To present the currently available evidence on transmission, clinical, diagnostic methods, treatment, and prevention methods of major arboviruses that occur in childhood.
Source of dataNon-systematic review carried out in MEDLINE (PubMed), LILACS (VHL), Scopus, Web of Science, Cochrane, CAPES Portal, and Google Scholar databases for the past five years using the search terms arboviruses, dengue, chikungunya, Zika, Mayaro, and West Nile fever, as well as child, newborn, and adolescent.
Synthesis of dataThe main characteristic of arboviruses is the fact that part of their replication cycle occurs inside insect vectors, thus being classically transmitted to humans through the bite of mosquitoes (hematophagous arthropods), although non-vector transmission of these viruses is also possible in specific situations. These diseases remain a major public health challenge, due to the lack of specific antiviral treatment, the co-circulation of different arboviruses in endemic/epidemic regions, the lack of effective and safe immunizations for the vast majority of these viruses, and the great difficulty in vector control, especially in large urban centers.
ConclusionsChildren are especially vulnerable to this group of diseases due to characteristics that facilitate the development of the most severe forms. More detailed knowledge of this group of diseases allows the pediatrician to diagnose them earlier, implement the correct treatment, monitor warning signs for the most severe forms, and establish effective preventive measures.
Apresentar as evidências atualmente disponíveis sobre transmissão, quadro clínico, métodos diagnósticos, tratamento e métodos de prevenção das principais arboviroses que ocorrem na infância.
Fonte de dadosRevisão não sistemática feita nas bases de dados Medline (Pubmed), Lilacs (BVS), Scopus, Web of Science, Cochrane, portal Capes e Google Scholar nos últimos cinco anos, com o uso dos termos arboviroses, dengue, chikungunya, zika, mayaro, febre do oeste do Nilo e criança, recém-nascido, adolescente.
Síntese de dadosOs arbovírus têm como característica principal o fato de parte de seu ciclo de replicação ocorrer em insetos vetores. Assim, são classicamente transmitidos aos seres humanos pela picada de mosquitos (artrópodes hematófagos), embora seja também possível a transmissão não vetorial desses vírus em situações específicas. Essas doenças ainda constituem um grande desafio na saúde pública, devido à inexistência de tratamento antiviral específico, à cocirculação de diferentes arbovírus em regiões endêmicas/epidêmicas, à falta de imunizações efetivas e seguras para a grande maioria desses vírus e à grande dificuldade do controle vetorial, em especial nos grandes centros urbanos.
ConclusõesAs crianças constituem um grupo especialmente vulnerável a esse grupo de doenças, pois têm características que facilitam o desenvolvimento das formas mais graves. O conhecimento mais detalhado desse grupo de doenças permite ao pediatra diagnosticar mais precocemente, instituir o tratamento correto, vigiar os sinais de alarme para as formas mais graves e colocar em prática efetivas medidas de prevenção.
In recent years, a significant increase in mosquito vector-borne diseases, especially arboviruses such as dengue, chikungunya, Zika, and yellow fever, has been occurring in many countries around the world. Human interference and modifications in ecosystems, disordered urban population growth, the process of globalization, the expansion of international exchange, and climate changes are some factors that probably contributed to this increase.1
Dengue fever is a severe public health problem, especially in tropical and subtropical regions, being the most common arbovirus worldwide.2 Over the past five years, there has been a 30-fold increase in the incidence of dengue fever, with the highest rates occurring in young adults and higher mortality rates in the elderly ;however, children constitute a special group, as they have a higher risk for the development of the severe form of the disease.3
The chikungunya virus was first described in 1952 during its epidemic in Makonde, a province in southern Tanzania. Since then, the chikungunya virus has caused millions of infections in humans in Africa, the islands of the Indian Ocean, Asia, Europe and the Americas.4
The first description of Zika virus infection in humans was made in 1952, after it was isolated in 1947 from a sentinel rhesus monkey used in studies on yellow fever in the Zika forest near Entebbe, the former capital of Uganda. Since then, this virus has been associated with infection in humans and has spread to different countries in Africa and Asia. The first epidemic of Zika virus infection occurred on Yap Island (Micronesia) in 2007. Subsequently, new epidemics occurred in 2013 in French Polynesia, in 2014 on Easter Island (Chile), and in 2015 in the Americas.5
More recently, one of the most significant events in the history of yellow fever in Brazil was observed during the 2017/2018 period, when the virus spread to the Brazilian east coast, in the region of the Atlantic Forest biome, close to large cities such as São Paulo and Rio de Janeiro, increasing the risk of re-urbanization of the disease.6,7
Mayaro fever and West Nile fever also represent important arboviruses, especially in the countries of the Americas.8
This group of diseases still constitutes a major challenge regarding diagnosis, and therapeutic/preventive approach, especially during childhood, due to the co-circulation of different arboviruses, the lack of specific antiviral treatment, the fact that treatment is only symptomatic, that its containment is limited to vector control, and the lack of effective immunization, with the exception of yellow fever.
