The use of broad-spectrum antimicrobials, such as third and fourth-generation, are responsible for emergence of multidrug-resistant microorganisms in neonatal units. Furthermore, antimicrobial daily doses are not standardized in neonatology. This study aimed to investigate the association between the use of antimicrobial broad spectrum to bacterial sensitivity profile in a referral unit of neonatal progressive care.
MethodsThis is a cohort study conducted in a referral neonatal progressive care unit from January 2008 to December 2016. The data of all hospitalized neonates was collected daily. The infection criteria used were the standardized national criteria, based on definitions of Center for Diseases Control and Prevention. In this study, the use of antimicrobials was evaluated as antimicrobial-day (ATM-day) and the ratio of multidrug-resistant microorganisms per 1000 ATM-day of broad spectrum was also calculated. The study was approved by the Institutional Review Board of the Universidade Federal de Minas Gerais (ETIC 312/08 e CAAE 58973616.2.0000.5149).
ResultsFrom 2008 to 2016, 2751 neonates were hospitalized, corresponding to 60,656 patient-days. The ratio of multidrug-resistant microorganisms per 1000 ATM-day of broad spectrum was 1,3 in the first period and 4,3 in the second period (p=0,005).
ConclusionIt was observed that use of broad-spectrum antimicrobials, especially those with coverage for Gram-negative bacteria, was associated with an increase of multidrug-resistant bacteria.
Healthcare-associated infections (HAI) are a main cause of morbidity and mortality in newborns.1–6 As an empirical guide to treat these types of infections, they are grouped into two categories, early and late onset-infections.1,3,7–12 For the treatment of early sepsis, the bacteria in the maternal genital-urinary tract are considered; for late sepsis, the microbiological profile of the neonatal unit must be taken into account.1,3,7–12
Usually, the symptoms of HAI are nonspecific and similar to other common conditions of neonates, such as respiratory, metabolic, hydro electrolytic, and thermoregulatory disorders.1,6–11 Timely treatment, however, is essential for improving the prognosis of infants. Antimicrobial therapy is usually initiated without laboratory confirmation.6–11 In addition, it is difficult to obtain two blood samples in volumes needed for culture, so sepsis treatment is often initiated based on clinical symptoms, leading to eight-fold increases in the levels of antimicrobial consumption.7,9
Recently, there has been an increase in HAI associated with resistant bacteria in neonatal units with high morbidity and mortality rates and raises hospital costs,1,4,9,13–15 with the progressive increase of resistance.2,8,10,16 It has been recognized that excessive use of antibiotics, especially broad-spectrum types, is a fundamental factor facilitating the onset of new bacterial resistance mechanisms.1,7,8,10,13–17
Well-designed studies on this topic are scarce, but some have shown the importance of stewardship programs in neonatal units.9,14,15,18,19 These studies have indicated that treatment should be precisely installed and should utilize antimicrobials with reduced spectrums. Therefore, the use of broad-spectrum antimicrobials, such as third- and fourth-generation cephalosporins, vancomycin, and carbapenems, should be indicated only for select cases to prevent the emergence of multidrug-resistant microorganisms.2,3,7,8,10,12,13
This study aimed to investigate the association between the use of broad-spectrum antimicrobials on bacterial sensitivity profiles in a referral unit of neonatal progressive care. In addition, the authors proposed an investigation of the association between the density of multidrug-resistant bacteria and the use of broad-spectrum antimicrobials (ATM) evaluated as ATM-day.
MethodsThis cohort study was conducted at the Neonatal Unit of the Hospital das Clínicas of Universidade Federal de Minas Gerais from January 2008 to December 2016, a tertiary referral university medical center.
Qualified and trained professionals of the hospital infection control commission (HICC) routinely and systemically collect, through active surveillance of medical records and discussion of cases with the care team, data regarding newborns considered to be under-risk for HAI, according to legislation recommendation. The infection criteria used here are the same as those standardized by the Brazilian Health Surveillance Agency (Agência Nacional de Vigilância Sanitária (ANVISA]) and are based on criteria formulated by the Centers for Disease and Control (CDC) and the National Healthcare Safety Network (NHSN).
