Enabling development of the ability to communicate effectively is the principal objective of cochlear implantation (CI) in children. However, objective and effective metrics of communication for cochlear-implanted Brazilian children are lacking. The Functioning after Pediatric Cochlear Implantation (FAPCI), a parent/caregiver reporting instrument developed in the United States, is the first communicative performance scale for evaluation of real-world verbal communicative performance of 2-5-year-old children with cochlear implants. The primary aim was to cross-culturally adapt and validate the Brazilian-Portuguese version of the FAPCI. The secondary aim was to conduct a trial of the adapted Brazilian-Portuguese FAPCI (FAPCI-BP) in normal hearing (NH) and CI children.
MethodsThe American-English FAPCI was translated by a rigorous forward-backward process. The FAPCI-BP was then applied to the parents of children with NH (n=131) and CI (n=13), 2-9 years of age. Test-retest reliability was verified.
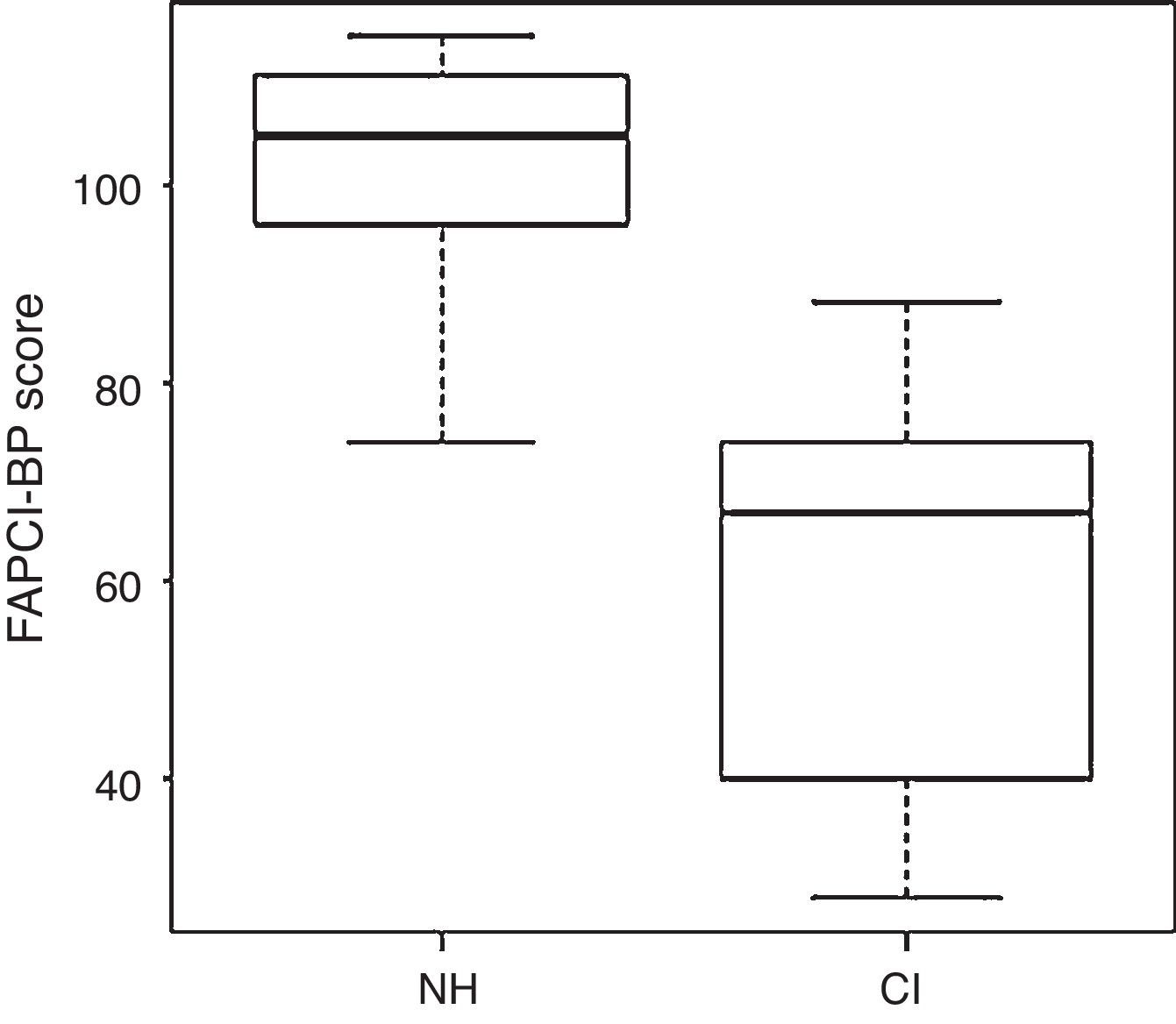
ResultsThe FAPCI-BP was confirmed to have excellent internal consistency (Cronbach's alpha > 0.90). The CI group had lower FAPCI scores (58.38±22.6) than the NH group (100.38±15.2; p<0.001, Wilcoxon test).
