To describe the dietary patterns and occurrence of metabolic disorders in children and adolescents with urolithiasis treatment at a referral hospital in southern Brazil in order to learn the features of urolithiasis in this population to better develop preventive actions.
MethodsDescriptive study conducted between 2016 and 2017 in a tertiary care referral hospital. Fourty patients aged 2–19 years old with urolithiasis proven by imaging were included. Clinical and dietary data were obtained through interviews and medical records. For statistical analyses, the chi-squared test was performed.
Results40 individuals were analyzed. Mean age at diagnosis was 7.2±4 years. 25% were overweight or obese. 95% had metabolic disorders, hypocitraturia being the predominant type. Protein intake was adequate in all participants and carbohydrate intake, in 70% of them; 37.5% had lipid intake above recommended and 65% had low fiber intake. The mean daily sodium intake was 2.64g (±1.74), with 55% of participants ingesting more than the recommended amount. A total of 52.5% had low potassium intake, with a mean of 4.79g/day (±2.49). Calcium intake was adequate in 27.5%. No significant differences were identified in relation to mean daily consumption among participants with or without the various metabolic disorders.
ConclusionPediatric urolithiasis is often accompanied by metabolic disorders; therefore, metabolic evaluation should be part of the diagnostic process and subsequent analysis of these patients’ dietary patterns, helping to optimize treatment and prevent recurrences and complications.
Descrever o padrão alimentar e ocorrência de distúrbios metabólicos em crianças e adolescentes portadoras de urolitíase acompanhadas em hospital de referências no sul do Brasil a fim de conhecer as particularidades da urolitíase nessa população para melhor desenvolver ações de prevenção.
MétodosEstudo observacional descritivo realizado entre 2016 e 2017 em centro de referência em atenção terciária. Foram selecionados 40 pacientes de 2 a 19 anos com urolitíase comprovada por exame de imagem. Dados clínicos e alimentares foram obtidos através de prontuário e entrevista. Para análise estatística, utilizou-se o teste qui-quadrado.
ResultadosForam analisados 40 indivíduos, 55% masculinos. Idade média ao diagnóstico 7,2±4 anos. 25% tinham sobrepeso ou obesidade. 95% tinham distúrbios metabólicos, predominando a hipocitratúria. O consumo proteico e de carboidratos foi adequado em 100% e 70% dos participantes, respectivamente. 37,5% apresentaram ingestão de lipídeos acima do recomendado e 65% apresentaram ingestão de fibras alimentares abaixo do recomendado. O consumo diário médio de sódio foi de 2,64g (±1,74), com 55% acima do recomendado. 52,5% apresentaram baixa ingestão de potássio com média de 4,79g/dia (±2,49). O consumo de cálcio foi adequado em 27,5%. Não foram identificadas diferenças significativas em relação ao consumo médio diário dos nutrientes entre os participantes com ou sem os diversos distúrbios metabólicos.
ConclusõesA urolitíase pediátrica é frequentemente acompanhada de distúrbios metabólicos, o que confirma a necessidade de avaliação metabólica adequada ao diagnóstico e análise do padrão alimentar a fim de identificar erros alimentares e otimizar o tratamento destes distúrbios, prevenindo recorrências e complicações.
Albeit less frequent in children, cases of urolithiasis (UL) have significantly increased in this population in the last decades.1 A recent study showed that the risk of UL doubled in the pediatric range between 1997 and 2012.2 The annual increment is currently between 5 and 10% among the child population.3
Several studies have tried to identify the causes of this new epidemiologic scenario. In addition to the normal infectious factors and anatomic changes of the urinary tract, studies have demonstrated the crucial role of metabolic disorders (MD) in the genesis of approximately 90% of the pediatric UL.1,3 Hypercalciuria, hypocitraturia, and hyperuricosuria are among the most studied MD.3
The literature presents evidence of the relationship between development of such disorders and food, particularly regarding low water, potassium, and calcium intake, as well as diets rich in animal protein and sodium.1,4 Recent studies suggest an association between UL and obesity in adult patients4; however, in the child population, there is no evidence of this relationship yet.4,5
Given the current overview of pediatric UL, this study aimed to describe the dietary pattern in children with UL monitored in a referral hospital in southern Brazil to identify potential nutritional alterations related to development of the associated MD in order to know the particularities of UL in this population to develop better preventive actions.