Microbiological identification was performed using an automated method (VITEK2) and with a diffusion sensitivity test in agar (Kirby Bauer) to confirm the resistance profile. The sensitivity profile of microorganisms was established by the CCIH and is based on the Clinical & Laboratory Standards Institute (CLSI) guidelines.
This study included the following variables for analysis: notified HAI frequency, number of patients at risk, patient-days, patients with HAI, HAI cumulative incidence (number of HAI for 100 patients at risk), and HAI incidence density (number of HAI per 1.000 patient-days). HAI incidence density was also stratified in a control chart according to the birth weight and topography of the infection. In addition, a chart control was created using the mean incidence density, the alert threshold (two standard deviations), and the control limit (three standard deviations) for follow-up reporting over time. Mean and endemic limits were calculated from monthly HAI rates per 1.000 patient-days. The model selected for the representation of the graphic rate was a U-type and was based on a Poisson probability distribution. The periods defined for comparison considered the control chart, which indicated an increase in the number of infections by multidrug-resistant microorganisms isolated after 2012. Thus, the two periods for comparison were, from 2008 to 2012 and from 2013 to 2016.
As antimicrobial consumption is not standardized for neonatology, in this study, the use of antimicrobials was evaluated by ATM-day, which is the sum of the total days of exposure to each antimicrobial. The same calculation was also performed for each broad-spectrum antimicrobial analyzed. The incidence density of multidrug-resistant microorganisms per 1000 ATM-days of broad-spectrum antimicrobials was calculated, along with the resistance density per group of bacteria (Gram-positive or Gram-negative) per ATM-specific day. Broad-spectrum antimicrobials were considered as those that were not the first choice for the treatment of the etiological agent in the microorganism sensitivity profile. The mid-test was used to compare incidence densities calculated between the periods. Statistical significance was considered if p≤0.05.
The study was approved by the Institutional Review Board of the Federal University of Minas Gerais and the need for a signed informed consent was waived, as the data included was institutional (ETIC 312/08 and CAAE 58973616.2.0000.5149).
ResultsFrom 2008 to 2016, 2751 neonates were hospitalized, corresponding to 60,656 patient-days. A total of 1398 infections were diagnosed, 1106 of which were systemic bacterial infections, requiring venous antimicrobial treatment. HAI incidence density throughout the study period was 23 per 1000 patient-day and the ATM-day/patient-day ratio was 0.367.
A total of 92% of patients admitted to the unit weighed above 1000g; however, the incidence density of HAI per weight range was higher in the group under 750g (34,6 per 1000 patients-day). When analyzing HAI by topography, a higher incidence density of laboratory-confirmed late-onset sepsis was observed (6.2 per 1000 patients-day).
During the nine-year period, 36 multidrug-resistant bacterial infections were identified. In 2009, no HAI from these bacteria was identified. The types of microorganisms identified and their distributions over the years investigated in this study are shown in Table 1.
Distribution of multidrug-resistant bacteria in late healthcare-associated infections, per year, in a neonatal reference unit, Belo Horizonte-MG, Brazil, 2008 to 2016.
| Multidrug-resistant bacteria | 2008 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | Total |
|---|---|---|---|---|---|---|---|---|---|
| Acinetobacter baumannii | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 5 |
| Acinetobacter lwoffii | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Enterobacter cloacae | 0 | 1 | 2 | 1 | 0 | 0 | 0 | 0 | 4 |
| Enterococcus faecalis | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 |
| Klebsiella pneumoniae | 0 | 0 | 0 | 2 | 1 | 3 | 11 | 0 | 17 |
| Pseudomonas aeruginosa | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| Serratia marcescens | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 2 |
| Staphylococcus aureus | 3 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 5 |
| Total | 4 | 2 | 2 | 4 | 5 | 5 | 12 | 2 | 36 |
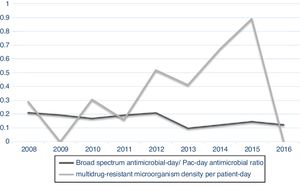
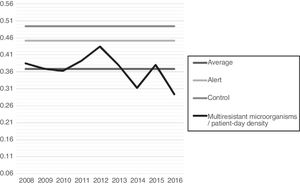
When analyzing antimicrobial-day/patient-day and broad-spectrum antimicrobials-day/patient-day, it was possible to observe a decreasing tendency for antimicrobial-day/patient-day use after 2012. Nevertheless, when this curve is superimposed on the multidrug-resistant microorganism density curve, progressive increases in the density of microorganisms can be observed (Fig. 1). The increased incidence density of multi-resistant microorganisms/patient-day can also be observed in the control chart (Fig. 2), which was associated with increases in the reporting of ESBL-producing Klebsiella pneumoniae and it reached alert level in 2015.