ConclusionThe present results indicate that the FAPCI-BP is a reliable instrument. It can be used to evaluate verbal communicative performance in children with and without CI. The FAPCI is currently the only psychometrically-validated instrument that allows such measures in cochlear-implanted children.
O principal objetivo do implante coclear (IC) em crianças é permitir o desenvolvimento da capacidade de se comunicar efetivamente. Contudo, não há objetivo nem parâmetros efetivos de comunicação para crianças brasileiras com o implante coclear. O Functioning after Pediatric Cochlear Implantation (FAPCI), instrumento de relato dos pais/prestadores de cuidados desenvolvido nos Estados Unidos, é a primeira escala de desempenho para avaliação do desempenho comunicativo verbal no mundo real de crianças de 2-5 anos de idade com implantes cocleares. Nosso principal objetivo era adaptar e validar a versão do FAPCI em português do Brasil de forma transcultural. Nosso objetivo secundário era realizar um teste da versão do FAPCI adaptada para o português do Brasil (FAPCI-PB) com grupos de crianças com audição normal (AN) e IC.
MétodosO FAPCI em inglês norte-americano foi traduzido por um processo rigoroso de tradução e retrotradução. O FAPCI-PB foi, então, aplicado aos pais das crianças com AN (N=131) e IC (N=13) de 2-9 anos de idade. Foi verificada a confiabilidade da reaplicação do teste.
ResultadosConfirmou-se que o FAPCI-PB tem excelente coerência interna (alfa de Cronbach > 0,90). O grupo com IC apresentou menores pontuações no FAPCI (58,38±22,6) que o grupo com AN (100,38±15,2; p<0,001, teste de Wilcoxon).
ConclusãoEsses resultados indicam que o FAPCI-PB é um instrumento confiável. Pode ser utilizado para avaliar o desempenho comunicativo verbal em crianças com e sem IC. O FAPCI é, atualmente, o único instrumento validado psicometricamente que possibilita essas medições em crianças com implante coclear.
Cochlear implantation (CI) is a treatment for severe-to-profound bilateral sensorioneural hearing loss, particularly for children with congenital and perilingual etiologies.1 It is recommended when traditional hearing aids (sound amplification appliances) cannot enable sound discrimination. Social communication is an essential human capacity and oral language is the most used form of complex communication. Ample evidence has shown that children who receive CI at a very young age are able to develop better performance in speech comprehension and production and achieve better academic and social behavior than children treated later.2 There is also a growing evidence that children with severe-to-profound bilateral hearing loss who receive CI bilaterally can perform almost as well as children with normal-hearing (NH).3 Early auditory deprivation, even if partial, has a deleterious effect on language development and on the development of central auditory processing skills in young children.4
Enabling hearing is the first goal of CI. Once adequate hearing has been established with CI, the development of oral language is expected to follow.5 Several factors can influence the outcome of CI, such as duration of deafness, age at implantation, the speech rehabilitation approach applied, and how these factors interact to influence neural plasticity.6 Many variables influence this process, and it is extremely important that physicians and speech therapists track the performance and progress of CI patients in the area of language development.