MethodsPatients monitored in the outpatient clinic of Pediatric Nephrology in the tertiary care referral center participated in a descriptive observational study carried out in the State of Santa Catarina, Brazil, between September 2016 and October 2017.
This study was approved by the Research Ethics Committee of the hospital (Protocol 029/2016) and did not receive any financial support.
Participants were selected through non-probabilistic sampling. The age of the included patients ranged from 2 to 19 years (age group defined due to the food frequency questionnaires [FFQ])6–8 and were diagnosed with UL, which was confirmed through imaging tests (ultrasonography or computed tomography).
The patients, accompanied by their legal guardians, were invited to participate in this study after a routine appointment; an informed consent form was signed. Demographics, clinic data, and anthropometric measurements (weight and height) were collected and a quantitative FFQ, validated for the Brazilian population, was administered according to the participant's age.6–8 The MD diagnosis and the urine and serum laboratory tests performed up to 6 months before the medical appointment were collected from the electronic medical records.
The nutritional status was evaluated through the body mass index (BMI); the Child Growth Pattern charts of the World Health Organization were used for the relevant classification of the participant.
The food analysis calculated the amount of proteins, lipids, carbohydrates, dietary fiber, calcium, magnesium, iron, sodium, and potassium in each food ingested by the patient. In order to do so, the amount in household measure and the reported consumption frequency were used; the Brazilian Food Composition Database ( TACO) was used as reference.9 The entire contents of each nutrient were added, and the total usual nutrient intake was obtained. In order to calculate the mean daily intake, the total was then divided by 30 days. This amount was classified according to the recommendations regarding age and sex of the Dietary Reference Intakes (DRI).10 The estimated average requirement and the tolerable upper intake level were established as the lower and upper limits, respectively, for the intake of each nutrient. Macronutrients were expressed as percentages of the total energy value (TEV) per day and other nutrients were expressed in grams or milligrams per day.10
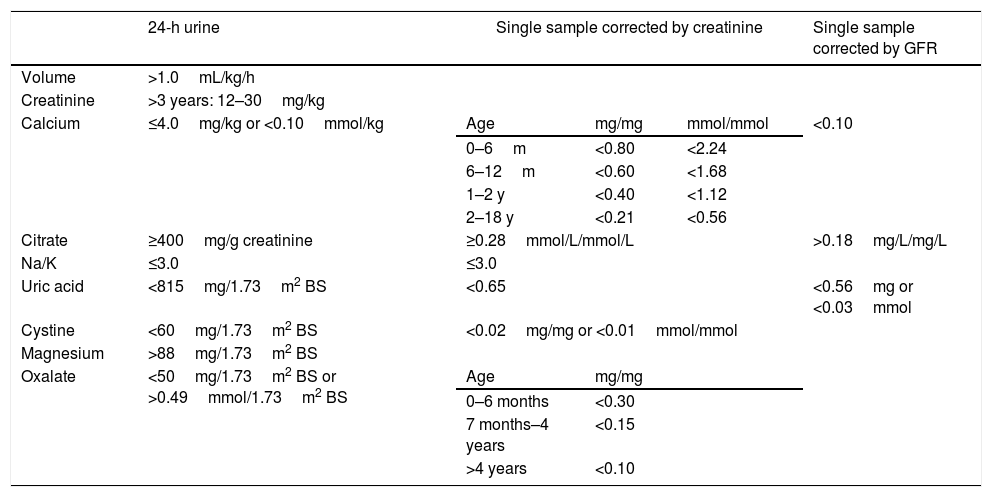
The diagnoses of MD were supported by abnormal 24-h urine test results and/or single urine sample, according to Table 1.11–14 The metabolic study was carried out at least one month after the diagnosis of UL, during asymptomatic periods and usual dietary and physical activity patterns. The standard biochemical tests adopted in the laboratory of the hospital studied were used to analyze the urine samples.
