AGA), length of hospital stay, and severity score (Score for Neonatal Acute Physiology, Perinatal Extension, Version II [SNAPPE II]).22 The hypothesis test was not applied when comparing these variables, in accordance with the standards of the Consolidated Standards of Reporting Trials (CONSORT Statement).23
Scores of motor, cognitive, and language domains were assessed by the Bayley Scale III at 12 months of CGA were also described. The mean scores of each development domain were calculated.
Multivariate analysis (simple linear regression) was used, despite the randomization, since some variables collected before that were not balanced between the two groups. Among them, the variables birth weight and gender, which have significant influence on the future development of preterm newborns, were used in the model.24,25 Although they were not balanced, the SNAPPE II variables and the length of hospital stay were not used in the model because they are considered collinear with birth weight. Moreover, as the outcome was measured several months after the end of the intervention, existing elements in this period could influence the final result.
Only one post-randomization variable was chosen to compose the model due to its importance for child development: “mother as primary caregiver of the child” (yes/no categories). It is known that the family unit is one of the most important factor in the child development.26 Resegue et al.27 give special emphasis to the maternal presence in the care of children in their first years of life, and consider maternal deprivation a risk factor for child development delay.
Maternal schooling was also considered to be essential for development26; however, having been collected before the randomization, this variable showed no significant difference in its distribution in both groups.
The simple linear regression analysis was performed, at first, in an unadjusted way only with the group variables (intervention or control) and score on the Bayley III scale for the three development domains (motor, cognitive and language). Then, a second analysis of simple linear regression was carried out, adjusted by the variables gender, birth weight and mother as primary caregiver of the child.
The variable corresponding to the scores of Bayley III Scale in the three domains had its normality verified by the Shapiro–Wilk test.
Data tabulation was carried out in EpiInfo 3.5 software (Epi Info™, Centers for Disease Control and Prevention, Atlanta, USA) and the statistical analyses were performed in STATA 11 (StataCorp. 2009. Stata Statistical Software: Release 11. College Station, TX: StataCorp LP).
