To assess whether the -675 4G/5G polymorphism in the plasminogen activator inhibitor-1 gene is associated with obesity and insulin resistance in Mexican children.
MethodsA cross-sectional study was performed in 174 children, 89 with normal-weight and 85 with obesity, aged from 6 to 13 years. All children were from state of Guerrero, and recruited from three primary schools in the city of Chilpancingo, state of Guerrero, Mexico. Insulin levels were determined by immunoenzymatic assay. The homeostasis model assessment was used to determine insulin resistance. The -675 4G/5G polymorphism in PAI-1 gene was analyzed by polymerase chain reaction-restriction fragment length polymorphism.
ResultsThe prevalence of insulin resistance in the obese group was higher (49.41%) than in the normal-weight group (16.85%). The 4G/5G PAI-1 polymorphism was found in Hardy Weinberg equilibrium. The 4G/5G genotype contributed to a significant increase in waist-hip ratio (β=0.02, p=0.006), waist circumference (β=4.42, p=0.009), and subscapular skinfold thickness (β=1.79, p=0.04); however, it was not related with insulin resistance.
ConclusionThe -675 4G/5G genotype of PAI-1 gene was associated with increase of body adiposity in Mexican children.
Elaboramos este estudo para avaliar se o polimorfismo -675 4G/5G no gene inibidor 1 do ativador do plasminogênio se associa à obesidade e à resistência insulínica em crianças mexicanas.
MétodosFoi realizado um estudo transversal em 174 crianças, 89 delas com peso normal e 85 obesas, variando sua idade de 6 a 13 anos. Todas as crianças eram do estado de Guerrero e foram recrutadas de três escolas primárias na cidade de Chilpancingo, México. Os níveis de insulina foram determinados por prova imunoenzimática. Foi usado o modelo de avaliação da homeostase para determinar resistência insulínica. O polimorfismo -675 4G/5G no gene PAI-1 foi analisado pelo método reação de polimerase em cadeia-polimorfismo no comprimento dos fragmentos de restrição.
ResultadosA prevalência de resistência insulínica no grupo obeso foi mais alta (49,41%) do que no grupo com peso normal (16,85%). O polimorfismo 4G/5G do PAI-1 foi encontrado em equilíbrio de Hardy Weinberg. O genótipo 4G/5G contribuiu para um aumento significativo da relação cintura-quadril (β=0,02, p=0,006), da circunferência da cintura (β=4,42, p=0,009) e da espessura da prega subescapular (β=1,79, p=0,04), mas não se relacionou com a resistência insulínica.
ConclusãoO genótipo -675 4G/5G do gene PAI-1 se associou a aumento da adiposidade corporal em crianças mexicanas.
Obesity is a complex, multifactorial chronic disease, frequently associated with insulin resistance, that appears to be the central characteristic to the pathogenesis of diabetes mellitus type 2; these metabolic disorders have been associated with increased plasminogen activator inhibitor-1 (PAI-1) levels in circulation.1
PAI-1 is the main inhibitor in the plasminogen activation system (PAS), which comprises an inactive proenzyme (plasminogen) that can be converted into its active form plasmin by the action of physiological plasminogen activators (PAs).2 Plasmin is the main enzyme that degrades fibrin into soluble products. Under physiological conditions PAI-1, is released into the circulation and into the extracellular space by certain cells, such as hepatocytes, smooth muscle cells, spleen cells, myocytes, adipocytes, monocytes, macrophages, and platelets, which are the main source of PAI-1.3–5 In pathological conditions, other tissues, such as tumor and endothelial cells secrete a large amount of PAI-1, mainly in response to upregulation by inflammatory cytokines; thus, PAI-1 is regarded as a marker in the course of inflammatory processes.3,6,7 The increase of PAI-1 levels in plasma is associated with risk factors such as obesity, glucose intolerance, hypertension, insulin resistance, and metabolic syndrome.4,8–10
Over 180 single nucleotide polymorphisms (SNPs) have been described in the PAI-1 gene. The -675 4G/5G polymorphism is characterized by an insertion/deletion of a single nucleotide guanine at position -675 of the promoter of PAI-1 gene.11 This polymorphism has been associated with high levels of PAI-1, obesity, hypertension, dyslipidemia, glucose intolerance, and insulin resistance.12 In a white European population it has been reported that subjects who are homozygous for the 4G allele (4G/4G genotype) have plasma concentrations of PAI-1 approximately 25% higher than subjects who are homozygous for the 5G allele (genotype 5G/5G).11 In another study, the -675 4G/5G polymorphism influenced the development of a state of insulin resistance and obesity, indicating that this polymorphism may be a marker of genetic susceptibility for these diseases.1,12 Therefore, this study was designed to assess whether the -675 4G/5G PAI-1 gene polymorphism is associated with obesity and insulin resistance in Mexican children.
