To estimate the prevalence and presentation of bladder, bowel, and combined bladder and bowel symptoms experienced by children with osteogenesis imperfecta and to describe the socio-demographic and clinical profile of these children.
MethodA descriptive study was conducted with a convenience sample of parent-child pairs of toilet-trained children aged from 3 to 18 years. Pairs were interviewed using three tools: (1) Socio-Demographic and Clinical Questionnaire; (2) Dysfunctional Voiding Scoring System; (3) Rome III Criteria along with the Bristol Stool Scale. Data were stratified by socio-demographic and clinical variables and analyzed using descriptive statistics.
ResultsThirty-one parent-child pairs participated in the study; 38.7% (n=12) children reported bowel symptoms, 19.4% (n=6) reported a combination of bladder issues (such as holding maneuvers and urgency) and bowel symptoms (such as hard or painful bowel movements and large diameter stools). There were no reports of isolated bladder issues. Among the child participants, 16 (51.7%) identified as female and 20 (64.5%) were 5–14 years old. The most prevalent type of osteogenesis imperfecta was type III (n=12; 38.7%) and eight (25.8%) children reported using a wheelchair.
ConclusionThis is the first study to examine the prevalence and presentation of bladder, bowel, and combined bladder and bowel symptoms in children with osteogenesis imperfecta, offering a preliminary socio-demographic and clinical profile of these children. This research is an important step toward effective screening, detection, and access to care and treatment, especially for clinicians working with this group of very fragile patients.
Estimar a prevalência e a apresentação de sintomas urinários, intestinais e urinários e intestinais combinados sofridos por crianças com osteogênese imperfeita e descrever o perfil sociodemográfico e clínico dessas crianças.
MétodoFoi realizado um estudo descritivo com uma amostra de conveniência de pares de pais-filhos de crianças treinadas para usar o banheiro com idades entre 3 e 18 anos. Os pares foram entrevistados utilizando três instrumentos: 1) o Questionário Sociodemográfico e Clínico; 2) o questionário Dysfunctional Voiding Scoring System; 3) os Critérios de Roma III juntamente com a Escala de Bristol para Consistência de Fezes. Os dados foram estratificados por variáveis sociodemográficas e clínicas e analisados utilizando estatísticas descritivas.
ResultadosParticiparam do estudo 31 pares de pais-filhos, 38,7% (n = 12) crianças relataram sintomas intestinais, 19,4% (n = 6) relataram uma combinação de problemas urinários (como segurar e urgência miccional) e sintomas intestinais (como fezes duras ou evacuações dolorosas e fezes de grande dimensão). Não houve relatos de problemas urinários isolados. Entre as crianças, 16 (51,7%) eram meninas e 20 (64,5%) tinham entre 5 e 14 anos. O tipo mais prevalente de osteogênese imperfeita foi o III (n = 12; 38,7%) e 8 (25,8%) crianças relataram usar cadeira de rodas.
ConclusãoEste é o primeiro estudo a examinar a prevalência e a apresentação de sintomas urinários, intestinais e urinários e intestinais combinados em crianças com osteogênese imperfeita e que mostra um perfil sociodemográfico e clínico preliminar dessas crianças. Nossa pesquisa é um passo importante com relação ao efetivo rastreamento, detecção e acesso ao cuidado e tratamento, principalmente para os profissionais de saúde que trabalham com esse grupo de pacientes tão frágeis.
Osteogenesis imperfecta (OI), also known as brittle bone disease, is a rare genetic condition (prevalence 1:10,000) characterized by bone fragility. There is no cure for OI; however, bisphosphonates are widely used to improve bone strength, decrease the occurrence of fractures, and improve quality of life.1
Children with OI may suffer from multiple fractures and limb deformities that may lead to impaired mobility,2,3 a risk factor for gastrointestinal problems such as constipation.4 This is a commonly reported symptom in children living with a physical disability.5 Moreover, the presence of constipation has a severe impact on urinary function due to the anatomical proximity of the bladder and intestine, which may develop into bladder and bowel dysfunction (BBD). In general, individuals with BBD do not recover proper bowel and bladder function unless the constipation is promptly and effectively managed.6
There appears to be a scarcity of evidence on the topic of elimination patterns in children with OI. In fact, the literature on elimination patterns in children with disabilities mostly concerns those with intellectual disabilities or neurological impairment.5 Specifically, children with OI may not only be at risk for the development of BBD due to their impaired mobility, but also at risk for bowel issues which are among the leading causes of mortality in OI patients.7 Given that, an investigation of the occurrence of bladder and bowel issues among children with OI offers a way forward. This study aimed to estimate the prevalence and presentation of bladder, bowel, and combined bladder and bowel symptoms in children with OI and to describe the socio-demographic and clinical profile of these children.