The research was approved through Edict N. 302/10 of the Research Ethics Committee of Hospital Universitário da Universidade Federal do Maranhão. This study consisted of a randomized clinical trial that followed the international standards established by the CONSORT Statement.23 The study was registered with the Brazilian Registry of Clinical Trials (REBEC) of the Brazilian Ministry of Health under No. U1111-1131-8413. It was based on the research project “Multicomponent supplementation of human milk for nutrition, growth, and development of preterm infants discharged from Neonatal Intensive Care Units”, edict MS/CNPq/FAPEMA – No. 477848/2007-9.
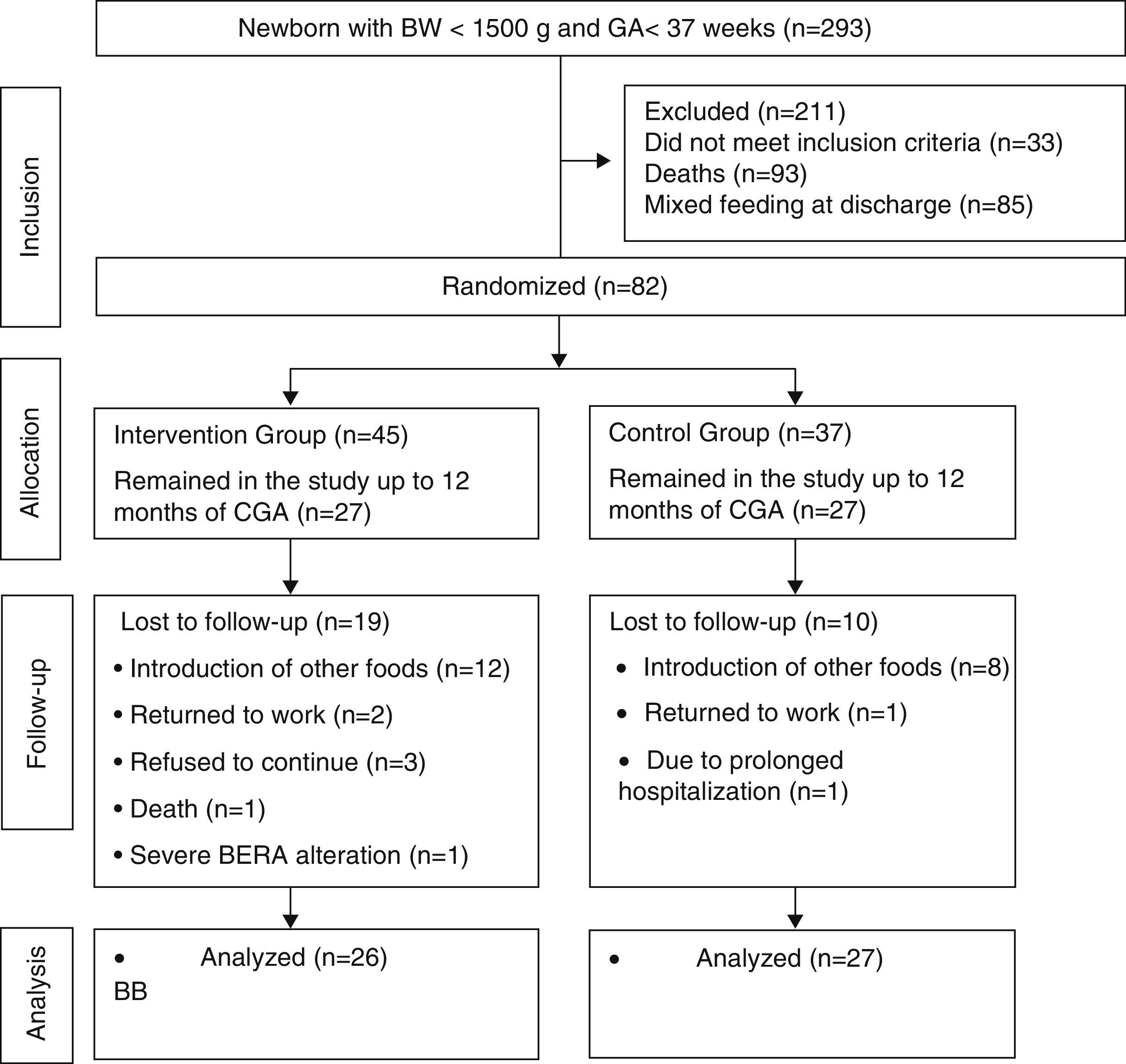
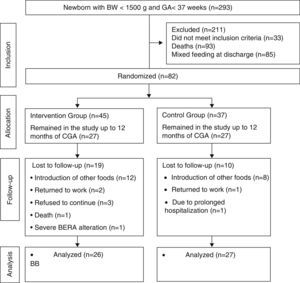
ResultsDuring the study period, 293 preterm infants were born weighing less than 1500g. A total of 118 newborns did not meet the inclusion criteria and 93 died. Randomization was performed in 82 infants. From the randomization to the end of the intervention, 29 children were lost to follow-up. Most of the losses were due to the mother's difficulty in maintaining EBF during the intervention period. These losses were statistically similar in both groups (p=0.12). The Bayley III scale for the development assessment was used to assess 53 children. The mean duration of the intervention was similar between the control and intervention groups: 5.84±0.54 months; 5.64±0.73 months; (p=0.42). Of the assessed children, 27 were in the control group and 26 in the intervention group (Fig. 1).
Flowchart of preterm infant randomization after hospital discharge.23 BW, birth weight; GA, gestational age; CGA, corrected gestational age; BERA, brain evoked response audiometry.
Of the children who completed the intervention, 20 (37.7%) were boys and 33 (62.3%) girls; most infants had mothers aged between 20 and 30 years (60.4%) with eight to 11 years of schooling (69.2%), and parents living in common-law marriage (60.4%).
The predominant family income was one to three minimum wages (49%), but with a high percentage of families living with up to one minimum wage (32%). Of the mothers who participated in the study, only 25% had a paid job when their child completed 12 months of CGA.
The mother was the primary caregiver of the child in 73.6% of cases. Only 18.9% of the families had more than one child living in the same household. Most homes (88.9%) had floor covering, allowing the child to play on the floor. Just over half of the population (52.8%) had access to educational toys (fitting and stacking toys).