MethodsA cross-sectional study was performed in 174 children, 89 with normal weight and 85 with obesity, aged between 6 and 13 years. All children were from the state of Guerrero, Mexico, and recruited from three primary schools in the city of Chilpancingo, Mexico. An informed consent was obtained from all parents before enrollment of children in the study. Approval for the study was obtained from the Research Ethics Committee of the University of Guerrero according to the ethical guidelines of the Declaration of Helsinki.
Body weight was determined using a Tanita body composition monitor (Tanita BC-553=Arlington, USA), and height was measured to the nearest 0.1cm using a stadiometer (Seca - Hamburg, Germany). Body circumferences were measured in duplicate using a diameter tape accurate to within ± 0.1cm (Seca 201 - Hamburg, Germany). Waist circumference was measured at the level of the umbilicus and the superior iliac crest. Hip circumference was measured at the maximum point below the waist, without compressing the skin. The thickness of four skinfolds was measured to the nearest 0.1mm, in duplicate, using a skinfold caliper (Dynatronics Co - Salt Lake City, USA): triceps, biceps, subscapular, and suprailiac. The duplicate measures were averaged. The classification of obesity was made using the 2000 Centers for Disease Control and Prevention growth charts, which defined normal weight as the 5th to 85th percentiles, and obesity as ≥ 95th percentile.
Blood samples were obtained from antecubital venipuncture after overnight fast. Serum glucose was analyzed with semi-automated equipment (COBAS MIRA). Insulin levels were determined by immunoenzymatic assay (GenWay INS-EASIA kit). The homeostasis model assessment was used to determine insulin resistance (IR) in children; this score was calculated with the following formula: fasting serum insulin (μU/mL) x fasting plasma glucose (mmol/L)/22.5. Insulin resistance was defined as a homeostasis model assessment for insulin resistance (HOMA-IR) above the 75th percentile for all children (HOMA-IR ≥ 2.4).