Source of dataA non-systematic review was carried out in the MEDLINE (PubMed), LILACS (VHL), Scopus, Web of Science, Cochrane, CAPES Portal, and Google Scholar databases, encompassing the last five years, using the following strategy: (“Arboviruses” OR “Arbovirus” OR “Arbovirus infection” OR “Arbovirus infections” OR “Flavivirus” OR “Dengue” OR “Zika” OR “Chikungunya” OR “Yellow Fever” OR “Mayaro” OR “West Nile Virus”) “AND” (“Child” OR “Children” OR “Minors” OR “Infant” OR “Newborn” OR “Neonate” OR “neonatal” OR “adolescent” OR “adolescence” OR “teen” OR “teenager” OR “youth”). After a careful evaluation based on the association with the proposed theme, articles that addressed arboviruses in the neonatal period, childhood, and/or adolescence were selected. The order of description of the different arboviruses below follows their frequency from the most to the least prevalent.
Dengue feverTransmissionThe main form of transmission of the dengue virus, as well as of chikungunya, Zika, and yellow fever in humans, is through the bite of infected females of Aedes aegypti mosquitoes.4,5,9,10 The Aedes aegypti is an urban diurnal mosquito, whose females lay their eggs in containers or natural breeding grounds that store rainwater. Once infected, the female will transmit the virus throughout life (six to eight weeks), with the possibility that part of its offspring will already be born carriers of the virus, due to transovarian transmission.11,12 The Aedes albopictus is the maintenance vector of arboviruses in Asia, already present in the Americas, and associated with the transmission of these viruses in these regions.13
Non-vector transmission of dengue can occur through blood transfusion, organ transplantation, accidents with perforating materials, and mucosal abrasions.9
Vertical transmission is common in pregnant women with viremia at the time of delivery. The dengue virus can be found in breast milk, which may be another way of transmission of dengue virus.10,12,14
PathogenesisThe transmissibility period comprises two cycles: one intrinsic, which occurs in humans, and another extrinsic, which occurs inside the vector.15 When the female mosquito feeds on human blood during the viremia period (24 h before fever onset up to the sixth day of the disease), the virus infects the middle intestine of the mosquito, where it replicates and spreads to the salivary glands. This extrinsic incubation period lasts from eight to 12 days. After this period, the virus can be transmitted to humans during future feedings (blood supply to obtain nutrients needed for ovarian development and egg maturation). Symptoms usually appear four to 12 days after the mosquito bite (intrinsic period).16 After the bite of the infected mosquito, the virus spreads to local lymph nodes, spreading to the reticuloendothelial system, where it proliferates and causes viremia. Viremia is associated with fever and the initial systemic symptoms, which last around four to five days.11
The severe form of dengue, which occurs after the defervescence period, is characterized by plasma leakage, leading to hypovolemic shock and fluid accumulation in several organs, producing signs of organ dysfunction in the heart, lungs, kidneys, liver, and/or central nervous system (CNS), in addition to the possibility of severe bleeding.17 The mechanisms responsible for the severe form of the disease are yet to be clearly understood, but it is accepted that a secondary infection by another serotype is one of the main risk factors.9
One explanation for this pathophysiology was first postulated by Halstead et al.,18 through the antibody-dependent enhancement model. This model describes that antibodies with cross-reactivity from a previous infection with a different virus serotype would facilitate virus endocytosis in immune system cells, such as monocytes, macrophages, and dendritic cells via Fc receptors expressed on their surface, resulting in amplification of the inflammatory cascade, which potentially leads to endothelial lesions, with consequent capillary leakage and disseminated intravascular coagulation.9
However, not all severe cases have previous infections by different dengue virus serotypes, which led to the postulation of other pathophysiological explanations for the severe form of the disease. Recent studies have sought mutations in the virus gene itself that would make it more resistant to the immune system, causing it to be more replicable in both humans and vectors.9 Some polymorphisms in the human gene also seem to play a role in granting more or less susceptibility to dengue virus infection, contributing to the genesis of its most severe form.9
ClinicalDengue fever in children may be asymptomatic or present as a febrile syndrome, with nonspecific signs and symptoms: adynamia, drowsiness, refusal of food and fluids, vomiting, and diarrhea. In children under 2 years of age, the disease onset may go unnoticed and the severe form of the disease may be identified as the first clinical manifestation, because warning signs are not so easily detected in this age group.17
Classically, three clinical phases can occur: febrile, critical, and recovery phases.