MethodsStudy design, setting, participants, and recruitmentFollowing institutional review board approval (#943.437, 02/03/2015), a descriptive study was conducted at a tertiary teaching hospital. This hospital is recognized nationally as a specialized center for OI treatment where children from the North, Northeast, and Central regions of Brazil are admitted to the inpatient unit. Bisphosphonate is administered during inpatient stay as an institutional standard care protocol. Potential study participants were enrolled and recruited over a four-month period (from February to May 2015), while they were receiving bisphosphonate treatment at the study site. Children were eligible for study if they were: (a) between the ages of 3 and 18 years, (b) diagnosed with any OI type, (c) toilet-trained, and (d) admitted to an inpatient unit at the study site during the data collection. Primary caregivers were eligible for study if: (a) their child met the inclusion criteria, (b) they were 18 years of age or older and (c) they were the primary child's caregiver during the time of toilet training. The children were excluded if they were not toilet trained and/or younger than 3 years. During their child's scheduled inpatient admission, all eligible primary caregivers were initially approached by a designated healthcare professional. When a primary caregiver expressed an interest, a member of the research team approached the primary caregivers to provide a study explanation, and if they agreed, informed consent was obtained, followed by assent from the child. Therefore, all participants approached were enrolled and recruited.
Data collection and instrumentsA single research assistant interviewed the parent-child pairs face-to-face, completing three instruments as data collection tools in a private room at the study site. It is worth noting that the research assistant adopted the following study guidelines to interview the pairs while filling out each of the forms. The data were primarily collected from a parental perspective and were only combined with the child's perspective for specific questions related to the child's toileting habits such as daily frequency of voiding, which the majority of parents were unaware of. The instruments consisted of the following:
Dysfunctional Voiding Scoring System (DVSS)The DVSS was used to identify abnormal voiding behaviors. The DVSS consists of ten items: Nine items for assessing bladder and bowel symptoms and one item assessing social and family-related issues.8 A DVSS score of six points for females and nine points for males suggests the child is suffering from abnormal voiding behaviors. The presence of abnormal voiding behaviors was confirmed when the participant reported experiencing a symptom “half of the time” or “almost every time” in a period of 30 days.
Rome III for the clinical diagnostic criteria of functional constipationThis instrument was used to capture a global picture of the child's bowel issues, associated with the Bristol Stool Chart. The presence of two or more positive answers out of six questions was indicative of functional constipation. The questions included: (1) two or fewer toilet defecations per week; (2) at least one episode of fecal incontinence per week; (3) history of retentive posturing or excessive volitional stool retention; (4) history of painful or hard bowel movements; (5) presence of a large fecal mass in the rectum; and (6) history of large diameter stools that may obstruct the toilet.9 The Bristol stool chart was used to facilitate participants’ understanding about stool consistency. This chart contains a drawing aid designed to classify feces into seven types: 1–2 (indicative of constipation), 3–4 (ideal stools), and 5–7 (indicative of diarrhea and urgency).10
Socio-demographic and clinical formBased on the instrument used in a study conducted by Martins et al.11 and adapted for the purpose of this study. This tool was also pilot-tested with three OI families for understandability. The form included questions pertaining to socio-demographics, family characteristics, and other clinically relevant information about the child's OI. Additional questions related to toilet training (TT) (not captured by the DVSS and Rome III) such as TT method (whether parent-based or child-based approach), duration, and difficulties encountered by the parents at the time of TT were also asked (Table 1).