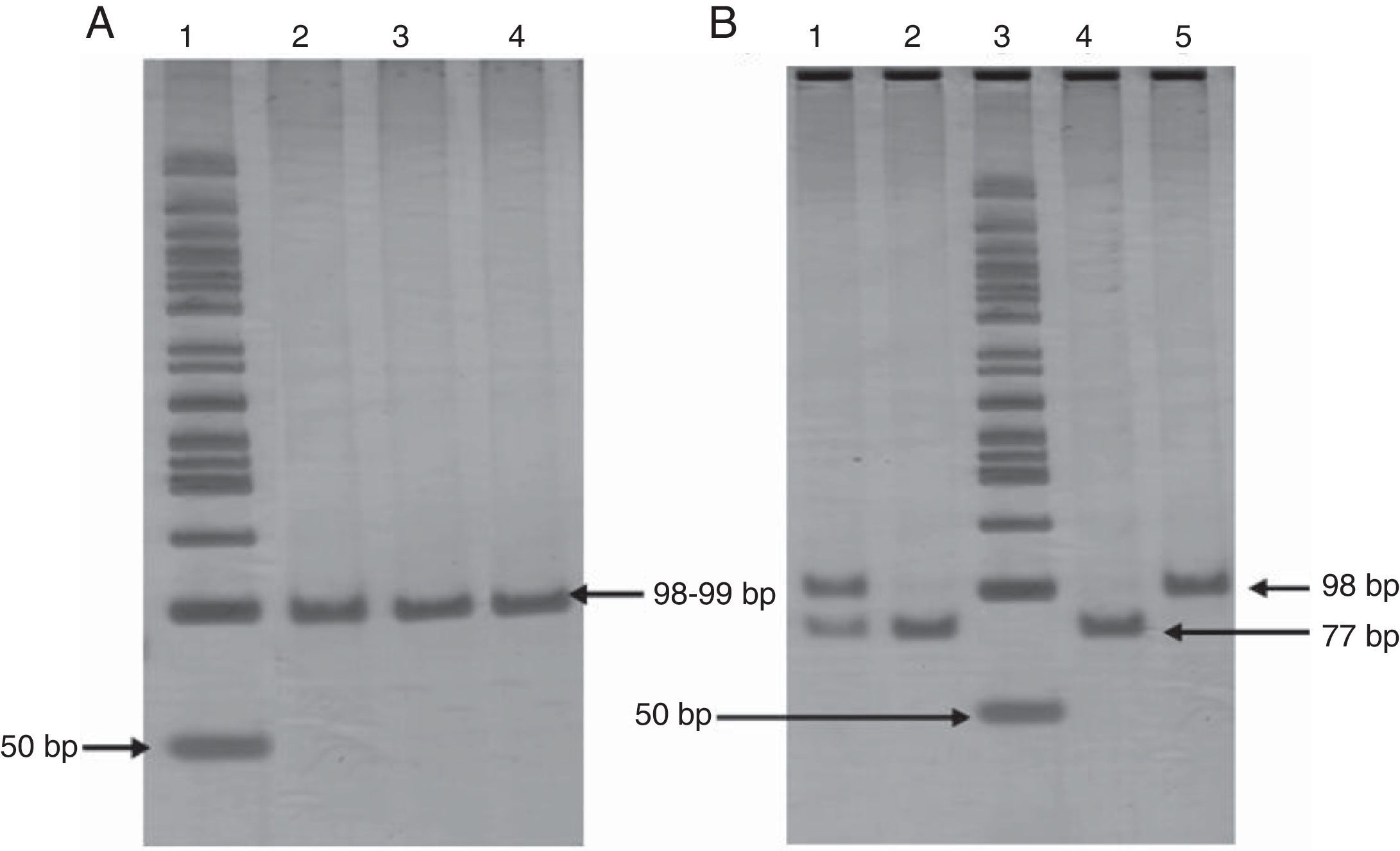
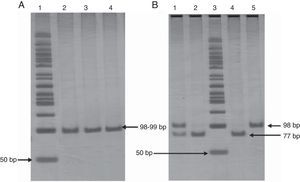
The extraction of genomic DNA (gDNA) was performed from leukocytes obtained from whole blood samples, according to the Miller method. The -675 4G/5G PAI-1 gene polymorphism was screened by the polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) method, using following primers: 5′CACAGAGAGAGTCTGGCCACGT3′ (forward), and 5′CCAACAGAGGACTCTTGGTCT3′ (reverse). PCR was carried out in a final volume of 25μL containing 1μg of DNA, 0.06μM of each oligonucleotide, 1.25 U/μL Taq DNA polymerase, supplied buffer enzyme 1X, 1.5mM MgCl2, and 0.1mM of each dNTP (Invitrogen™ life technologies). PCR was performed by initial denaturation at 94°C during 3min, 30 cycles of amplification at 94°C during 30 s for denaturation, at 60°C during 30 s for annealing, and at 72°C during 30 s for extension. Finally, 72°C during 1min was used for end extension, resulting in a fragment of 99bp for the 5G or 98bp for the 4G. These were analyzed on a 6% polyacrylamide gel (Invitrogen™ life technologies) stained with silver nitrate. Amplified fragments were digested for 2h and 30min at 55°C with 3 U of Bsl I (New England Biolabs) restriction enzyme. Afterwards, restriction fragments were analyzed by electrophoresis on 6% polyacrylamide gel (Invitrogen™ life technologies) stained with silver nitrate. PAI-1 genotyping was done in duplicate in all cases (Fig. 1). To confirm the results, were random selected a few genotypes and analyzed for sequencing.