Several studies have investigated the effects of implantation age and the outcome of language development skills. Not surprisingly, earlier implantation has been demonstrated to lead to better language outcomes.7,8 Other factors that play a role in language development after CI include family involvement in rehabilitation therapy and the educational level of the family. Geers et al.9 argued that children with congenital deafness should receive CI no later than 2 years of age, while electrophysiological studies and the brain plasticity literature define the critical period for CI as extending to about 3.5 years of age.10 In successive studies of the long-term effects auditory deprivation on language development, Davidson et al.11 found that a long period of deprivation before CI had only a minor negative effect on vocabulary acquisition, but hindered syntax and prosody severely. The language development of children who received stimulation via sound amplification equipment and sign language was better following CI than those who did not receive such interventions, but outcomes are improved more by early CI than by these interventions.11
Electrophysiological research by Gilley et al.6 has shown that children with congenital deafness who receive CI within the critical maximally plastic period for central auditory pathway development develop cortical electrical potentials with latencies that are close to latencies observed in hearing children within six months of stimulation.6 In contrast, children with congenital deafness who received CI after 7 years of age show cortical electrical potentials with latencies that are consistently longer than those of age-matched children with NH; outcomes in children who received CI between 3.5 years and 7 years of age were highly variable. These findings are consistent with prior neurophysiological and functional imaging studies in indicating a critical period for neuroplasticity of the auditory system before the age of 3.5 years.12,13
Families and physicians need to be able to determine whether or not the objectives of CI have been met. Traditionally, clinicians have used speech perception and discrimination tests to evaluate communicative capacity in children following CI.14 However, these measurements may not reflect the child's ability to communicate in a real-world environment with background noise and non-ideal listening conditions.15 The World Health Organization's International Classification of Functioning (ICF) distinguishes between communicative capacity, the ability to communicate in a standardized environment, and communicative performance, the ability to communicate in real-world environments.16 Measuring communicative performance after CI is very difficult, particularly in young children, and this challenge has created a demand for validated assessments tools. The widely available questionnaires used for assessment of communicative performance after CI were designed to measure communicative capacity, that is, the child's ability to understand lexicon, grammar, and syntax.15 Examples of this type of instrument include the Reynell Developmental Language Scales (RDLSs), the MacArthur Communicative Development Inventories (MCDIs), and the Meaningful Use of Speech Scale (MUSS). The RDLSs are used to assess expressive and receptive language, the MCDIs are used to evaluate lexical development, and the MUSS is used to assess oral language use in children with hearing impairments. Communicative capacity can be measured in a clinical setting, but such testing is not sufficient to establish whether patients are able to use their communication skills well enough to function in a normal social environment in their daily lives.15
Currently, there are no instruments with reliable parameters that can be used to evaluate the communicative performance of pediatric cochlear implant users in Brazilian Portuguese. Children who have received CI in Brazil are still evaluated primarily in terms of the results of auditory and language assessments applied in an isolated environment.17 Speech perception and language skills are measured by direct behavioral observation or, more commonly, a proxy assessment, such as the MUSS or RDLSs, mentioned above, or the Infant-Toddler Meaningful Auditory Integration Scale for children younger than 24 months.