Reference values regarding volume and solute excretion in a 24-h urine and urine sample in children and adolescents.11–14
| 24-h urine | Single sample corrected by creatinine | Single sample corrected by GFR | |||
|---|---|---|---|---|---|
| Volume | >1.0mL/kg/h | ||||
| Creatinine | >3 years: 12–30mg/kg | ||||
| Calcium | ≤4.0mg/kg or <0.10mmol/kg | Age | mg/mg | mmol/mmol | <0.10 |
| 0–6m | <0.80 | <2.24 | |||
| 6–12m | <0.60 | <1.68 | |||
| 1–2 y | <0.40 | <1.12 | |||
| 2–18 y | <0.21 | <0.56 | |||
| Citrate | ≥400mg/g creatinine | ≥0.28mmol/L/mmol/L | >0.18mg/L/mg/L | ||
| Na/K | ≤3.0 | ≤3.0 | |||
| Uric acid | <815mg/1.73m2 BS | <0.65 | <0.56mg or <0.03mmol | ||
| Cystine | <60mg/1.73m2 BS | <0.02mg/mg or <0.01mmol/mmol | |||
| Magnesium | >88mg/1.73m2 BS | ||||
| Oxalate | <50mg/1.73m2 BS or >0.49mmol/1.73m2 BS | Age | mg/mg | ||
| 0–6 months | <0.30 | ||||
| 7 months–4 years | <0.15 | ||||
| >4 years | <0.10 | ||||
GFR, glomerular filtration rate; BS, body surface.
Data collected were registered in a database using Excel® 2007 (Excel®, Microsoft, WA, USA). Statistical analysis was performed through Epi Info version 7.2 (Centers for Disease Control and Prevention, GA, USA). Nominal variables were described as absolute value and proportions, and categorical variables were described as measures of central tendency and dispersion. The chi-squared test was used to compare the qualitative variables, and a significance level of 95% (<0.05) was adopted.
ResultsOf the 41 patients selected, one did not agree to participate, and the other 40 patients remained.
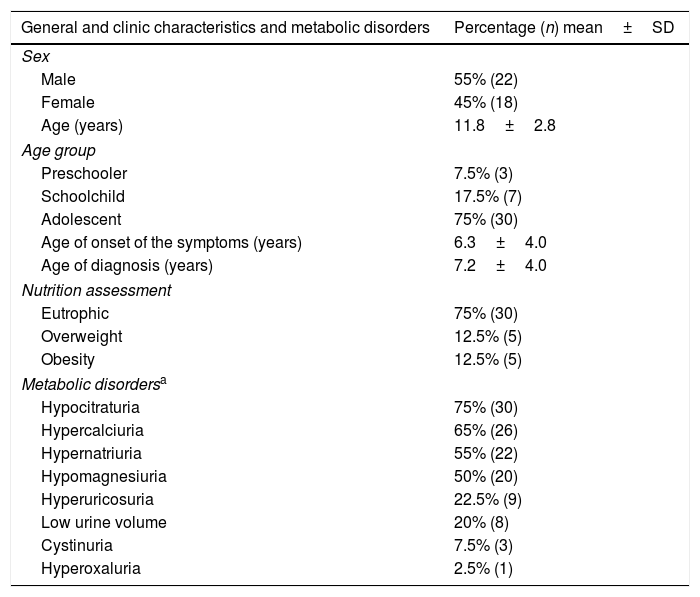
Table 2 shows the general characteristics, demographics, and clinical data of the patients.