Panel A. Polyacrylamide gel electrophoresis showing polymerase chain reaction amplification of 99 pb fragment of -675 4G/5G polymorphism. Lane 1: 50bp DNA ladder; lanes 2, 3, and 4 PCR product 98-99bp. Panel B. Polyacrylamide gel electrophoresis showing pattern restriction of -675 4G/5G polymorphism. Lane 1: 4G/5G genotype; lanes 2 and 4: 5G/5G genotype; lane 3: 50bp DNA ladder; lane 5: 4G/4G genotype.
The statistical analysis was performed using the statistical software STATA v. 9.2. For the descriptive analysis, nominal variables were expressed as frequencies, continuous variables normally-distributed as mean and standard deviation, and those not normally-distributed were expressed as medians and 5th and 95th percentiles. The chi-squared test was used to compare proportions between groups (normal weight and obese children), and Student's t-test and/or Mann-Whitney test were used to compare quantitative measurements between groups. Genotype and allele frequencies for the polymorphism -675 4G/5G PAI-1 gene were determined by direct counting, and the significance of the differences between the biochemical and anthropometric parameters for each genotype was determined using ANOVA and by the Kruskal-Wallis test; the chi-squared test was used to evaluate the Hardy-Weinberg equilibrium. To evaluate the effect of polymorphism, linear regression models were used. Differences were considered statistically significant when p<0.05.
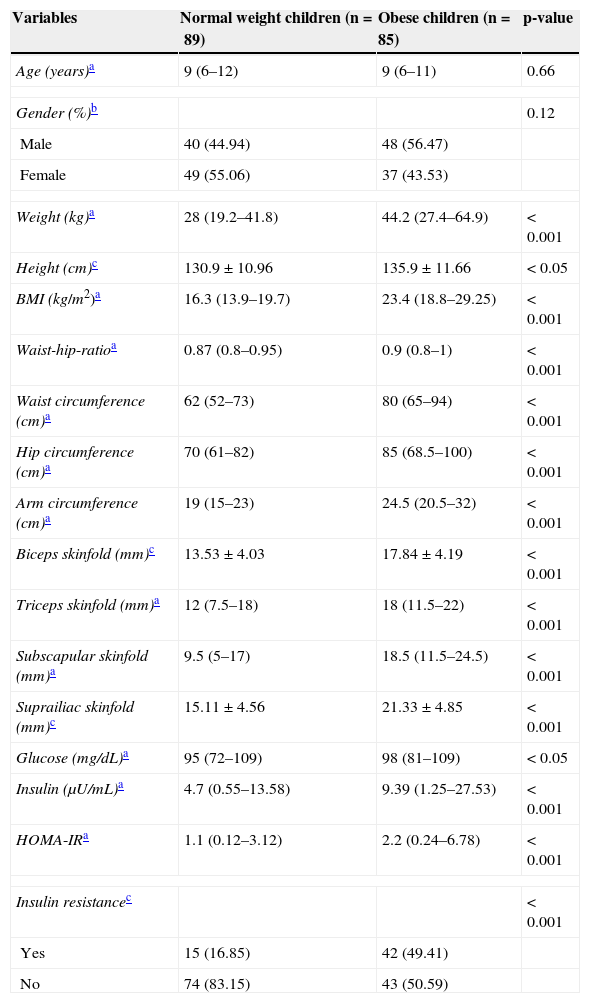
ResultsThe comparison of the clinical and anthropometric variables between both groups revealed, in the obese group, a significant increase of glucose and insulin levels, measures of central and peripheral adiposity, as well as systolic and diastolic blood pressures. The prevalence of insulin resistance the in obese group was 49.41%, versus the 16.85% of the group with normal-weight (Table 1).