- 1)
Febrile phase:
The first classic manifestation of dengue is fever lasting two to seven days, usually high (39 °C–40 °C), with associated systemic symptoms. Exanthema is present in 50% of the cases, predominantly maculopapular, affecting mainly the face, trunk, and limbs, not sparing the soles of the feet and palms of the hands.17
- 2)
Critical phase:
It may be present in some patients and may progress to the severe form of the disease. It occurs after defervescence, between the third and seventh days of the disease. Warning signs in this phase should be routinely screened and considered, as their presence indicates immediate hospitalization.17
The severe form of the disease is characterized by the presence of shock secondary to capillary leakage, severe bleeding, and/or severe organ dysfunction (hepatitis, encephalitis, or myocarditis).8
Individual risk factors determine the severity of the disease, including age. Younger children are less able to compensate for capillary leakage and are consequently at higher risk of shock secondary to dengue fever.12
- 3)
Recovery phase:
After the critical phase there is a gradual reabsorption of the leaked content, with progressive clinical improvement. Care with hyperhydration is essential at this stage. A new skin rash, with or without pruritus, may occur. Secondary bacterial infections occur at this stage, contributing to disease lethality.17
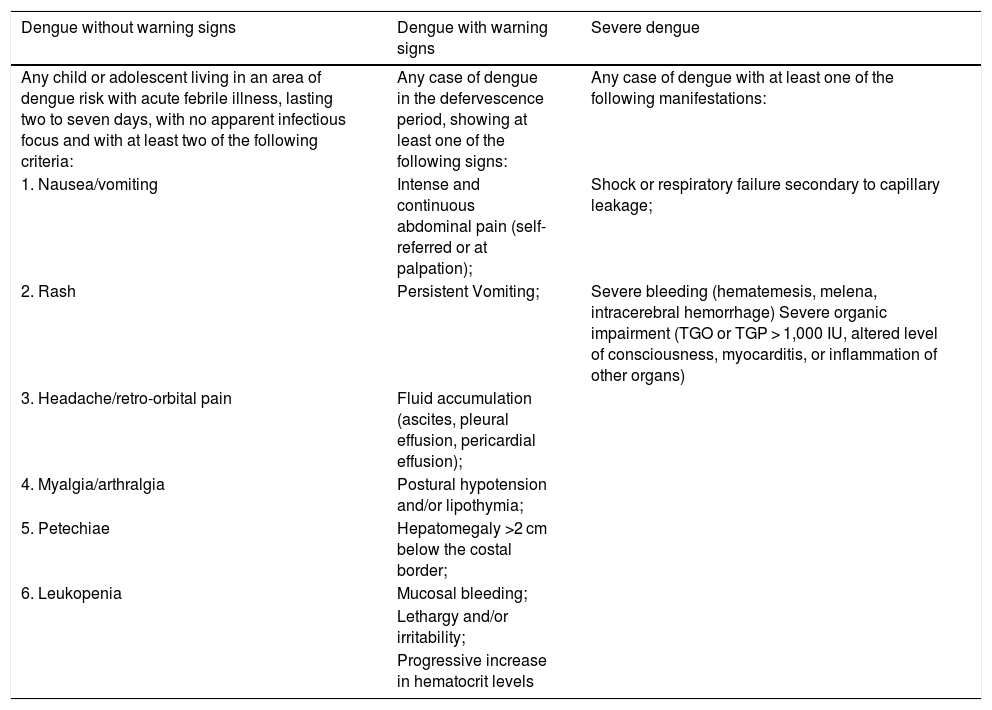
Table 1 describes the main clinical and laboratory characteristics of dengue classification in childhood.
Dengue classification in childhood.
| Dengue without warning signs | Dengue with warning signs | Severe dengue |
|---|---|---|
| Any child or adolescent living in an area of dengue risk with acute febrile illness, lasting two to seven days, with no apparent infectious focus and with at least two of the following criteria: | Any case of dengue in the defervescence period, showing at least one of the following signs: | Any case of dengue with at least one of the following manifestations: |
| 1. Nausea/vomiting | Intense and continuous abdominal pain (self-referred or at palpation); | Shock or respiratory failure secondary to capillary leakage; |
| 2. Rash | Persistent Vomiting; | Severe bleeding (hematemesis, melena, intracerebral hemorrhage) Severe organic impairment (TGO or TGP > 1,000 IU, altered level of consciousness, myocarditis, or inflammation of other organs) |
| 3. Headache/retro-orbital pain | Fluid accumulation (ascites, pleural effusion, pericardial effusion); | |
| 4. Myalgia/arthralgia | Postural hypotension and/or lipothymia; | |
| 5. Petechiae | Hepatomegaly >2 cm below the costal border; | |
| 6. Leukopenia | Mucosal bleeding; | |
| Lethargy and/or irritability; | ||
| Progressive increase in hematocrit levels |
Paixão et al.19 showed that symptomatic dengue in pregnant women is associated with a higher risk of miscarriage, stillbirth, preterm birth, and low birth weight. When the mother's clinical condition occurs near the time of delivery, the fetal or neonatal disease may be severe, simulating a condition of neonatal sepsis associated with thrombocytopenia.