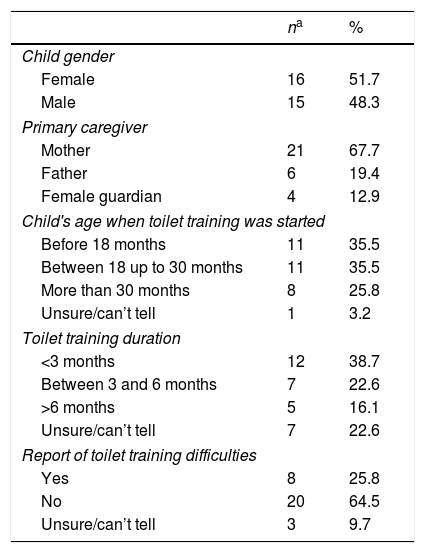
Descriptive characteristics of sample.
| na | % | |
|---|---|---|
| Child gender | ||
| Female | 16 | 51.7 |
| Male | 15 | 48.3 |
| Primary caregiver | ||
| Mother | 21 | 67.7 |
| Father | 6 | 19.4 |
| Female guardian | 4 | 12.9 |
| Child's age when toilet training was started | ||
| Before 18 months | 11 | 35.5 |
| Between 18 up to 30 months | 11 | 35.5 |
| More than 30 months | 8 | 25.8 |
| Unsure/can’t tell | 1 | 3.2 |
| Toilet training duration | ||
| <3 months | 12 | 38.7 |
| Between 3 and 6 months | 7 | 22.6 |
| >6 months | 5 | 16.1 |
| Unsure/can’t tell | 7 | 22.6 |
| Report of toilet training difficulties | ||
| Yes | 8 | 25.8 |
| No | 20 | 64.5 |
| Unsure/can’t tell | 3 | 9.7 |
OI children were diagnosed with BBD if they had at least two positive answers out of the six questions from the Rome III criteria, in addition to at least a total DVSS score of 6 points for females and 9 points for males, which represented the following urinary symptoms: storage symptoms (increased or decreased voiding frequency, incontinence, urgency, and nocturia), voiding symptoms (hesitancy, straining, weak stream, and intermittency) and/or other symptoms (holding maneuvers, incomplete emptying, post-micturition dribbling, and genital/lower urinary tract pain). It is worth noting that this sample was composed by children aged 4 years and above; therefore, these findings are aligned with the use of both validated tools: Rome III criteria, which sets the age 4, and the DVSS, which adopts the age of 3 years old or if the child has already been toilet trained as their diagnostic cut-off values.
Data analysisDescriptive data analyses were performed with SPSS (IBM SPSS Statistics for Windows, version 19.0, Armonk, NY, USA) using means, frequencies, and percentages to estimate the prevalence and presentation of bladder, bowel, and BBD symptoms in the sample, and also to describe participants’ characteristics such as socio-demographic (child gender, age, distance to hospital, yearly family income, birth order, number of children), clinical (OI type, fractures to interview date, ambulatory status, mobility assistive devices, and Rome III and DVSS scores) and TT-related variables (starting age, duration, presence of difficulties, method, characteristics of bladder and bowel control acquisition). These variables were chosen based on a literature review, as they are associated with BBD development during childhood.11–14
ResultsSample characteristicsAll potential participants eligible for study were enrolled and recruited to participate in this study. In total, 21 mothers, six fathers, and four female primary caregivers of 31 children (n=16, female), with a mean age of 11.2 years ranging from 5–14 years (Table 1). All of these primary caregivers were the primary caregivers during the time of toilet training. The children had OI type I (n=8), type III (n=12), type IV (n=5), or an unknown type (n=6).
TT always began within the child's first 18–30 months of life, and lasted up to six months for the majority of children (71%, n=22). Nearly all parents (97%, n=30) opted for the removal of the child's diapers during the day as their preferred TT method. Eight parents (25.8%) encountered challenges while training their children (three females with OI type III [n=2] and unknown [n=1] and five males with OI type IV [n=1], type I [n=1], type III [n=1], and unknown [n=2]). Challenges included: (a) delayed signs of readiness for nocturnal continence and independent toilet usage; (b) coping with multiple fractures and severe constipation; and (c) diaper usage during school time due to severe mobility impairment and poor school infrastructure (i.e., absence of accessible washrooms at school).