Clinical and biochemical characteristics in normal weight and obese children.
| Variables | Normal weight children (n=89) | Obese children (n=85) | p-value |
|---|---|---|---|
| Age (years)a | 9 (6–12) | 9 (6–11) | 0.66 |
| Gender (%)b | 0.12 | ||
| Male | 40 (44.94) | 48 (56.47) | |
| Female | 49 (55.06) | 37 (43.53) | |
| Weight (kg)a | 28 (19.2–41.8) | 44.2 (27.4–64.9) | < 0.001 |
| Height (cm)c | 130.9 ± 10.96 | 135.9 ± 11.66 | < 0.05 |
| BMI (kg/m2)a | 16.3 (13.9–19.7) | 23.4 (18.8–29.25) | < 0.001 |
| Waist-hip-ratioa | 0.87 (0.8–0.95) | 0.9 (0.8–1) | < 0.001 |
| Waist circumference (cm)a | 62 (52–73) | 80 (65–94) | < 0.001 |
| Hip circumference (cm)a | 70 (61–82) | 85 (68.5–100) | < 0.001 |
| Arm circumference (cm)a | 19 (15–23) | 24.5 (20.5–32) | < 0.001 |
| Biceps skinfold (mm)c | 13.53 ± 4.03 | 17.84 ± 4.19 | < 0.001 |
| Triceps skinfold (mm)a | 12 (7.5–18) | 18 (11.5–22) | < 0.001 |
| Subscapular skinfold (mm)a | 9.5 (5–17) | 18.5 (11.5–24.5) | < 0.001 |
| Suprailiac skinfold (mm)c | 15.11 ± 4.56 | 21.33 ± 4.85 | < 0.001 |
| Glucose (mg/dL)a | 95 (72–109) | 98 (81–109) | < 0.05 |
| Insulin (μU/mL)a | 4.7 (0.55–13.58) | 9.39 (1.25–27.53) | < 0.001 |
| HOMA-IRa | 1.1 (0.12–3.12) | 2.2 (0.24–6.78) | < 0.001 |
| Insulin resistancec | < 0.001 | ||
| Yes | 15 (16.85) | 42 (49.41) | |
| No | 74 (83.15) | 43 (50.59) | |
BMI, body mass index; HOMA-IR, homeostasis model assessment for insulin resistance; SD, standard deviation.
The 4G/5G PAI-1 polymorphism was found in Hardy Weinberg equilibrium (X2=0.95, p=0.4). The distribution of genotype and allele frequencies of -675 4G/5G PAI-1 polymorphism was as follows: in the obese group, 8.24% 4G/4G, 49.41% 4G/5G and 42.35% 5G/5G, for 4G allele 32.94% and 5G allele 67.06%, whereas in the normal-weight group, 8.99% 4G/4G, 34.83% 4G/5G and 56.18% 5G/5G, for 4G allele 26.40% and 5G allele 73.60%. In both groups, the 5G/5G genotype and the 5G allele were the most frequently identified. The comparison between both groups showed no significant differences in genotype (χ2=3.91, p=0.14) and allele frequencies (χ2=1.78, p=0.18).