General and clinic characteristics and metabolic disorders of children and adolescents with diagnosis of urolithiasis.
| General and clinic characteristics and metabolic disorders | Percentage (n) mean±SD |
|---|---|
| Sex | |
| Male | 55% (22) |
| Female | 45% (18) |
| Age (years) | 11.8±2.8 |
| Age group | |
| Preschooler | 7.5% (3) |
| Schoolchild | 17.5% (7) |
| Adolescent | 75% (30) |
| Age of onset of the symptoms (years) | 6.3±4.0 |
| Age of diagnosis (years) | 7.2±4.0 |
| Nutrition assessment | |
| Eutrophic | 75% (30) |
| Overweight | 12.5% (5) |
| Obesity | 12.5% (5) |
| Metabolic disordersa | |
| Hypocitraturia | 75% (30) |
| Hypercalciuria | 65% (26) |
| Hypernatriuria | 55% (22) |
| Hypomagnesiuria | 50% (20) |
| Hyperuricosuria | 22.5% (9) |
| Low urine volume | 20% (8) |
| Cystinuria | 7.5% (3) |
| Hyperoxaluria | 2.5% (1) |
SD, standard deviation; n, number of participants.
All of the 38 patients (95%) submitted to laboratory tests to evaluate the metabolic change presented at least one MD, and the metabolic serum tests were within the normal values of the local laboratory. The frequency of MD can be found in Table 2.
The nutritional assessment showed that all preschoolers, six schoolchildren (85.7%), and 21 adolescents (70%) were eutrophic. Among the overweight patients, four were adolescents (80%). All obese patients were adolescents.
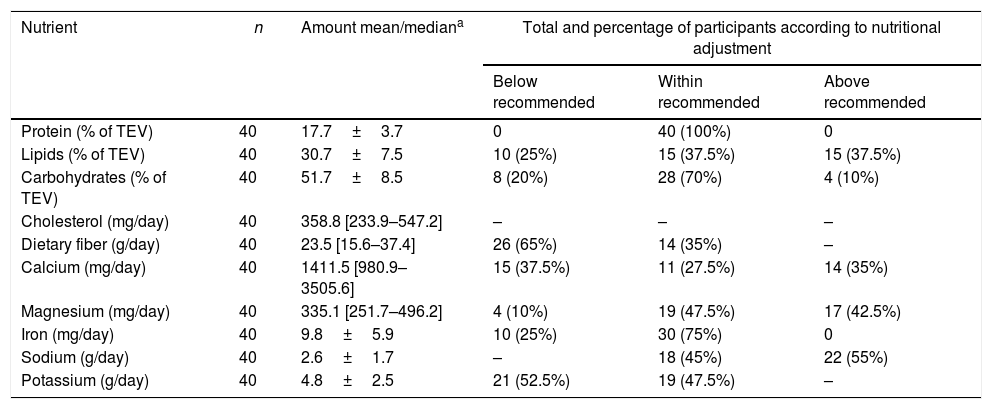
The mean intake of each nutrient assessed based on FFQ, as well as the percentage of patients with average intake within, above, or below the recommendations regarding age and sex according to DRI, are presented in Table 3.
Intake and classification of nutrient intake according to DRI's recommendations for sex and age, based on FFQ assessment for age group.
| Nutrient | n | Amount mean/mediana | Total and percentage of participants according to nutritional adjustment | ||
|---|---|---|---|---|---|
| Below recommended | Within recommended | Above recommended | |||
| Protein (% of TEV) | 40 | 17.7±3.7 | 0 | 40 (100%) | 0 |
| Lipids (% of TEV) | 40 | 30.7±7.5 | 10 (25%) | 15 (37.5%) | 15 (37.5%) |
| Carbohydrates (% of TEV) | 40 | 51.7±8.5 | 8 (20%) | 28 (70%) | 4 (10%) |
| Cholesterol (mg/day) | 40 | 358.8 [233.9–547.2] | – | – | – |
| Dietary fiber (g/day) | 40 | 23.5 [15.6–37.4] | 26 (65%) | 14 (35%) | – |
| Calcium (mg/day) | 40 | 1411.5 [980.9–3505.6] | 15 (37.5%) | 11 (27.5%) | 14 (35%) |
| Magnesium (mg/day) | 40 | 335.1 [251.7–496.2] | 4 (10%) | 19 (47.5%) | 17 (42.5%) |
| Iron (mg/day) | 40 | 9.8±5.9 | 10 (25%) | 30 (75%) | 0 |
| Sodium (g/day) | 40 | 2.6±1.7 | – | 18 (45%) | 22 (55%) |
| Potassium (g/day) | 40 | 4.8±2.5 | 21 (52.5%) | 19 (47.5%) | – |
DRI, dietary reference intakes; FFQ, food frequency questionnaire; TEV, total energy value; amounts expressed as mean±standard deviation or median [interquartile range].