Prevalence of functional constipation (Rome III diagnostic criteria)More than half of the sample (n=16, 51%) reported a history of hard or painful evacuation. Eight children (26%) reported feces of large diameter that obstructed the toilet. Over a third of the children (n=12, 38%) reported at least two out of the six symptoms for functional constipation (Table 2). Bowel symptoms were more prevalent than lower urinary tract symptoms. This finding suggests that functional constipation was a common co-morbidity associated with OI.
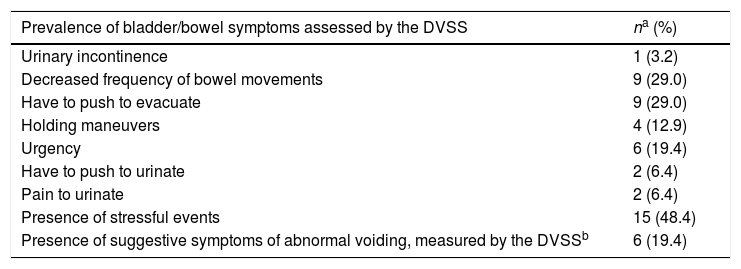
Prevalence of bladder, bowel, and combined bladder and bowel symptoms.
| Prevalence of bladder/bowel symptoms assessed by the DVSS | na (%) |
|---|---|
| Urinary incontinence | 1 (3.2) |
| Decreased frequency of bowel movements | 9 (29.0) |
| Have to push to evacuate | 9 (29.0) |
| Holding maneuvers | 4 (12.9) |
| Urgency | 6 (19.4) |
| Have to push to urinate | 2 (6.4) |
| Pain to urinate | 2 (6.4) |
| Presence of stressful events | 15 (48.4) |
| Presence of suggestive symptoms of abnormal voiding, measured by the DVSSb | 6 (19.4) |
| Prevalence of bowel symptoms assessed by the Rome IIIc | n (%) |
|---|---|
| Two or fewer evacuations per week | 3 (9.7) |
| At least one episode of fecal incontinence per week | 3 (9.7) |
| History of retentive posture or voluntary retention | 4 (12.9) |
| History of hard or painful evacuation | 16 (51.6) |
| Presence of large fecal mass in rectum | 5 (16.1) |
| Feces of large diameter that obstruct the toilet | 8 (25.8) |
| Presence of suggestive symptoms of functional constipation, measured by the Rome III criteriac | 12 (38.7) |
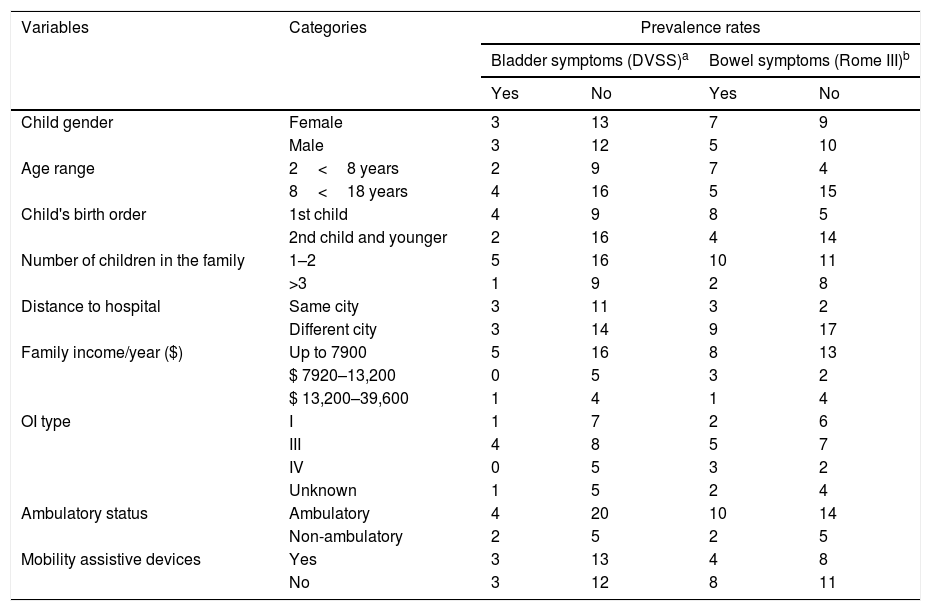
The majority of the sample did not present with reports suggestive of lower urinary tract symptoms. In this sample, the mean DVSS score for female children (n=13, 81%) was less than 6 points and for male children (n=12, 80%) was less than 9 points. The highest scoring report captured by the DVSS for the entire sample was associated with the question “Have to push to evacuate” in both males (n=5, 53%) and females (n=4, 37%), and this situation occurred more than half of the time or all of the time in the last 30 days (Table 2). The mean DVSS score for all six symptomatic children was 6 points (SD: 1.5), which was considered to be mild, since it was very close to the diagnostic cut-off value8 for female children. These children reported urinary issues such as holding maneuvers and urgency, along with bowel symptoms suggestive of functional constipation. The prevalence of urinary (3.2%) and fecal incontinence (9.7%) was very low in this sample (Table 2). The three symptomatic female children ranged from five to 14 years and had OI type III (n=2) or unknown (n=1) and presented with DVSS scores ranging from 7 to 10. The three symptomatic male children ranged from six to 14 years and had OI types I (n=1) or III (n=2), and presented with DVSS scores ranging from 10 to 11 points. Bowel and bladder issues were more common in patients with OI type 3, although no statistically significant correlation was found with any specific OI type (Table 3).
Demographic and clinical characteristics across prevalent bladder and bowel symptoms.
| Variables | Categories | Prevalence rates | |||
|---|---|---|---|---|---|
| Bladder symptoms (DVSS)a | Bowel symptoms (Rome III)b | ||||