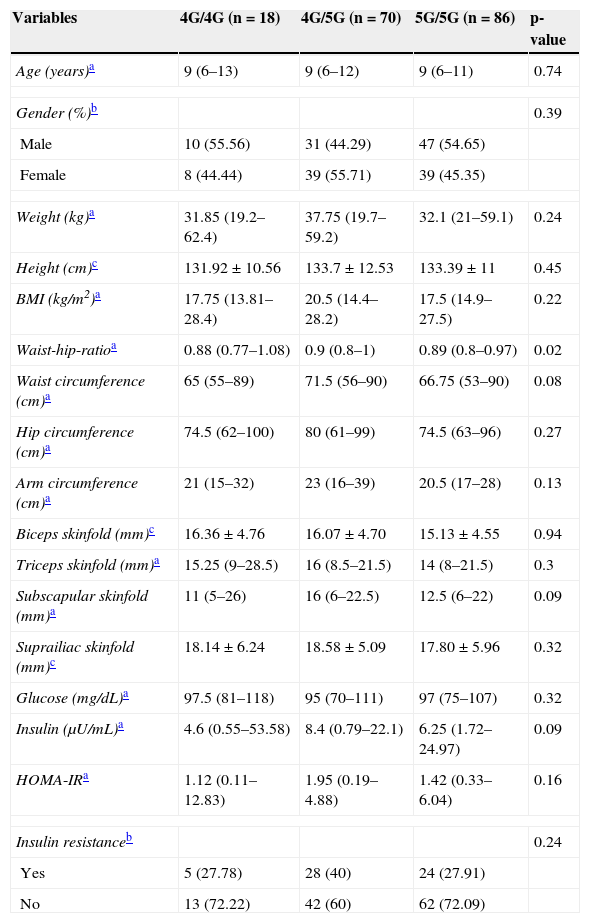
Clinical and biochemical variables were compared by genotypes of -675 4G/5G polymorphism. Carriers of the 4G/5G genotype showed a significant increase in waist-hip ratio (p=0.02), and trends for the increase in waist circumference (p=0.08) and subscapular skinfold thickness (p=0.09) compared with carriers of genotypes 4G/4G and 5G/5G (Table 2).
Clinical and biochemical characteristics stratified by -675 4G/5G polymorphism in the PAI-1 gene.
| Variables | 4G/4G (n=18) | 4G/5G (n=70) | 5G/5G (n=86) | p-value |
|---|---|---|---|---|
| Age (years)a | 9 (6–13) | 9 (6–12) | 9 (6–11) | 0.74 |
| Gender (%)b | 0.39 | |||
| Male | 10 (55.56) | 31 (44.29) | 47 (54.65) | |
| Female | 8 (44.44) | 39 (55.71) | 39 (45.35) | |
| Weight (kg)a | 31.85 (19.2–62.4) | 37.75 (19.7–59.2) | 32.1 (21–59.1) | 0.24 |
| Height (cm)c | 131.92 ± 10.56 | 133.7 ± 12.53 | 133.39 ± 11 | 0.45 |
| BMI (kg/m2)a | 17.75 (13.81–28.4) | 20.5 (14.4–28.2) | 17.5 (14.9–27.5) | 0.22 |
| Waist-hip-ratioa | 0.88 (0.77–1.08) | 0.9 (0.8–1) | 0.89 (0.8–0.97) | 0.02 |
| Waist circumference (cm)a | 65 (55–89) | 71.5 (56–90) | 66.75 (53–90) | 0.08 |
| Hip circumference (cm)a | 74.5 (62–100) | 80 (61–99) | 74.5 (63–96) | 0.27 |
| Arm circumference (cm)a | 21 (15–32) | 23 (16–39) | 20.5 (17–28) | 0.13 |
| Biceps skinfold (mm)c | 16.36±4.76 | 16.07±4.70 | 15.13±4.55 | 0.94 |
| Triceps skinfold (mm)a | 15.25 (9–28.5) | 16 (8.5–21.5) | 14 (8–21.5) | 0.3 |
| Subscapular skinfold (mm)a | 11 (5–26) | 16 (6–22.5) | 12.5 (6–22) | 0.09 |
| Suprailiac skinfold (mm)c | 18.14 ± 6.24 | 18.58 ± 5.09 | 17.80±5.96 | 0.32 |
| Glucose (mg/dL)a | 97.5 (81–118) | 95 (70–111) | 97 (75–107) | 0.32 |
| Insulin (μU/mL)a | 4.6 (0.55–53.58) | 8.4 (0.79–22.1) | 6.25 (1.72–24.97) | 0.09 |
| HOMA-IRa | 1.12 (0.11–12.83) | 1.95 (0.19–4.88) | 1.42 (0.33–6.04) | 0.16 |
| Insulin resistanceb | 0.24 | |||
| Yes | 5 (27.78) | 28 (40) | 24 (27.91) | |
| No | 13 (72.22) | 42 (60) | 62 (72.09) | |
BMI, body mass index; HOMA-IR, homeostasis model assessment for insulin resistance; PAI-1, plasminogen activator inhibitor 1; SD, standard deviation.