It was also observed an increase in the density of multidrug-resistance to broad-spectrum antimicrobials after 2012. This increase was observed when the analysis was performed including all antimicrobials and also for broad-spectrum antimicrobials for Gram-negative bacteria.
When evaluating the use of all broad-spectrum antimicrobial groups, the use of carbapenems and third- and fourth-generation cephalosporins provided a significant increase in multidrug-resistant microorganism density for antimicrobial-day in the second period of study. However, for aminopenicillin associated with the β-lactamase inhibitors, the opposite trend was observed, with significant reductions in multi-resistant microorganism density by antimicrobial-day. Regarding Gram-positive bacteria, no statistically significant differences were observed in relation to antimicrobials administered, even when vancomycin was analyzed separately, despite the increase in incidence density use (Table 2).
Multidrug-resistant bacteria frequency over antibiotic-day by antimicrobial for all multidrug-resistant bacteria and for Gram-negative and Gram-positive bacteria, in two periods, in a neonatal reference unit, Belo Horizonte-MG, Brazil, from 2008 to 2016.
| Antibiotic | MR bacteria | ATM-day | MR/ATM-day | P (MID test) | |||
|---|---|---|---|---|---|---|---|
| 2008−2012 | 2013−2016 | 2008−2012 | 2013−2016 | 2008−2012 | 2013−2016 | ||
| Meropenem/imipinem | 6 | 13 | 970 | 761 | 6.2 | 17.1 | 0.0364 |
| 3rd and 4th generation cephalosporin | 6 | 13 | 1.922 | 674 | 3.1 | 19.3 | 0.0001 |
| Ampicillin and sulbactam OR Amoxicillin with clavulanate | 6 | 13 | 4 | 204 | 1.500 | 63.5 | <0.0000 |
| Vancomycin/teicoplamine | 2 | 2 | 4.229 | 2.117 | 0.5 | 0.9 | 0.5192 |
| All broad-spectrum antibiotics (Gram positive) | 2 | 2 | 6.241 | 3.486 | 0.3 | 0.6 | 0.5865 |
| All broad-spectrum antibiotics (Gram negative) | 6 | 13 | 6.241 | 3.486 | 1.0 | 3.7 | 0.0048 |
| All broad-spectrum antibiotics (All bacteria) | 8 | 15 | 6.241 | 3.486 | 1.3 | 4.3 | 0.0050 |
Significance of bold value: Statistical significance was considered if p≤0,05.
In the referral neonatal unit where the present study was conducted, a higher HAI incidence density was observed in newborns with birth weights below 1000g, especially late onset sepsis. Preterm infants generally undergo more invasive procedures and are hospitalized for a greater length of time than higher birth weight infants; the literature indicates that these are risk factors for late sepsis and high mortality.6,20 These factors justify the greater use of ATM in this age group, especially in newborns weighing less than 750g.
A review showed that global consumption of antibiotics has risen 36% from 2000 to 2010. BRICS countries (Brazil, Russia, India, China, and South Africa) contributed to 75% of this increase while accounting for only 40 % of the world’s population. The same review cited one study including 226 hospitals in Europe, Africa, and South East Asia that reported a 40% of pathogens resistant to the first-line antibiotics as recommended by World Health Organization (WHO).14 In Senegal, Landre-Peigne et al.21 observed a positive association of increased consumption of antibiotics and number of multidrug-resistant bacteria. Conversely, in the present unit an increase of multidrug-resistant bacteria was observed over the time, despite the reduction in the use of broad-spectrum antimicrobials when ATM-day was considered. This reduction is likely associated with the modification of the vancomycin to oxacillin empirical scheme for late sepsis treatment in the second period of study in this neonatal unit. International studies,6,20,22,23 as well another research in the same neonatal unit,24 have demonstrated that replacing vancomycin with oxacillin can be done safely and without increasing mortality when considering coagulase-negative Staphylococcus. Moreover, the overall incidence of multidrug-resistant Gram-positive bacteria remained relatively constant throughout the present study, with no statistical difference observed. This was probably due to the low incidence of HAI caused by Staphylococcus aureus and Enterococcus sp. in the neonatal unit of this study and the modification of empirical therapy as described.