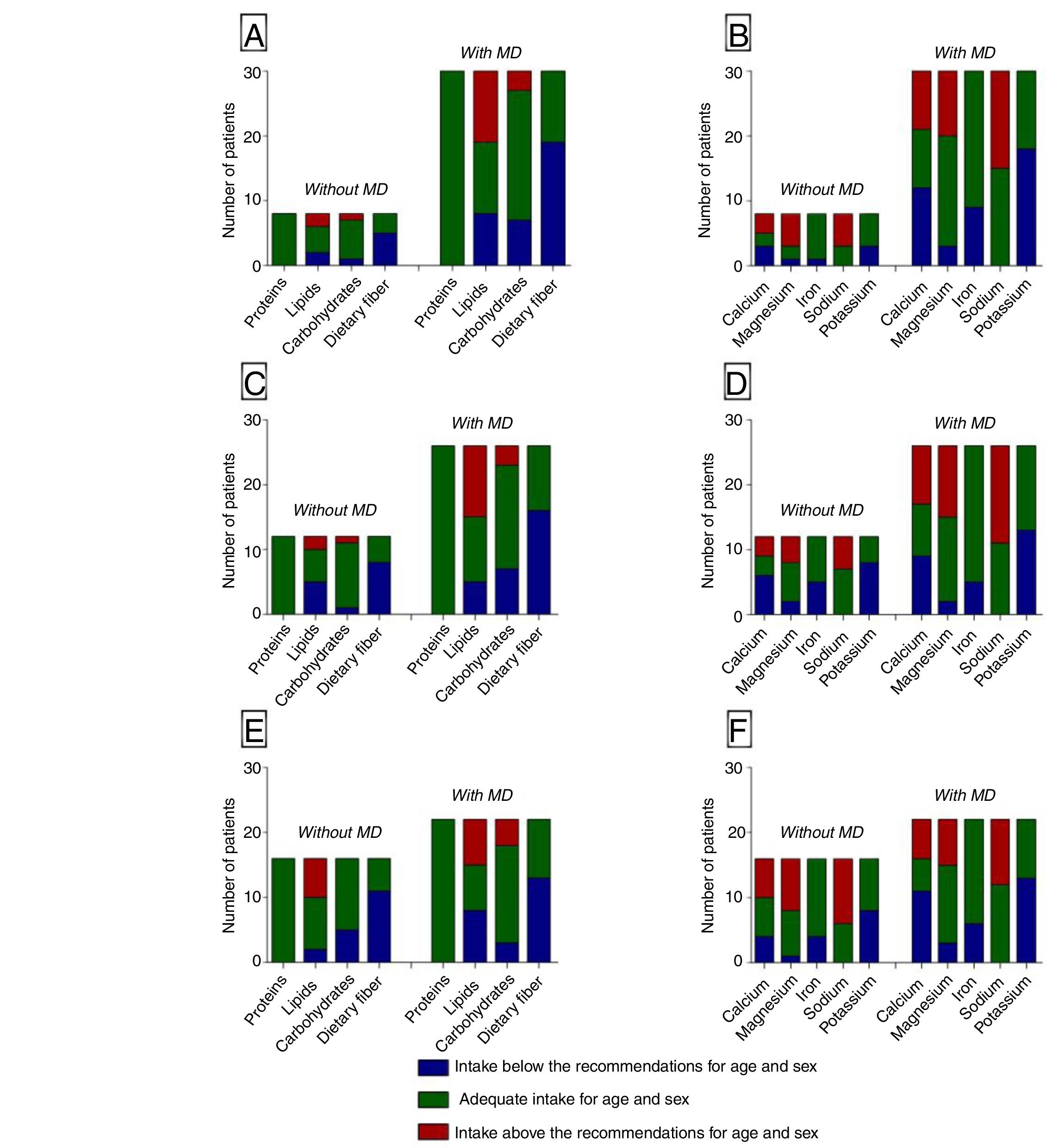
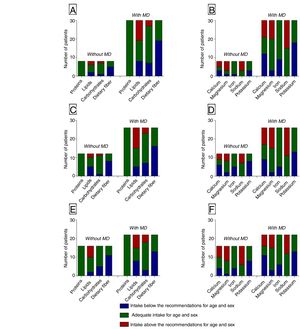
The correlation between the dietary pattern and the most frequently observed MDs is shown in Fig. 1. No significant differences were identified regarding the mean daily intake of each nutrient in the presence or absence of MD, in accordance with the previously observed patterns.