DiagnosisLeukopenia with atypical lymphocyte increase and thrombocytopenia are common in the febrile phase. At the critical phase, hematocrit concentration and altered coagulogram can be observed, as well as the persistence of leukopenia and thrombocytopenia.9
Increased transaminases may occur at any stage of the disease, reflecting both muscle and liver involvement. Hypoproteinemia and hypoalbuminemia may occur in the critical phase, but may be masked by hemoconcentration.9
The method used for the laboratory confirmation of dengue infection will depend on the number of days of the disease:
- 1)
Viral antigen detection: NS1, viral isolation, reverse transcriptase reaction, followed by reverse transcription polymerase chain reaction (RT-PCR) and immunohistochemistry – up to the fifth day of symptom onset. A positive result confirms the disease; a negative result indicates serology collection.8,17
- 2)
Serology (enzyme-linked immunosorbent assay method [ELISA]) – from the sixth day of symptom onset.8,17 The IgM for dengue virus is detected from the fourth day of the disease, with a peak between ten and 14 days, disappearing after three months.9 The IgG shows low concentrations at the end of the first week, with gradual increase over time and positivity for the rest of one’s life.9
There is a high chance of cross-reaction of dengue serological tests with other flavivirus infections, such as Zika virus and yellow fever.9 This is one of the main challenges in dengue diagnosis.
TreatmentThere is no specific treatment for dengue. Treatment is symptomatic and based on the classification of the disease into four distinct groups: group A (dengue without warning signs), group B1 (dengue without warning signs but with comorbidity or social risk), group B2 (dengue with warning signs), and group C (severe form of the disease).8
Group A patients should receive oral hydration and rest, paracetamol, and/or dipyrone for fever and/or pain; they should be advised not to use salicylates or non-steroidal anti-inflammatory drugs.17
Patients in groups B and C should be referred to inpatient units for test collection, symptomatic treatment, and monitoring. Hospitalization is also indicated in those who refuse food and fluids, in patients with respiratory impairment, those with decompensated comorbidities, in pregnant women, and in children under 2 years old.8,9,11,17 Hospital discharge is linked to the presence of the following criteria: hemodynamic stabilization for 48 h, absence of fever for 48 h without antipyretics, clinical improvement, normal hematocrit levels stable for 24 h, and platelets >50,000/mm3.17
It is important to recall that the adequate management of children and adolescents with dengue fever depends on early recognition of warning signs, continuous monitoring, re-staging of outpatient cases or hospital inpatient follow-up, and prompt fluid replacement.
ChikungunyaTransmissionIn addition to vector transmission by the Aedes species (A. aegypti and A. albopictus), vertical transmission in pregnant women with peripartum viremia and transmission through the transfusion of blood components from individuals in the viremia phase of the disease have also been described.20,21
PathogenesisClinical signs appear two to four days after a bite from an infected mosquito. The virus reproduces in the reticuloendothelial system. Fever is due to the initial viremia and most of the clinical symptoms present in the first week of the disease are due to the process of viral elimination by the immune system, with an increase mainly of type 1 interferon. The classic joint involvement involves vascular proliferation, perivascular macrophages, and synovial hyperplasia. Synovitis, enthesopathy, and periosteal reactions may also occur. The exact mechanism of arthritis is still unknown, but autoimmune mechanisms and viral persistence in synovial macrophages may be part of the pathophysiology of the chronic phase of the disease.11,22
ClinicalAfter an incubation period of one to 12 days, the acute phase of the disease is classically described as fever >39 °C associated with symmetric polyarthralgia, with spontaneous resolution between seven and ten days. Other common symptoms are headache, nausea and vomiting, myalgia, rash, and non-purulent conjunctivitis. More severe cases have been described: encephalitis, autoimmune neurological diseases (Guillain–Barre syndrome and facial paralysis), uveitis, retinitis, myocarditis, hepatitis, and nephritis.8
The post-acute (from the fourth week to the end of the third month) and chronic (from the fourth month onward) phases of the disease are mainly characterized by the persistence of joint symptoms, often requiring drug interventions to relieve symptoms and the resulting worsening in the quality of life.8
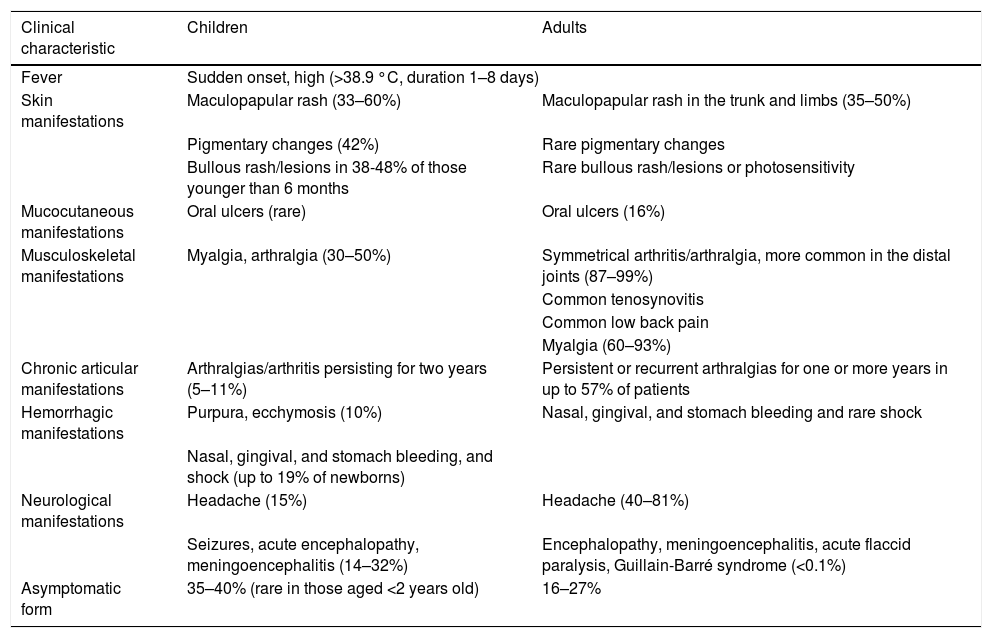