To improve auditory (re)habilitation such that communicative performance of children with CI is maximized, there needs to be a sufficient understanding of the instruments’ functional limitations.18 The Functioning Inventory after Pediatric Cochlear Implantation (FAPCI) instrument was developed in the USA, in American English, to enable more objective evaluation of the auditory communicative performance of 2-5-year-old children with CI. It was designed to probe the child's use of communication skills in his or her interactions with linguistically fluent individuals.18
The Speech, Spatial, and Qualities of Hearing Scale (SSQ)19 is a widely used structured scale that evaluates hearing ability in everyday situations. Originally designed for adults, it has been adapted for use with children, parents, and teachers.20 It is composed of three sections, A, B, and C. Section A assesses the ability of the individual to understand oral language in a quiet setting, with background noise, in reverberant environments, and on the phone. Section B evaluates how well an individual perceives his or her position and movement away from a sound source. Section C asks the individual to identify sounds and voices with the aim of determining whether sounds can be understood and segregated with ease. SSQ findings are relevant for receptive language assessment, but the SSQ does not provide information about expressive language or the quality of oral language communication, as the FAPCI does. Furthermore, the SSQ was developed for adults and then adapted to a parent/teacher version for proxy assessment of 5-11-year-old children, and adapted to a self-report child version for children over 11 years old. Hence, the SSQ is not suitable for use with children in the 2-5-year-old age band, whereas the FAPCI is.
The FAPCI models various situations of everyday life, and allows communicative performance to be assessed by professional care providers or family members.18 The instrument consists of a 23-item questionnaire that is answered by parents or guardians probing the language development of children with cochlear implants who are in the 2-5-year-old age band. Respondents answer questions with a five-point scale. The FAPCI is being utilized in several ongoing NIH-funded studies of pediatric CI and has already been translated into German.21 There is no child self-report version of the FAPCI.
A series of studies carried out by the group that developed the FAPCI15,22,23 showed that despite the establishment of good communicative capacity, children with CI were not communicating on par with their peers and were struggling to communicate with oral language in social environments, including school. Therefore, the primary aim of this study was to translate, adapt, and test the reliability of the FAPCI for use with Brazilian children. The second aim of this work was to test the sensitivity of the FAPCI translated and adapted to Brazilian Portuguese (FAPCI-BP) in the evaluation of language development in NH children versus children using CI.
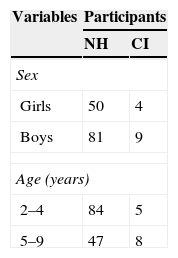
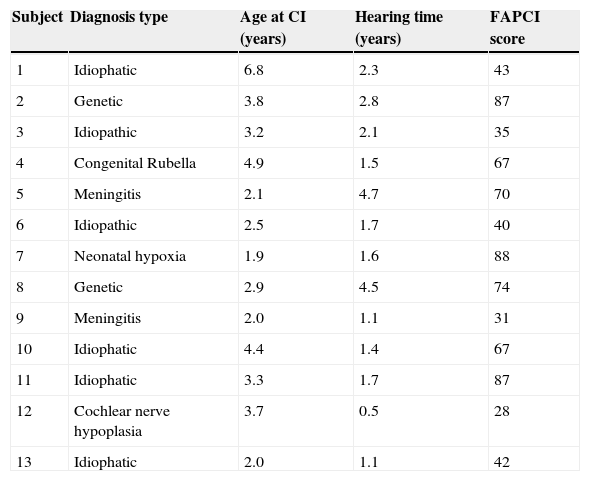
MethodsParticipantsThis research was approved by the Institutional Research Committee for Human Subjects. The study included children between 2 and 9 years of age, of both sexes, who were treated as outpatients at the Pequeno Príncipe Children's Hospital in the city of Curitiba, Brazil. The CI sample included children aged 2-9 years who had undergone CI and had been living with activated implants for at least six months. The NH group consisted of similarly aged children with no otological, neurological, or neuropsychiatry complaints. Parents or guardians accompanying the children provided written informed consent and answered the FAPCI-BP. Table 1 summarizes the demographic characteristics of the children in the NH and CI groups, and Table 2 details the clinical characteristics of the CI participants. The FAPCI-BP was completed by a total of 131 parents of 144 children. The clinical characteristics of the 13 cochlear-implanted children of the CI group are summarized in Table 3.