DiscussionThe current evidence shows an increased incidence of UL in the pediatric population.3 The association between UL and MD is frequent in this population, and the role of diet in the formation of kidney stones is suggested.1,3 While it is well-known in adults,15 the association between obesity and UL is still questioned among children and adolescents16 and demands a more detailed investigation on the dietary pattern of this population. Within this scope, this study quantitatively and qualitatively described the dietary pattern of patients monitored in a state referral center for pediatric nephrology.
General characteristics, demographics, clinical, and image dataMost patients were male, in agreement with the findings of other investigations17 (Table 2). While it is historically more frequent in this sex,1 the incidence of UL among the female population is already higher in some studies.1,2,18,19 Nonetheless, American data showed a uniform distribution between the genders,17,18,20 suggesting there is no causal relationship between them and the occurrence of UL.
The mean age at the diagnosis was 7.2 years old, confirming the findings by Akin et al.,21 who demonstrated that this age usually varies between 4.2 and 9.4 years old. Conversely, other studies showed a variation of 11.3–11.8 years old, a higher average than that observed in the present study.18,20 This lower age at the diagnosis of the Brazilian patients was already verified by Penido et al.17 in a cohort study with American and Brazilian children and adolescents with UL. The authors showed that the Brazilian patients are usually younger at the diagnosis.
Metabolic disordersMD are usually associated with UL, especially in the pediatric population, and may result in recurring episodes of calculi formation.1,21 Studies show high rates of MD in this population, ranging from 76% to 91%,18,22 which is agreement with the present study, where 95% of the patients showed at least one MD. The high rates of hypocitraturia and hypercalciuria were expected, given that it was also the finding of several authors.17,18,23,24 Penido et al.17 hypothesized that, although hypercalciuria is very relevant in UL and is frequently the most prevalent MD, it cannot always individually justify the calculi formation, unless associated with a low level of citrate.25
Recent studies have evidenced increased urine excretion of sodium as a risk factor for UL.1,24 The findings of the present study are in agreement with the literature, and 55% of the patients showed hypernatriuria in the urine analysis. In turn, hypomagnesiuria, whose prevalence is usually low,23 was observed in 50% of the cases in this study, despite the fact that 57.9% of the children with this MD showed adequate magnesium intake.
It is known that proper water intake prevents supersaturation of substances in the urine and calculi formation.1,21 Penido et al.18 showed high prevalence of low urine volume and, in 24% of the children and adolescents evaluated, this was the only metabolic change. Another study between American and Brazilian children showed oliguria in 63% and 49% of the patients, respectively.17 In the present study, oliguria was observed in 20% of the cases. This lower percentage may be justified by the adherence of the patients to the insistent recommendation regarding water intake in the outpatient clinic where the study was carried out.