In 2002, the Center for Diseases Control12 published the twelve-step recommendation for bacterial resistance prevention, which reinforces the need for a careful choice of antimicrobial, which should be used for the shortest time required, as well as restriction and adequacy according to microbiological results. Stewardship programs also recommend not only reducing the use of antimicrobials, but also using them for the shortest time possible and with spectrum restrictions aiming to produce the best possible outcomes. Although studies designed to evaluate the impact of antimicrobial use in the neonatal population are scarce, a previous systematic review has demonstrated the association between inadequate use of antimicrobial agents and increase of multidrug-resistant bacterial HAI in neonatal units.19 Thus, it is recommended to reduce the practice of empirical antimicrobial treatments and to include treatment of clinical sepsis for five days, the use of oxacillin in an initial regimen for late sepsis rather than vancomycin, and adequacy of treatment based on culture and not clinical severity.2,8,12,15,21
In this study, a significant increase in the density of broad-spectrum antimicrobial multidrug-resistance was observed when all antimicrobials within the third and fourth generation of cephalosporin and carbapenems group were considered. The relationship between the increase in multidrug-resistant Gram-negative bacteria and these two groups of antimicrobials was already recognized in the literature.2,5,8,11–13,16–18,25–29 It is mainly reported with respect to the increase of bacteria-producing ESBL and the use of cephalosporins of the third and fourth generations.2,5,11,16–18,26–29 In 2015, the studied neonatal unit presented an alert as a result of increases in ESBL-producing Klebsiella pneumoniae, reinforcing the hypothesis that inadequate empirical treatment occurred after 2012.
A reduction in the incidence density of resistant microorganisms when using aminopenicillins, associated with an increase in the use of β-lactamase inhibitors was observed in the second period. There was a shortage of ampicillin, which is indicated as the treatment of choice for early sepsis, and the institution allowed the use of ampicillin and sulbactam. In a study conducted in the Netherlands,30 different empiric antimicrobial regimes for sepsis were used in neonatal units (penicillin-tobramycin or flucloxacillin versus tobramicyn regimen and amoxicillin-cefotaxime regimen). Gram-negative bacillus resistance to empiric therapy was 1798 greater (CI 95% 5,57–58,01) when a large-spectrum regimen was used. However, the increased number of ESBL microorganisms in the present study was observed in the second period of follow-up. As a result, the study may not have carried out long enough to observe associations between the occurrence of these ATM use and prevalence of ESBL strains.
The present study has limitations, due to its prospective observational design with multifactorial predictors, in which there was no control of other care factors that could influence the incidence of late neonatal infections by multidrug-resistant bacteria. Such factors include hand hygiene, cleaning and sterilization of instruments, distance between hospital beds, and professional/patient relationship recommended by the level of care provided, affecting quality of assistance.
Although there is no standardization of antimicrobial consumption in neonatal units, it was observed that use of broad-spectrum antimicrobials, especially those with coverage for Gram-negative bacteria, was associated with increases in multidrug-resistance. It should be noted that this association was identified in the second period of the study, considering antimicrobial-day density when broad-spectrum antibiotics were used as an empirical treatment.
Stewardship programs are essential in neonatal units, especially when considering treatment for clinical neonatal sepsis, to reduce the empirical use of antimicrobials and to indicate a rational use of antimicrobial agents.
FundingThis work was supported by an Institutional Scholarship from Universidade Federal de Minas Gerais and Pró-Reitoria de Pesquisa (PRPq) da Universidade Federal de Minas Gerais.
Conflicts of interestThe authors declare no conflicts of interest.
Study conducted at Universidade Federal de Minas Gerais (UFMG), Belo Horizonte, MG, Brazil.