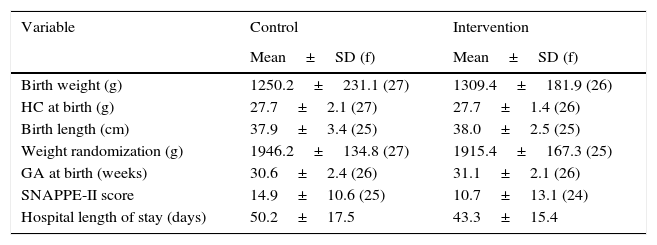
In the analysis of pre-randomization characteristics, the infants from the control and intervention groups showed similar profiles, except for the variables birth weight, SNAPPE-II, length of hospital stay, and gender (Table 1).
Distribution of perinatal characteristics of very low birth weight preterm infants. São Luís, Maranhão, Brazil, 2010–2014.
| Variable | Control | Intervention |
|---|---|---|
| Mean±SD (f) | Mean±SD (f) | |
| Birth weight (g) | 1250.2±231.1 (27) | 1309.4±181.9 (26) |
| HC at birth (g) | 27.7±2.1 (27) | 27.7±1.4 (26) |
| Birth length (cm) | 37.9±3.4 (25) | 38.0±2.5 (25) |
| Weight randomization (g) | 1946.2±134.8 (27) | 1915.4±167.3 (25) |
| GA at birth (weeks) | 30.6±2.4 (26) | 31.1±2.1 (26) |
| SNAPPE-II score | 14.9±10.6 (25) | 10.7±13.1 (24) |
| Hospital length of stay (days) | 50.2±17.5 | 43.3±15.4 |
| % (f) | % (f) | |
|---|---|---|
| SGA NB | 38.5 (10) | 38.5 (10) |
| Male gender | 33.3 (09) | 42.3 (11) |
| Maternal schooling <8 years | 50 (21) | 50 (21) |
SD, standard deviation; f, frequency; HC, head circumference; GA, gestational age; SNAPPE-II, Score for Neonatal Acute Physiology-Perinatal Extension, Version II; NB, newborn; SGA, small for gestational age.
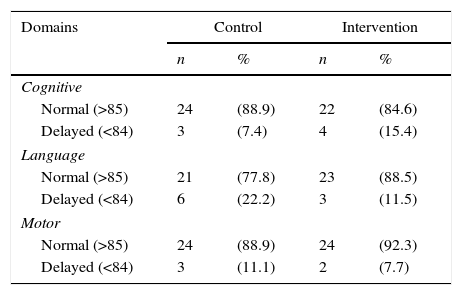
The assessment results of the 53 children using the Bayley III Scale in the three domains of development at 12 months of CGA are shown in Table 2. In 16 cases (30.8%), some NPMD delay was observed: nine (33, 3%) in the control group and seven (28%) in the intervention group.
Normality classification in the three development domains assessed by the Bayley Scale III. São Luís, Maranhão, Brazil, 2010–2014.
| Domains | Control | Intervention | ||
|---|---|---|---|---|
| n | % | n | % | |
| Cognitive | ||||
| Normal (>85) | 24 | (88.9) | 22 | (84.6) |
| Delayed (<84) | 3 | (7.4) | 4 | (15.4) |
| Language | ||||
| Normal (>85) | 21 | (77.8) | 23 | (88.5) |
| Delayed (<84) | 6 | (22.2) | 3 | (11.5) |
| Motor | ||||
| Normal (>85) | 24 | (88.9) | 24 | (92.3) |
| Delayed (<84) | 3 | (11.1) | 2 | (7.7) |
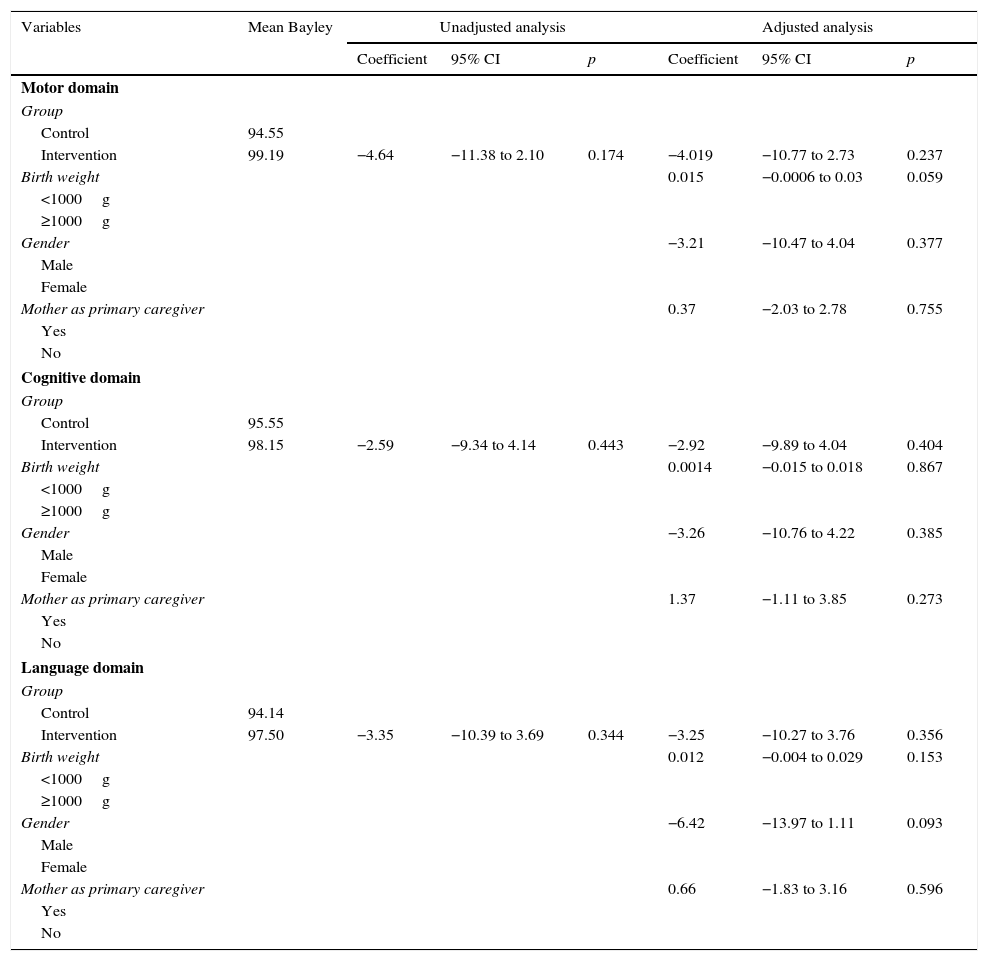