Chikungunya virus infection in children has some clinical differences when compared to adults. Children have more asymptomatic forms of the disease. Symptomatic, dermatological, and neurological symptoms are more common, while joint symptoms are less frequent.23,24Table 2 summarizes the main differences in the clinical between children and adults.
Clinical presentation of the chikungunya in children vs. adults.
| Clinical characteristic | Children | Adults |
|---|---|---|
| Fever | Sudden onset, high (>38.9 °C, duration 1–8 days) | |
| Skin manifestations | Maculopapular rash (33–60%) | Maculopapular rash in the trunk and limbs (35–50%) |
| Pigmentary changes (42%) | Rare pigmentary changes | |
| Bullous rash/lesions in 38-48% of those younger than 6 months | Rare bullous rash/lesions or photosensitivity | |
| Mucocutaneous manifestations | Oral ulcers (rare) | Oral ulcers (16%) |
| Musculoskeletal manifestations | Myalgia, arthralgia (30–50%) | Symmetrical arthritis/arthralgia, more common in the distal joints (87–99%) |
| Common tenosynovitis | ||
| Common low back pain | ||
| Myalgia (60–93%) | ||
| Chronic articular manifestations | Arthralgias/arthritis persisting for two years (5–11%) | Persistent or recurrent arthralgias for one or more years in up to 57% of patients |
| Hemorrhagic manifestations | Purpura, ecchymosis (10%) | Nasal, gingival, and stomach bleeding and rare shock |
| Nasal, gingival, and stomach bleeding, and shock (up to 19% of newborns) | ||
| Neurological manifestations | Headache (15%) | Headache (40–81%) |
| Seizures, acute encephalopathy, meningoencephalitis (14–32%) | Encephalopathy, meningoencephalitis, acute flaccid paralysis, Guillain-Barré syndrome (<0.1%) | |
| Asymptomatic form | 35–40% (rare in those aged <2 years old) | 16–27% |
Modified from Ritz et al.24
Neonatal infection by the chikungunya virus occurs in approximately 50% of pregnant women with peripartum viremia.20 In newborns, symptoms tend to occur between 3 and 7 days of age, ranging from mild (43%) to severe clinical presentation, with viral encephalitis (53%) requiring intensive neonatal treatment.25 The most commonly observed symptoms in this age group are fever, irritability, hyperalgesia, limb edema, rash, meningoencephalitis,26 and clinical that are similar to neonatal sepsis.20 The estimated lethality in newborns is around 2.8% and long-term neurodevelopmental involvement may occur in 50% of symptomatic cases.20
DiagnosisLeukopenia (especially lymphopenia), thrombocytopenia, increased transaminases, and hypocalcemia can be observed.23 The method used for laboratory confirmation will depend on the number of days of the disease:
- 1)
Viral antigen detection by RT-PCR – up to the fifth day after symptom onset. A positive result confirms the disease, a negative result indicates serology collection.8
- 2)
Serology (ELISA method) – from the sixth day of symptom onset.8 Serological methods for chikungunya virus may yield false positive results, due to cross-reaction with other viral infections such as the Mayaro virus; therefore, in areas where both viruses circulate, more specific methods should be used (plaque-reduction neutralization test [PRNT]).4,23 The peak increase in IgM occurs between three and five weeks after symptom onset, with a decline after two months.24 IgG is detected one week after symptom onset and persists for life.24
There is no specific antiviral treatment for chikungunya virus. Spontaneous symptom resolution occurs between seven and ten days in most cases.23 The recommended treatment is hydration and control of fever and pain symptoms.8
In children, the initial use of analgesic drugs such as acetaminophen or dipyrone is recommended.4 Second-line analgesic drugs that may be tried include tramadol and codeine (the latter only in those aged 12 years or older).8 The use of non-steroidal anti-inflammatory drugs (especially acetylsalicylic acid) is not recommended in the acute phase of the disease, especially in areas where dengue virus infections coexist, due to the risk of bleeding.4,8,23 Corticosteroids are not recommended in this phase.4,8,23
In cases that progress to the chronic phase of the disease, the use of non-steroidal anti-inflammatory drugs and corticosteroids may be tried. In more refractory cases, drugs used for neuropathic disease such as gabapentin and pregabalin may be tried.27 Motor physiotherapy is also indicated in chronic cases.8
In symptomatic cases caused by vertical transmission, hospitalization with newborn monitoring and symptomatic care should be implemented. Asymptomatic newborns born to mothers with confirmed peripartum viremia should be monitored for at least five days, including thermal curve, breastfeeding quality, and signs of pain, while paying attention to skin abnormalities and hydration.8