| Yes | No | Yes | No | ||
| Child gender | Female | 3 | 13 | 7 | 9 |
| Male | 3 | 12 | 5 | 10 | |
| Age range | 2<8 years | 2 | 9 | 7 | 4 |
| 8<18 years | 4 | 16 | 5 | 15 | |
| Child's birth order | 1st child | 4 | 9 | 8 | 5 |
| 2nd child and younger | 2 | 16 | 4 | 14 | |
| Number of children in the family | 1–2 | 5 | 16 | 10 | 11 |
| >3 | 1 | 9 | 2 | 8 | |
| Distance to hospital | Same city | 3 | 11 | 3 | 2 |
| Different city | 3 | 14 | 9 | 17 | |
| Family income/year ($) | Up to 7900 | 5 | 16 | 8 | 13 |
| $ 7920–13,200 | 0 | 5 | 3 | 2 | |
| $ 13,200–39,600 | 1 | 4 | 1 | 4 | |
| OI type | I | 1 | 7 | 2 | 6 |
| III | 4 | 8 | 5 | 7 | |
| IV | 0 | 5 | 3 | 2 | |
| Unknown | 1 | 5 | 2 | 4 | |
| Ambulatory status | Ambulatory | 4 | 20 | 10 | 14 |
| Non-ambulatory | 2 | 5 | 2 | 5 | |
| Mobility assistive devices | Yes | 3 | 13 | 4 | 8 |
| No | 3 | 12 | 8 | 11 | |
These findings show the prevalence of functional constipation (38.7%) was higher as compared to the general pediatric population, given that the global prevalence of pediatric functional constipation has been estimated between 0.3% and 29.6%.15 With regard to the prevalence rates, the prevalence of bladder symptoms in children with functional constipation varies from 37% to 64%16 and the present study showed that children with OI have a lower prevalence of 19.4% for the co-occurrence of bladder and bowel symptoms. Additionally, this study showed that children with OI did not report isolated urinary symptoms. The reported prevalence of urinary (3.2%) and fecal (9.7%) incontinence was lower in children with OI compared to children with neurological impairments (38% with urinary and 19% with fecal incontinence)17 as well as with intellectual disabilities (39.2% with urinary and fecal incontinence during the day).18 Although this study showed lower prevalence's rates of urinary and fecal incontinence in children with OI, the prevalence of functional constipation was higher as compared to healthy children. For example, an Australian population-based study conducted with school-aged children found the following overall prevalence's rates: 16.9% for daytime urinary incontinence, 9.4% for constipation, and 21% for fecal incontinence/encopresis.19
In chronically ill children, any mobility impairment is considered a potential risk factor for unsuccessful TT and subsequent incontinence.20,21 In the present study, 35% (n=11) families who started TT after 18 months reported the presence of TT difficulties. This finding highlights the fact that some children may suffer from dysfunctional voiding had symptoms such as infrequent voiding, constipation, and urinary tract infections before starting TT.22 According to Hodges et al.,22 abnormal elimination behaviors may be associated with the starting age of TT and the presence of constipation. Since constipation also happens to lead to lengthy and difficult TT, Hodges et al.22 proposed it was this combination of factors that lead to BBD. In the present study, 26% (n=8) of the parents who reported facing difficulties with their child's TT reported the presence of severe constipation and multiple fractures as the principal reasons for unsuccessful TT. These findings align with Hodges et al.’s work and show that constipation may contribute to BBD development later on in life, and not only at the child's age when TT is initiated.