Clinical characteristics of CI group participants.
| Subject | Diagnosis type | Age at CI (years) | Hearing time (years) | FAPCI score |
|---|---|---|---|---|
| 1 | Idiophatic | 6.8 | 2.3 | 43 |
| 2 | Genetic | 3.8 | 2.8 | 87 |
| 3 | Idiopathic | 3.2 | 2.1 | 35 |
| 4 | Congenital Rubella | 4.9 | 1.5 | 67 |
| 5 | Meningitis | 2.1 | 4.7 | 70 |
| 6 | Idiopathic | 2.5 | 1.7 | 40 |
| 7 | Neonatal hypoxia | 1.9 | 1.6 | 88 |
| 8 | Genetic | 2.9 | 4.5 | 74 |
| 9 | Meningitis | 2.0 | 1.1 | 31 |
| 10 | Idiophatic | 4.4 | 1.4 | 67 |
| 11 | Idiophatic | 3.3 | 1.7 | 87 |
| 12 | Cochlear nerve hypoplasia | 3.7 | 0.5 | 28 |
| 13 | Idiophatic | 2.0 | 1.1 | 42 |
CI, cochlear implantation; FAPCI, Functioning after Pediatric Cochlear Implantation.
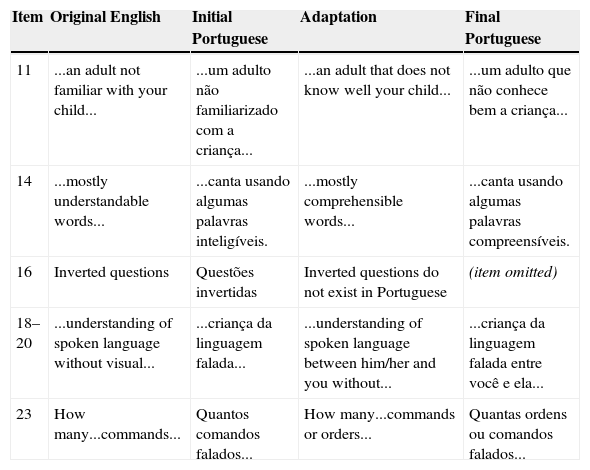
Item changes in the adaptation of the FAPCI to Brazilian Portuguese.
| Item | Original English | Initial Portuguese | Adaptation | Final Portuguese |
|---|---|---|---|---|
| 11 | ...an adult not familiar with your child... | ...um adulto não familiarizado com a criança... | ...an adult that does not know well your child... | ...um adulto que não conhece bem a criança... |
| 14 | ...mostly understandable words... | ...canta usando algumas palavras inteligíveis. | ...mostly comprehensible words... | ...canta usando algumas palavras compreensíveis. |
| 16 | Inverted questions | Questões invertidas | Inverted questions do not exist in Portuguese | (item omitted) |
| 18–20 | ...understanding of spoken language without visual... | ...criança da linguagem falada... | ...understanding of spoken language between him/her and you without... | ...criança da linguagem falada entre você e ela... |
| 23 | How many...commands... | Quantos comandos falados... | How many...commands or orders... | Quantas ordens ou comandos falados... |
FAPCI, Functioning after Pediatric Cochlear Implantation.
A two-step strategy was implemented: (1) translation, retro-translation, and adaptation of the FAPCI; and (2) administration of the FAPCI-BP to children with CI and children with NH.
FAPCIThe FAPCI is a 23-item written questionnaire designed to measure verbal communicative performance in children 2 to 5 years of age after CI.18 It is completed by the parents or the guardians of the subjects and can be finished in about 5-10minutes. There are three response mode formats: frequency (response levels never, rarely, sometimes, often, always); quantity/proportion (number or percentage of occurrences, e.g. 0-4%, 5-24%, 25-49%, 50-95%, or 96-100%); and examples (responses offer descriptions or examples of behaviors, and levels correspond to an ordinal scale).15 Each answered item is translated into a score ranging from 1 point (e.g. for never) to 5 points (e.g. always), regardless of the type of question, and the unanswered questions are scored as 0 points, yielding a maximum total score of 115. If the number of unanswered questions exceeds two, the questionnaire is considered invalid. If more than one answer is marked, the higher answer is taken. Mean scores were compared between the two groups and reported with standard deviations (SDs). This instrument was developed to complement other tests of spoken language competence to enable assessment of communicative capacity in children with CI.