Zika virusTransmissionIn addition to the vector transmission by the Aedes aegypti and Aedes albopictus mosquitoes, sexual and transplacental transmission have been documented in the literature.5 Sexual transmission can occur from man to woman, from woman to man, and from man to man. The Zika virus is present in semen for up to 141 days and in vaginal secretions for 14 days.28,29
Vertical transmission can occur at any time during pregnancy. The Zika virus causes placental infection and can also be found in the amniotic fluid of infected pregnant women.5 The Zika virus has also been isolated from human milk,30 urine, saliva, and tears, but infections through these routes have not yet been reported.31
ClinicalThe acute clinical of Zika virus infection in children seems to be very similar to that in adults.32 The incubation period lasts three to 14 days, and it is estimated that 80% of cases are asymptomatic.32 Fever is usually low (<38.5 °C) and maculopapular rash appears in a cephalocaudal pattern, affecting the palms and soles. Pruritus is very common and can be intense.8
Two recent reviews of postnatal manifestations of Zika virus infection in childhood have been published33,34 and have shown that the most common symptoms in this age group are rash, fever, conjunctivitis, and arthralgia. Other reported symptoms include myalgia, gastrointestinal complaints, retro-orbital pain, and upper respiratory tract symptoms. In these case series, neurological complications or deaths were not reported, and there was only one report of a patient with dengue virus co-infection with hypotension and thrombocytopenia.34
Neurological complications associated with Zika virus infection reported in the literature include Guillain-Barré syndrome, peripheral polyneuropathy, acute myelitis, and meningoencephalitis.5,35
A number of case series have described some congenital and postnatal characteristics of intrauterine Zika virus infection. In addition to microcephaly, this group of children may have intrauterine growth retardation, reduced brain volume, brain malformations (especially subcortical calcifications, ventriculomegaly, and cortical migration defects), craniofacial disproportion, and skin accumulation in the occipital region.36 The development of postnatal microcephaly in children exposed to intrauterine Zika virus infection has also been reported,37 in addition to negative effects on child growth and development.38,39 Other characteristics of congenital Zika virus infection are eye abnormalities, hyperexcitability, hypertonia, irritability, epilepsy, and arthrogryposis.36,39
The prevalence of congenital defects and/or neurodevelopmental abnormalities related to intrauterine exposure to Zika virus is between 6% and 9% in exposed newborns.40 The lethality of confirmed or probable congenital infection by Zika virus was between 4% and 6% in a cohort of Brazilian newborns.41
DiagnosisThe Centers for Disease Control and Prevention (CDC)42 recommends the following for the diagnosis of Zika virus infection:
- •
<14 days after symptom onset: RT-PCR in serum and/or urine;
- •
From 14 days after symptom onset: Zika virus IgM through the MAC-ELISA method, including plaque reduction neutralization test for differential diagnosis with other arboviruses. IgM can be detected in the first week of infection and persists for up to eight to 12 weeks;42
- •
Congenital Zika virus infection: RT-PCR and IgM for the Zika virus in the newborn’s serum and urine. These same tests can be ordered in the cerebrospinal fluid, placenta, and amniotic fluid.42
The treatment of symptomatic infections in childhood is supportive, including hydration and antipyretics/analgesics; the use of non-steroidal anti-inflammatory drugs should be avoided due to the risk of dengue virus co-infection.8
In cases of congenital infection, newborns should be followed by a multidisciplinary team. The neurological examination should be performed at birth and repeated within the first two years of life, and the child should be referred to a follow-up with a neuropediatrician. Eye examination should be performed within the first month of life and repeated at three months. The brainstem auditory evoked potential should be performed in the first month of life and repeated within four to six months. Early stimulation with therapies such as motor physiotherapy and speech therapy should be indicated.5
Yellow feverTransmissionThe transmission of the yellow fever virus to humans occurs through the bite of the Haemagogus or Sabethes mosquito in forested areas and the Aedes aegypti mosquito in urban areas.8
ClinicalThe incubation period ranges from three to 15 days; the transmissibility period varies from 24 to 48 h before to up to three to five days after symptom onset.43 The infected mosquito transmits the virus for six to eight weeks.43
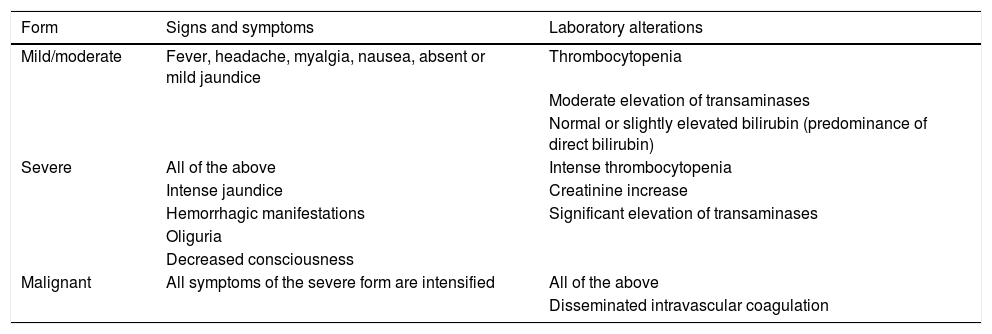
The clinical spectrum can range from asymptomatic infections to severe and fatal conditions (Table 3).