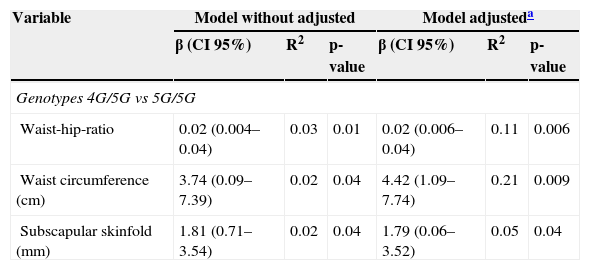
To estimate the contribution of polymorphism to anthropometric and metabolic variables, multiple linear regression models were used. After adjustment for age and gender, it was determined that the 4G/5G genotype contributed to a significant increase in waist-hip ratio (β=0.02, p=0.006), waist circumference (β=4.42, p=0.009), and subscapular skinfold thickness (β=1.79, p=0.04) (Table 3). However, no relationship with insulin levels, HOMA-IR, or insulin resistance was found (data not shown).
Effect of -675 4G/5G polymorphism on body measures.
| Variable | Model without adjusted | Model adjusteda | ||||
|---|---|---|---|---|---|---|
| β (CI 95%) | R2 | p-value | β (CI 95%) | R2 | p-value | |
| Genotypes 4G/5G vs 5G/5G | ||||||
| Waist-hip-ratio | 0.02 (0.004–0.04) | 0.03 | 0.01 | 0.02 (0.006–0.04) | 0.11 | 0.006 |
| Waist circumference (cm) | 3.74 (0.09–7.39) | 0.02 | 0.04 | 4.42 (1.09–7.74) | 0.21 | 0.009 |
| Subscapular skinfold (mm) | 1.81 (0.71–3.54) | 0.02 | 0.04 | 1.79 (0.06–3.52) | 0.05 | 0.04 |
It is currently known that insulin resistance is an important predictor of diabetes mellitus type 2, and is one of the main factors involved in the development of insulin resistance related to increased adipose tissue and its release of adipocytokines; a protein that is secreted by adipocytes in great amounts is PAI-1.13 Previous studies in other populations to investigate the contribution of PAI-1 polymorphism with obesity and insulin resistance have reported inconsistent results.14 In the present study, it was found that the -675 4G/5G polymorphism is related with measures of body adiposity, but not with insulin resistance, in Mexican children.
The results indicate that this sample of obese children had increased glucose and insulin levels, measures of central and peripheral adiposity, and a high prevalence of insulin resistance (49.41%); however, 16.85% of normal-weight children had insulin resistance.