Biochemical urine profiles vary around the world and depend on genetic, and environmental factors, diet, and urinary tract infections.1,17 Nonetheless, it was observed that most children with UL has some type of MD.1,18,21,24
Nutritional assessmentOne-fourth of the patients in this study were considered to be overweight or obese, and 80% of patients with overweight and 100% of the obese were adolescents. Despite the lack of evidence in the literature about the association between BMI and age group in the pediatric population with UL, data from this study suggest that the older the population, there will be more overweighed or obese persons.
Food consumption assessmentThe association between food and MD causing UL has been assessed for a long time, and some associations have been established. Among the macronutrients associated with lithogenesis, proteins are noteworthy.1 In this study, all patients showed mean protein intake within the amounts recommended by the literature. Studies indicate that diets rich in protein, especially those of animal origin, lead to increase in the kidney excretion of calcium, hyperuricosuria, and decrease in citraturia,1 as protein metabolism generates elevated acid loads in the blood, resulting in increased bone remodeling and hypercalciuria.21 Among the acid loads generated by protein metabolism, the most important is uric acid, which contributes to hyperuricosuria.19 Based on this fact, and considering the proteins required to proper development, current recommendations aim at diets with normal protein levels, without a restrictive component for children with UL.1,21
The other two macronutrients, carbohydrates and lipids, while not directly related to lithogenesis, directly contribute to maintainance of adequate weight, since they are the main energetic sources of the usual diet. It is known that overweight/obesity, as well as one of their main consequences, metabolic syndrome, are connected to UL formation, a fact that supports the current recommendations for a balanced diet for these patients.24 In the present study, most patients (70%) showed a mean carbohydrate intake within the recommended rates for age and sex; however, more than one-third of them presented a lipid intake above the recommended rate. Considering that the caloric load of lipids is more than double that of carbohydrates, hyperlipid diets significantly contribute with increased overweight/obesity rates in childhood.
Assessment of lipids, regardless of the corresponding percentage of each subtype of fat molecule (saturated or unsaturated fatty acids, for instance), is restricted, since each perform a different role in the metabolism and, thus, may result in beneficial or harmful effects to the human body. In this study, the lipid subtypes that were part of the patient's food were not assessed; however, the mean cholesterol intake, an important class of lipid, was quantified. The current recommendations of The National Academies of Sciences Engineering Medicine, which elaborates the DRI, indicate no minimum reference amount for cholesterol in the daily intake.10 However, the lowest possible intake is recommended, considering the important role of dyslipidemia as a cardiovascular risk factor.26 The patients of this study showed a median cholesterol intake of 358.8mg/day, and the range among the patients was quite heterogeneous.
In the fight against dyslipidemia, one of the major agents is dietary fibers, an important element in a healthy diet. Studies with adults showed that diets rich in fibers are related to a smaller incidence of UL.27 However, the Brazilian population's diet is low in fibers, which is reflected in the children's food habit. In the present study, 65% of the patients had a diet low in fibers, with a median of 23.5g/day. Considering the patient's nutritional status, it is possible to identify a trend for higher fiber intake among eutrophic individuals when compared with their overweight and obese peers, with a median of 27.9g/day (16.3–38.5), 16.7g/day (15.5–21.8), and 14.5g/day (13.6–15.9), respectively. Regarding inadequate diets, all overweight individuals and 80% of the obese showed a daily intake below the recommended for their age and sex; among the eutrophic, such percentage was of 56.6%.