Common clinical and laboratory manifestations of yellow fever.43
| Form | Signs and symptoms | Laboratory alterations |
|---|---|---|
| Mild/moderate | Fever, headache, myalgia, nausea, absent or mild jaundice | Thrombocytopenia |
| Moderate elevation of transaminases | ||
| Normal or slightly elevated bilirubin (predominance of direct bilirubin) | ||
| Severe | All of the above | Intense thrombocytopenia |
| Intense jaundice | Creatinine increase | |
| Hemorrhagic manifestations | Significant elevation of transaminases | |
| Oliguria | ||
| Decreased consciousness | ||
| Malignant | All symptoms of the severe form are intensified | All of the above |
| Disseminated intravascular coagulation |
Suspected cases should be considered in the following situations: children with exposure in recently affected areas (outbreak) or in rural and/or wild environments, with up to seven days of acute fever, accompanied by two or more of the following signs and symptoms: headache, myalgia, low back pain, malaise, chills, nausea, jaundice, and/or hemorrhagic manifestations, in those without proof of yellow fever vaccination or those who received the first dose less than 30 days before.43
Perinatal transmission of the yellow fever virus has been reported, as well as antenatal transmission, but both conditions are extremely rare.44
There is a risk of transmission of the vaccine virus to the child through breast milk if the mother receives the vaccine during breastfeeding up to the child’s 6th month of life. If the mother needs vaccination during this period, it is recommended that breastfeeding be suspended for ten days.43
DiagnosisThe following tests should be requested: complete blood count, transaminases, bilirubin, coagulation tests, and abnormal elements in urine. Albuminuria is present in 90% of patients, which helps in the differential diagnosis with hepatitis.8
RT-PCR should be ordered up to the fifth day after disease onset for virus detection and disease confirmation. The presence of IgM against yellow fever virus in unvaccinated children or a four-fold or greater increase in IgG titers in vaccinated children also confirms the disease.8
TreatmentThe initial management will depend on the clinical and laboratory findings:43
- 1.
Outpatient follow-up with symptomatic drugs and oral hydration can be performed when the following are observed:
- •
Mild or moderate clinical forms;
- •
Patients in good general condition, hydrated, with no vomiting, no history of bleeding, and with normal level of consciousness;
- •
Normal or mildly altered laboratory tests;
- •
Possibility of return to the health service;
- •
Presence of people at home who can see signs of worsening (persistence of high fever for more than four days and/or onset of signs: jaundice, bleeding, vomiting, decreased diuresis).
- 2.
Hospitalization is indicated in the presence of at least one of the following situations: dehydrated patient, presence of vomiting, active bleeding, altered level of consciousness, leukopenia, thrombocytopenia, hemoconcentration, transaminases greater than two-fold the upper limit, bilirubin greater than 1.5-fold the upper limit, presence of proteinuria, and abnormal coagulation tests.