In the analysis by simple linear regression, there were no statistically significant associations in any of the models corresponding to the three domains, although the mean values of the Bayley Scale score among the supplemented children were always higher than those of non-supplemented children (Table 3).
Adjusted and unadjusted analyses of the association between breast milk supplementation and development assessed by the Bayley III scale for the motor, cognitive, and language domains. São Luís, Maranhão, Brazil, 2010–2014.
| Variables | Mean Bayley | Unadjusted analysis | Adjusted analysis | ||||
|---|---|---|---|---|---|---|---|
| Coefficient | 95% CI | p | Coefficient | 95% CI | p | ||
| Motor domain | |||||||
| Group | |||||||
| Control | 94.55 | ||||||
| Intervention | 99.19 | −4.64 | −11.38 to 2.10 | 0.174 | −4.019 | −10.77 to 2.73 | 0.237 |
| Birth weight | 0.015 | −0.0006 to 0.03 | 0.059 | ||||
| <1000g | |||||||
| ≥1000g | |||||||
| Gender | −3.21 | −10.47 to 4.04 | 0.377 | ||||
| Male | |||||||
| Female | |||||||
| Mother as primary caregiver | 0.37 | −2.03 to 2.78 | 0.755 | ||||
| Yes | |||||||
| No | |||||||
| Cognitive domain | |||||||
| Group | |||||||
| Control | 95.55 | ||||||
| Intervention | 98.15 | −2.59 | −9.34 to 4.14 | 0.443 | −2.92 | −9.89 to 4.04 | 0.404 |
| Birth weight | 0.0014 | −0.015 to 0.018 | 0.867 | ||||
| <1000g | |||||||
| ≥1000g | |||||||
| Gender | −3.26 | −10.76 to 4.22 | 0.385 | ||||
| Male | |||||||
| Female | |||||||
| Mother as primary caregiver | 1.37 | −1.11 to 3.85 | 0.273 | ||||
| Yes | |||||||
| No | |||||||
| Language domain | |||||||
| Group | |||||||
| Control | 94.14 | ||||||
| Intervention | 97.50 | −3.35 | −10.39 to 3.69 | 0.344 | −3.25 | −10.27 to 3.76 | 0.356 |
| Birth weight | 0.012 | −0.004 to 0.029 | 0.153 | ||||
| <1000g | |||||||
| ≥1000g | |||||||
| Gender | −6.42 | −13.97 to 1.11 | 0.093 | ||||
| Male | |||||||
| Female | |||||||
| Mother as primary caregiver | 0.66 | −1.83 to 3.16 | 0.596 | ||||
| Yes | |||||||
| No | |||||||
The comparison of the NPMD assessed by Bayley III Scale between the control and intervention groups showed no statistically significant difference in any of the studied domains: motor, cognitive, and language. The score values were always higher in the intervention group than in the control group in the three domains.