Sodium is one the micronutrients with proven relationship with UL; excess consumption leads to increased calciuria level and favors calcium stone formation.1,21 The sodium intake among children and adults is high, especially due to the large amount of processed food included in contemporary diet. According to information provided by the Household Budget Survey 2008–2009, the sodium intake of 70% of the adolescents was above the tolerable amounts (tolerable upper intake level, according to DRI).28 The findings of the present study are not different; 55% of the patients presented a higher sodium intake than the recommended for age and sex. The mean daily intake found was of 2.6g/day, a rate higher than the maximum level recommended for the adult population (2.3g/day).10 Among the patients with hypercalciuria, 57.7% had a daily mean sodium intake higher than the recommended rate. In turn, in the group without this type of MD, few patients (41.7%) consumed sodium in an amount higher than the recommended. This finding shows a relation between excessive sodium intake and hypercalciuria, despite the lack of statistical significance, which can probably be explained by the sample. Therefore, limiting this mineral intake is an important measure to reduce calciuria in the clinical practice.1
Sodium and potassium are known for having inverse effects in the systemic blood pressure. This trend is also verified regarding calciuria, as the potassium salts reduce calcium urine excretion.21 In the present study, the potassium intake was inversely related to the sodium intake, with 52.5% of the patients with an intake below the recommended and a mean intake of 4.8g/day.
Dietary calcium is related to UL, but its action is not related to hypercalciuria, but to hyperoxaluria. Diets low in calcium may increase the intestinal absorption of oxalate and raise risk of UL, since such mineral is frequent in the calculi formation. A classic study carried out by Curhan et al.29 assessed the diet of more than 45,000 adults for four years and found a significantly lower risk of kidney calculi formation in individuals with a diet rich in calcium when compared to those with a diet low in this mineral. Within this scope, the current recommendations highlight the need for diets with normal amounts of calcium.1,21 Only 27.5% of the patients had a proper diet, the others were similarly below and above the normal rate. Hyperoxaluria is not one of the most common MD,18 as noted in this study, where only one patient had such disorder and showed a calcium intake below the amount indicated for age and sex.
Fruit and vegetables consumption, which are important sources of magnesium, citrate, and potassium, must always be encouraged in the overall population, especially in those with UL. Studies have shown that, in individuals with hypocitraturia, a diet rich in these components increases kidney excretion of magnesium and citrate, increasing the urinary anti-lithogenic factors.30 The present study found that only 10% of the patients had a magnesium intake below the recommended rate, and most of them were overweight/obese.
Most individuals (75%) also showed a proper daily iron intake, averaging 9.8mg/day. It is noteworthy that the mean iron intake decreased as BMI increased, with 10.4mg/day (±6.4) among the eutrophic, 8.8mg/day (±2.6) among those overweight, and 7.2mg/day (±4.4) among the obese. However, no data about such association was retrieved, emphasizing the need for additional studies.
Assessing the food intake is a hard task, considering the several nuances presented by the current assessment methods. Among the most used methods, there are the three-day food diary, the 24-h dietary recall, and the FFQ. In this study, the FFQ was used because, although it is not the best method available, it is among the most accepted alternatives to assess the dietary pattern of a population, being able to quantify and assess the intakes for longer periods.
The limitations of the study are mainly its sample size and the lack of a control group, which makes it impossible to analyze whether the dietary pattern and the MD are typical of UL in the southern of Brazil and whether the composition of the diet reflects the dietary pattern of children and adolescents in this region. Regarding eating habits, lipid subtypes and daily water intake, the latter being part of the initial approach in the treatment of UL, were not assessed. Another potential restriction was that, during the interview, patients had already received general food instructions in a previous medical appointment with the pediatric nephrology team.
Pediatric UL is frequently followed by MD. This fact confirms the need for a metabolic assessment suitable for the diagnosis, as well as an analysis of the dietary pattern. A low intake of dietary fiber and potassium and a high intake of sodium were observed in this sample. However, they were not significantly associated with MD. The lack of data in the literature about the eating habits in children and adolescents with UL, as well as the pending association between pediatric UL and obesity, should stimulate further research on the topic, aiming to improve the understanding and the therapeutic approach to this condition, whose prevalence is increasing in this population.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Vieira MS, Francisco PC, Hallal AL, Penido MG, Bresolin NL. Association between dietary pattern and metabolic disorders in children and adolescents with urolithiasis. J Pediatr (Rio J). 2019. https://doi.org/10.1016/j.jped.2018.11.008
Study conducted at Hospital Infantil Joana de Gusmão, Serviço de Nefrologia, Florianópolis, SC, Brazil.