Moreover, constipation is generally associated with neurological impairments and confounding factors such as immobility as well as pain in children living with a physical disability.23 In the case of children with OI, the underlying reasons for constipation are related to mobility impairments, bone deformities, excessive perspiration, and side effects associated with bisphosphonate treatment.24 In addition, fractures may lead to bone remodeling and pelvic narrowing, the latter being associated with acetabular protrusion in up to two-thirds of adults with OI type III.25 These OI adults are known to experience chronic constipation and abdominal pain.25 Recently, the OI Foundation has identified underemphasized health conditions in adults with OI, and 65–97% (n=623–930 of 959) of the respondents reported “urinary tract” or “gastrointestinal system” as the organ systems that had the most significant negative impact on their quality of life.26 Furthermore, gastrointestinal problems are cited among the leading causes of mortality in OI individuals.7 Thus, early detection of bowel issues and a timely referral to a gastrointestinal specialist is crucial to avoid these symptoms later on.27
Although the present study is the first to estimate the prevalence and presentation of bladder, bowel, and BBD symptoms in a sample of OI children, this research has some limitations. Firstly, these findings did not explore the role of psychosocial factors such as stressful life events, which have been shown to contribute to abnormal elimination disorders in children.28 Possibly, the presence of BBD symptoms may carry a greater impact on children's lives than their bone fragility and the mobility impairments per se, more precisely due to the associated stigma.29,30 Additionally, due to its small sample size and its descriptive design, these findings should be interpreted with caution. There is a risk of selection bias as this study was carried out at only one tertiary institution. There is also a risk of recall bias because the data collected were dependent on the accuracy of participants’ memory regarding past events; this could be considerable for parents of older children.
To the authors’ knowledge this is the first study to investigate the prevalence of BBD in children with OI. In this sample, there were no reports of isolated bladder issues; however, 38.7% (n=12) reported bowel symptoms and 19% (n=6) reported a combination of bladder issues (such as holding maneuvers and urgency) and bowel symptoms (such as hard or painful bowel movements and large diameter stools).
Given the variability in needs and outcomes related to bladder and bowel control of children with OI, clinicians should monitor continence and abnormal elimination behaviors in these children even before or as soon as TT begins. It is worth noting that the main reasons for unsuccessful TT in children with OI, reported by the caregivers in this study, were severe constipation and mobility impairments associated with multiple fractures.
Therefore, increased awareness about the occurrence of BBD symptoms, especially constipation, in children with OI is warranted. Given the growing body of knowledge on the complications of unmanaged BBD symptoms in individuals with OI, conservative interventions, including lifestyle changes and behavioral therapies, have not been examined specifically in children with OI experiencing BBD and represent an optimal opportunity to provide relief and improve quality of life. Further research should also explore the perspectives of children with OI and their caregivers to gain an understanding of common beliefs and behaviors associated with bladder and bowel management as key drivers of seeking treatment, as well as the impact of such symptoms on their quality of life.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Martins G, Siedlikowski M, Coelho AK, Rauch F, Tsimicalis A. Bladder and bowel symptoms experienced by children with osteogenesis imperfecta. J Pediatr (Rio J). 2020;96:472–8.