Only one study similar to the present trial has been identified in the literature to date. Both studied similar populations consisting of preterm infants, discharged from the NICU, with mean birth weight of 1279g (present study) and 1287g,20 on EBF at hospital discharge, and both studies aimed at using multinutrient supplementation to provide more energy and protein intake. However, the way the supplementation was administered differed between them. Unlike the present study, Aimone et al.20 added supplementation to approximately half the daily intake of breast milk, which represented 10% more energy and 20% more protein. In both studies, breast milk was obtained by the mother's own milking. In the present trial, a special formula for preterm infants was used (Pré-Nan®, Nestlé, Vevey, Switzerland), different from that study, which used a human milk multinutrient fortifier in powder form (Similac HM fortifier, Abbott Nutrition®, Ohio, USA).
In both trials, there was no influence of supplementation on the duration of EBF.
The study by Aimone et al.20 assessed the development at 18 months of CGA through the Bayley II Scale, finding no statistically significant differences between groups. They found, however, borderline associations in the language (0.053) and motor (0.067) domains. Similar percentages of children with developmental delay were found in both groups.
The present study confirmed these results, as similar mean scores were obtained on the Bayley III Scale in both groups, in the three evaluated domains, at 12 months of CGA, with no statistically significant difference. In the study by Aimone et al.,20 developmental delay was distributed similarly in both groups.
Some factors may have contributed to this result. The non-monthly correction of supplementation may have relatively reduced the effect of the 4g of supplement on infant development. Another point that may have contributed to the reduction of differences between the groups, which is characteristic of intervention studies with follow-up, is that, despite the careful and systematic follow-up of the dyads, it is virtually impossible to ensure that the correct frequency of supplemented breast milk administration for domestic use had been followed.
A third factor is that the infants in both groups probably ingested similar amounts of calories and nutrients, as those who did not receive supplement supposedly would nurse for a longer period, receiving a larger amount of hindmilk. As for those in the supplemented group, they would tend to be satiated earlier, nursing for a shorter period of time than the control group.
Finally, although the initial sample calculation indicated that the number of individuals was sufficient to detect differences between groups, it is possible that the extent of these differences has been overestimated, and larger samples are required to detect them.
The strengths of this study include its experimental design, with NPMD evaluator blinding, and the fact that only one other study was found in the literature testing a similar hypothesis.
This study faced some obstacles. The difficulties of follow-up and the introduction of other foods made it impossible to follow a larger number of children. The possibility of bias due to the long time between the end of the intervention and the development assessment and the different percentage of losses in both groups were minimized by the use of adjusted analysis.
Considering the results of this study, it can be concluded that, according to the current state of knowledge, there is still no evidence of an association between post-discharge multicomponent supplementation and NPMD of very low birth weight preterm infants.
FundingFinancial support for the equipment and materials used in the study: MS/CNPq/FAPEMA – n.477848/2007-9.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: da Cunha RD, Lamy Filho F, Rafael EV, Lamy ZC, Queiroz AL. Breast milk supplementation and preterm infant development after hospital discharge: a randomized clinical trial. J Pediatr (Rio J). 2016;92:136–42.
Study conducted at Universidade Federal do Maranhão (UFMA), São Luís, MA, Brazil.