Step 1: Cross-cultural adaptation and validation processAuthorization by the original instrument's author for the translation, adaptation, and validation of the FAPCI for the Brazilian population was obtained, and the process was conducted in accordance with the guidelines established by Beaton et al.24 The FAPCI was translated from English to Portuguese by a professional translator familiar with both languages. Small changes were necessary in order to adapt the verbiage to Brazilian culture, but the original essence of the questions was maintained as much as possible. The FAPCI-BP is presented in its entirety as an appendix with the approval of the developers.
The adapted questionnaire was sent to another professional translator who was unfamiliar with the original questionnaire for retro-translation into English. An equivalence of construction analysis was performed in which the original and retro-translated English versions were compared to determine whether there were significant differences in the content of the questions, that is, whether the FAPCI-BP was faithful to the structure of the original questionnaire.
To test for internal consistency, a subgroup of 34 parents of children with NH and 13 parents of children with CI completed the FAPCI-BP twice with a time interval of at least two weeks, but not more than one month. Cronbach's alpha was used to verify the internal consistency of the instrument's items between the first and second runs. A construct can be validated indirectly with an internal base of consistency or no relation between the questions that make up part of the scale, allowing the conclusion that the scale has a valid construction.15 Cronbach's alpha coefficient is the simplest and best-known measure of internal consistency, and is the primary approach used in scale validation. In general, a group of items that explore a common factor should have a high alpha value. The minimum acceptable value for the alpha coefficient is 0.70; alpha values greater than 0.80 are preferable.25
Step 2: Applicability of the FAPCI-BPThe FAPCI-BP was answered by parents of NH children and parents of CI children. The results were subjected to statistical analyses in R software, version 3.0.1 (R Project for Statistical Computing, University of California, CA, USA). The data were verified in relation to normality and descriptive analyses using Wilcoxon tests. Comparisons were considered significant when they had two-tailed p-values<.001.
ResultsAdaptation and internal consistencyAfter comparison of the retro-translation to the original English and consideration of cultural linguistic use, it was determined that several items needed to be adapted and one item needed to be withdrawn (question 16), as shown in Table 1, to obtain a final version of the adapted questionnaire that was consistent with the original. The Cronbach's alpha for internal consistency was 0.948 for the NH group and 0.964 for the CI group, with no questions observed to be outside the expected average, indicating that the instrument had good internal consistency.
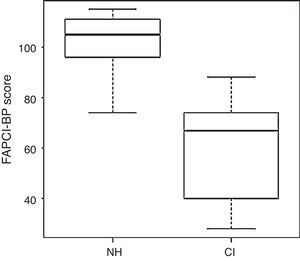
FAPCI-BP trialComparison of the groups’ overall mean scores±SDs revealed that the NH group performed significantly better on the FAPCI-BP (100.38±23.5) than did the CI group (63.00±21.0; p<.001, Wilcoxon test). The mean scores±SDs obtained for children in the NH group divided by chronological age are reported in Table 3 together with score data for children in the CI group, separated by chronological age and the children's ages at the time hearing was established. The mean scores by age of hearing within each age year bin are presented as means without SDs, since the subgroups were small and irregular. The group medians and distributions are illustrated in a boxplot graph in Fig. 1.
DiscussionThe goal of CI is not only that children will gain functional auditory processing skills, but also that they will develop the skills needed to communicate effectively with spoken language. The FAPCI is the only currently available instrument that allows the impact of CI on communication skills to be measured in children 2-5 years old. The present study produced a Portuguese-language FAPCI version adapted for use in the Brazilian population (see appendix for the final version of FAPCI-BP). Consistent with the American18 and German21 versions, the Brazilian FAPCI had excellent internal consistency (Cronbach's alpha > 0.90). Additionally, the expected gap in communicative performance was observed between children with CI and children with NH.