In hospitalized patients, in addition to administration of symptomatic drugs and venous hydration, monitoring and correction of electrolytes, glycemia, acid-base balance, and coagulation disorders are recommended.43
Mayaro feverThe Mayaro virus is mainly transmitted by mosquitoes of the genera Haemagogus, Culex, Mansonia, Aedes, Psorophora, Sabethes, and Coquillettidia.8,45 The acute phase is characterized by the presence of fever for three to five days, headache, retro-ocular pain, myalgia, and prostration. Arthralgia is the main disease manifestation, usually symmetrical, affecting mainly the wrists, toes, ankles, and feet. At the end of the febrile phase, arthralgia is accompanied by rash or petechial lesions.8,45 There are no reports in the literature of prenatal or perinatal Mayaro virus infection.25
The diagnosis is made by RT-PCR or ELISA (IgM) for the Mayaro virus in blood. Cross-reaction in serological tests may occur with the chikungunya virus.8 In areas where both viruses circulate, more specific methods should be used, such as PRNT.46 The IgM for Mayaro virus appears three days after symptom onset, persisting for approximately three months, while the IgG may persist for years.45 Treatment is solely symptomatic.8,45
West Nile feverThe West Nile virus is typically transmitted through the bite of Culex and Aedes mosquitoes. Transmission through blood products and organ transplantation have also been reported in the literature.8
After an incubation period of two to 15 days, West Nile virus infection is mild and self-limiting in more than 80% of cases. In less than 20% of cases the disease presents as fever, headache, fatigue, prostration, myalgia, weakness, gastrointestinal symptoms, lymphadenomegaly, and maculopapular rash without pruritus in the trunk and extremities, sparing the palms and soles. This clinical is self-limiting in most cases.46
In less than 1% of cases, West Nile virus neuroinvasive disease occurs, characterized by encephalitis, meningitis, and flaccid paralysis. The cerebrospinal fluid shows lymphocytic pleocytosis.47 This group of patients may experience a full recovery, but may also have neurological sequelae or evolve to death.8
Although extremely rare, there have been reports of vertical transmission, both during pregnancy and in the perinatal period. Infected newborns may have rash, chorioretinitis, and/or encephalitis.25
The diagnosis is hindered by the high rate of viral mutations, low viral loads, short viremia time (up to seven days), and cross-reaction with other viruses, such as dengue fever and Japanese encephalitis virus, as well as those vaccinated for yellow fever.46 RT-PCR and ELISA for specific IgM and IgG in blood are recommended and, in cases of neuroinvasive disease, also in cerebrospinal fluid.8 The treatment is also symptomatic and supportive.8,46
Prevention of arbovirusesCurrent arboviruses transmission control and prevention measures are focused on fighting vectors. Aedes aegypti is a domestic mosquito with diurnal habits. Only the female feeds on human blood, especially at dawn and dusk, to reproduce. Reproduction occurs in standing water (clean or dirty). Thus, community measures to fight mosquitoes should be encouraged, such as weekly hygiene and cleaning of water supply sites, keeping water reservoirs covered, and disposal of containers with unnecessary standing water.47
Topical repellents applied to the skin are part of the preventive care against arboviruses. The most commonly used repellents are N, N-diethyl-3-methylbenzamide (DEET), IR3535, and Icaridin.47 IR3535 at a concentration of 20% can be used from 6 months of age.48 DEET and Icaridin products should not be used in children under 2 years. Between 2 and 12 years, the maximum concentration of the product should be 10% and the applications should be restricted to three times a day. Repellents should be applied to exposed areas of the body and clothing and spray should be applied to the face, or in children, by first applying the product to the palms and then spreading on the face or body, followed by adequate hand washing.47
Another important preventive measure is the development of vaccines, which can disrupt the natural cycle of epidemics and the spread of arboviruses. The only WHO-approved dengue vaccine is the CYD-TDV, a quadrivalent attenuated virus vaccine. Despite the proven efficacy from the age of 9 in seropositive patients for one of the dengue serotypes, an increased risk of hospitalization and development of severe disease has been observed in seronegative patients. Thus, the World Health Organization (WHO) recommends CYD-TDV only in patients between 9 and 45 years of age who are seropositive for the disease.49 Two new dengue vaccines are at phase 3 of their research: TV003/TV005, NCT02406729 and NCT02747927, with promising results.9
To date, there are no available vaccines against the chikungunya and Zika viruses. Phase 1 and 2 clinical trials are ongoing.4,50 Vaccines against the Mayaro and West Nile viruses are not available either.45,46
The vaccine against yellow fever is recommended for all inhabitants or travelers to countries or areas at risk of disease transmission. It is a single-dose, live-attenuated virus vaccine, which confers immunity for life. The effectiveness of the vaccine is close to 100%. Vaccination is recommended from 9 months of age onwards, but it may be indicated between 6 and 8 months of life in areas where the risk of contamination is very high.8,43
ConclusionThe control of arboviruses in Brazil and worldwide is extremely imperative, due to the limited therapeutic arsenal and the lack of vaccines that prevent this group of infections. Control can be achieved through ongoing epidemiological and entomological surveillance, continuing education of health professionals and the population, and efficient vector control.
Children are especially vulnerable to this group of diseases, due to characteristics that facilitate the development of the most severe forms. More detailed knowledge of this group of diseases allows the pediatrician to diagnose them earlier, implement the correct treatment, monitor warning signs for the most severe forms, and establish effective preventive measures.
Conflicts of interestThe authors declare no conflicts of interest.