Regarding the genotype and allele frequency, it was observed that this polymorphism is inversely distributed compared to those reported in white populations, in which the 4G/4G genotype (> 25%) was more common than the 5G/5G genotype. In the present population, the 5G/5G genotype was more frequent (42.35%), and the 4G/4G genotype was less common (8.24%). These differences can be attributed to the racial influence, which may be related to the genetic background of the population. It is known that the Mexican population originated from a mixture of European and African populations with Amerindian groups, giving origin to the Mexican mestizo population, which has a great genetic diversity in the distribution of this and other polymorphisms.15 This can explain the differences in the distribution of both genotypic and allelic frequencies of this population with other populations in the world. The frequencies reported in the present study are consistent with those reported in a previous study in a mestizo population of western Mexico, where a high frequency of 5G allele was observed.16 Similarly, in ethnic groups of Mexico, the 5G allele is predominant (≥ 50%) compared with the 4G allele,17 which suggests that the high frequency of 5G allele may be due to the contribution of Amerindian genes in the genetic background of the Mexican population.
This study described, for first time in Mexican children, that -675 4G/5G polymorphism in the PAI-1 gene is not associated with insulin resistance, but with increased body adiposity, determined by increase in the waist-hip ratio, waist circumference, and subscapular skinfold thickness, in carriers of 4G/5G genotype. In contrast, some authors have reported a relationship between the 4G/4G genotype with insulin resistance and increased adipose tissue in white populations,14 where the 4G allele is considered the risk allele, since it was associated with high plasma levels of PAI-1 due to the lack of a binding site for a transcriptional repressor gene.11,18
It is well known that obese adults and children have higher plasma levels of PAI-1 than non-obese controls. Other studies have shown that high BMI, dyslipidemia, and insulin resistance are associated with high PAI-1 levels in obese adults.14,19–22 This is possibly because adipose tissue produces PAI-1, and increases circulating PAI-1 levels in both obese and insulin-resistant subjects. Furthermore, studies of PAI-1 knockout mice have shown an effect of PAI-1 on weight gain and increased adipose cellularity associated with high-fat die.23 Besides disruption of the PAI-1 gene reducing the adiposity of the obese ob/ob mice, this suggests that the PAI-1 gene can control fat mass. Although the mechanism of action is not yet known, it has been proposed that the proliferation of adipocytes may be related to the expression of genes, such as tumor necrosis factor alpha (TNF-α), transforming growth factor beta (TGF-β), leptin, and insulin.24
In the present study, the -675 4G/5G polymorphism in the PAI-1 gene was associated with increased body adiposity, and not with insulin resistance. This is probably because the -675 4G/5G polymorphism in the PAI-1 gene does not contribute directly to the development of insulin resistance in normal-weight and obese children; as the insulin resistance may be of multifactorial origin, in which environmental and genetic factors are involved. That could be influenced by other polymorphisms related to alterations in energetic metabolism, and to the development of obesity in children. Although the data suggest that the -675 4G/5G polymorphism in PAI-1 gene is linked to body adiposity, further studies are needed to clarify this role.
Even though in this study an association between genotype 4G/5G polymorphism in the PAI-1 gene and body adiposity was found, a limitation of the present study is that PAI-1 plasma levels were not measured; thus, the association of the genotypes with PAI-1 levels remains uncertain in this population. Future studies in Mexican children are necessary to determine this parameter.
In summary, the -675 4G/5G genotype of the PAI-1 gene was associated with measures of body adiposity but not with insulin resistance, which suggests that this genotype may confer susceptibility for obesity in Mexican children.
FundingThis research was supported by grants No. 106734 from the Fondo de Investigación Básica SEP-CONACYT 2008-01 and No. 147778 from the Fondo Mixto CONACYT-Gobierno del Estado de Guerrero 2010-01.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: De la Cruz-Mosso U, Muñoz-Valle JF, Salgado-Bernabé AB, Castro-Alarcón N, Salgado-Goytia L, Sánchez-Corona J, et al. Body adiposity but not insulin resistance is associated with -675 4G/5G polymorphism in the PAI-1 gene in a sample of Mexican children. J Pediatr (Rio J). 2013;89:492–8.