After translation and retro-translation of the original FAPCI, very few questions needed cultural adaptation, and only one item was omitted (question 16). The Cronbach's alpha value for internal consistency that was obtained for the 22-item FAPCI-BP was similar to that of the original 23-item version, which was validated in a study of 75 families (alpha=0.86).18,25 The alpha value serves as an index of an instrument's reliability in situations where the researcher is not able to perform additional interviews of the individuals in question, but requires an estimate of the average degree of error.25
Examining the scores by age enabled several inferences to be made. Firstly, it was noticed that FAPCI-BP performance was relatively stable across ages within the NH group, especially among children from age 3 through 8 years. Only the youngest (2-year-olds) and oldest (9-year-olds) had non-overlapping SDs, which is not altogether surprising given that, normally, children exhibit a great linguistic expansion between 2 and 3 years of age and are still developing basic linguistic skills. Regardless, there was at least one child within each age year bin achieved the maximum possible score (115). The greater the age, the more children achieved the maximum possible score. Yet, even in the upper age groups, there were always some children that performed below the maximum, raising the question of whether the instrument may also be valid beyond the stated upper limit of 9 years of age. Regardless, it should be noted that there were quite small numbers of children in the age 7-, 8-, and 9-year subgroups. Thus, the data for those subgroups is likely less reliable than the data for the lower age subgroups, which were substantially larger.
The comparison of scores between the NH and CI groups demonstrated a significant lingering communicative deficit among children with CI. Moreover, examining the scores of the individual children with CI, it is apparent that they had not achieved communicative performance on par with their peers, There are several factors that may affect the ability of children with CI to achieve optimum-level communicative performance, including age of onset of deafness, age at CI activation, use of speech therapy/rehabilitation, underlying pathology, and the presence of other disabilities, not to mention inter-individual variation in general, which can be substantial.26 Age of implantation appears to be a particularly important factor in language outcome. Children implanted when they were 16–24 months of age had Preschool Language Scale scores matching their hearing peers at ages 4 and 6.7 In the present study, only one child received CI before 24 months of age, and only three children received CI at approximately 24-25 months old. The majority of participants in this study received CI after 3 years of age. Thus, the comparison of NH and CI groups can be considered preliminary; more studies are needed with a larger sample of CI children receiving CI before 24 months of age.
It is expected that the sooner the CI is performed, the better the outcome will be. The present study's CI group was quite heterogeneous in terms of age of CI, pathology, and hearing rehabilitation. Most children in the CI group were implanted and activated between 3 and 4 years of age, which is considered borderline for the critical period of auditory pathway development in the brain. Two of the children (No. 5 and No. 9) lost their hearing as a sequela of meningitis very early in life, when they were considered to be prelingual. The critical period for language development is thought to end at about 3.5 years of age for children with congenital deafness; children who received CI after their fourth birthday show greater variation in outcome than children who received CI when they were younger than 4 years old.17,21
To minimize the duration of deafness and lack of critical auditory stimulation, CI surgery should be performed before 2 years of age.27,28 However, even when impairment is detected with a newborn hearing test, progression of referrals to hearing specialists in Brazil can be quite slow and the frequency of CIs performed is inadequate in many places (i.e. two per month in the state of Paraná, only one per month each at the Pequeno Príncipe Hospital and the Hospital das Clínicas, personal communication). Although CI is covered by the Brazilian public health system, children needing CI often have aged beyond the critical period for optimum results by the time they receive the intervention, due to long waiting times. Newborn screening programs in Brazil are also inadequate. It is particularly important for infants to be screened in neonatal intensive care units, given their elevated prevalence of affected newborns. For example, one study reported that, among 979 newborns in an intensive care unit, 10.2% presented with unilateral and 4.9% presented with bilateral auditory brainstem response impairments.29
Furthermore, after CI, children should commence immediately with auditory rehabilitation and proper speech therapy, in addition to stimulation by the family through communicative experiences. Children are subjected to a variety of therapies, which are not always the most suitable for rehabilitation from deafness and language development. Speech therapists are not present in every city, and even when properly trained therapists are present, often there are not a sufficient number to accommodate the need. Moreover, it is important that the therapy be individualized to meet the specific needs of each child and be of sufficient duration to allow the child to absorb the treatment.
In evaluations of candidates for CI, psychologists and social workers consider the commitment and involvement of the families in the treatment, and assess whether the families are capable of bearing the expense of maintaining the appliance. Rechargeable batteries have reduced the expenses. However, in order to conserve batteries and delay replacement, some children do not use their implants throughout the day, but rather turn them on only when they are in school. Another difficult issue for families is the cost of spare parts, such as cables and antennas, which when damaged prevent the use of the implants, leaving children without auditory stimulation.
This study has some limitations. First, because the sample of CI children was small and heterogeneous, it is not possible to extrapolate the results to all CI children. Clearly, larger studies are needed to enable multiple variables to be controlled. Also, studies comparing FAPCI-BP results with the results of traditional widely-used tests of language use are needed, since the FAPCI-BP is a new instrument. In principle, it is expected that FAPCI-BP scores should increase within children with implants over time in relation to increases in cumulative therapy and stimulation experience, as observed with the American18 and German versions.21 Likewise, the FAPCI-BP should be useful for phonoaudilogical monitoring of patients, particularly in relation to revealing which communicability areas may be lagging. The aforementioned limitations notwithstanding, this study established a Brazilian version of the FAPCI with excellent internal consistency. Second, even though the FAPCI was designed originally to test the communicative skills of children in the 2-5-year-old age band, this study included older children, up to 9 years of age. This was done in order to compare their FAPCI-BP results with results obtained for NH children, similar to previous studies validating other versions the FAPCI which included subjects up to 10 years of age.21 The scores of the small number of children over 5 years old examined here did not appear to differ markedly from the scores of younger children. This result is not surprising given that, normally, the bulk of fundamental language development is thought to occur by the age of 5 years.6
In conclusion, the recently developed FAPCI-BP is the first instrument to allow functional language development to be measured in Brazilian children using cochlear implants. After translation and adaption, the FAPCI-BP showed excellent internal consistency and demonstrated the expected gap between NH and CI groups, indicating that it is valid for use in Brazilian children. This work paves the way for future studies in Brazil, such as applying the FAPCI-BP to develop score growth curves in NH children to serve as framework for interpreting scores in children with CI. Although the number of subjects with CI in this study was small, it was possible to establish that the FAPCI-BP could be very useful to Brazilian physicians and healthcare providers as a reliable metric of the development of communication skills in their CI patients. The FAPCI-BP may be particularly useful for clarifying diagnoses as well as for directing and revising rehabilitative plans, and thus bettering the prospects of a good quality of life for children with CI.
FundingSecretaria da Ciência, Tecnologia e Ensino Superior do Paraná (SETI-PR).
Conflicts of interestThe authors declare no conflicts of interest.
This study was supported by the Department of Science and Technology of the State of Paraná. The authors thank psychologist Cassia Benko for her assistance, and the children and their families for participating in the study. They also thank Dr. John K. Niparko for providing the opportunity to translate and adapt the FAPCI to Portuguese.
Please cite this article as: Vassoler T, Cordeiro ML. Brazilian adaptation of the Functioning after Pediatric Cochlear Implantation (FAPCI): comparison between normal hearing and cochlear implanted children. J Pediatr (Rio J). 2015;91:160–7.
Article reffers to master's project of otorhinolaryngologist medical doctor Trissia Vassoler, under orientation of professor doctor Mara Lucia Cordeiro.