HIV-infected individuals (HIVI) are threatened by meningococcal infection and presented lower response to vaccines. Data are scarce on long-term persistence of human serum bactericidal antibody (hSBA) after a meningococcal C conjugate (MCC) vaccine in HIVI youth; the authors aimed to describe this persistence in HIVI.
MethodsHIVI and HIV uninfected individuals (HIVU), aged 2–18 years, CD4 >15% were recruited. Seroprotection (hSBA ≥1:4) at baseline and at 12–18 months after immunization was evaluated and the association of the different factors with the long-term persistence was calculated using logistic regression.
ResultsA total of 145 HIVI, 50 HIVU were recruited and immunized, and their median age was 11 years (median age in HIVI group was 12 years, and 10 years in HIVU group, p-value=0.02). 85 HIVI (44%) had undetectable viral load (UVL). Seroprotection rate was 27.2%: 24.1% in HIVI and 36% in HIVU 12–18 months after immunization (p=0.14). Baseline immunity (odds ratio [OR]=70.70, 95% CI: 65.2–766.6); UVL at entry (OR: 2.87, 95% CI: 0.96–8.62) and lower family income (OR: 0.09, 95% CI: 0.01–0.69) were associated with seroprotection among HIVI.
ConclusionSeroprotection at 12–18 months after single dose of MCC was low for both groups, and higher among individuals who presented baseline immunity. Among HIVI, vaccine should be administered after UVL is achieved.
As pessoas infectadas pelo HIV (HIVI) estão sujeitas a infecção meningocócica e apresentam menor resposta a vacinas. São escassos os dados a respeito da persistência de longo prazo do anticorpo bactericida no soro humano (hSBA) após vacina conjugada meningocócica C (MCC) em HIVI jovens, e visamos descrever essa persistência em HIVI.
MétodosForam recrutadas pessoas HIVI e pessoas não infectadas por HIV (HIVU), com idades entre 2 e 18 anos, CD4>15%. A seroproteção (hSBA ≥ 1:4) basal aos 12–18 meses após a imunização foi avaliada e a associação dos diferentes fatores com a persistência de longo prazo foi calculada utilizando a regressão logística.
Resultados145 HIVI e 50 HIVU foram recrutados e imunizados e sua idade média foi determinada em 11 anos (a idade média no grupo HIVI foi 12 anos e no grupo HIVU foi 10 anos, valor de p=0.02). 85 HIVI (44%) apresentaram carga viral indetectável (CVI). A taxa de seroproteção foi 27.2%: 24.1% no grupo HIVI e 36% no grupo HIVU 12-18 meses após imunização (p=0.14). A imunidade basal [razão de chance (RC)=7070, IC: 65,2–7666]; CVI no momento da participação (RC: 2,87, IC de 95%: 0,96-8,62) e renda familiar mais baixa (RC: 0,09, IC de 95%: 0,01-0,69) foram associadas a seroproteção entre as pessoas HIVI.
ConclusãoA seroproteção aos 12-18 meses após única dose de MCC mostrou-se baixa em ambos os grupos e mais elevada entre as pessoas que apresentaram imunidade basal. Entre as pessoas HIVI, as vacinas devem ser administradas após a CVI ser atingida.
Meningococcal disease (MD) is an important cause of septicemia and meningitis, and a major public health challenge worldwide.1,2 People living with human immunodeficiency virus (HIV) infection have an increased risk for MD. Studies have demonstrated a five to ten times higher risk of MD among HIV-infected (HIVI) children and almost ten-fold higher among adolescents and adults when compared with HIV-uninfected individuals (HIVU).3,4 Furthermore, HIVI children and adolescents have lower vaccine response rates despite being on combination antiretroviral therapy (cART).5–7 Vaccine-induced antibodies also may wane more quickly in persons with HIV than in healthy individuals, indicating that immune reconstitution was not sufficient to ensure long-term protection and highlighting the importance of evaluating long-term immunogenicity to meningococcal vaccines and factors that may be associated with a better response.8,9
The persistence of antibodies after meningococcal serogroup C (MCC) conjugate vaccines has been studied in many high-risk populations with different degrees of immunosuppression, but there are few studies in HIVI.10–12
The aims of this study were: (1) to assess the persistence of serum bactericidal antibody against MCC, using human complement (hSBA) in HIVI children and adolescents, 12–18 months after a single dose of MCC, and compared with HIVU participants; and (2) to evaluate factors associated with antibody persistence.
Materials and methodsStudy design and populationThis was a prospective cohort study of HIVI and HIVU children and adolescents followed at the Instituto de Puericultura e Pediatria Martagão Gesteira (IPPMG), a pediatric hospital of the Universidade Federal do Rio de Janeiro, reference center for HIV care in Rio de Janeiro, Brazil, from February 2011 to December 2012. They were recruited at the clinic's waiting room.
Eligibility criteria were: individuals aged 2–18 years who had not received any previous MCC vaccine, had not received a live vaccine within 4 weeks before entry, and were not scheduled to receive other vaccines within 2 weeks. For the HIVI group, additional eligibility criteria were HIV infection (defined as two reactive anti-HIV tests in children >2 years old), absence of World Health Organization clinical stages 3 or 4 HIV clinical disease at entry, and CD4+ T-lymphocyte cell (CD4) count at or above 350cell/mm3 and/or 15% at study entry. HIVU were recruited among healthy children at the clinic at the same hospital who had a negative HIV serology result after 18 months of age; the test was again required at entry for those who were sexually active. Individuals in both groups had to live in the same geographic area.
The exclusion criteria included pregnancy; presence of any other immunosuppressive disease; use of systemic immunosuppressive drugs; use of antibiotics up to 3 weeks or immunoglobulin therapy within the last 6 months before entry; bleeding disorders precluding intramuscular injection; adverse reactions to any vaccine components; and psychiatric disorders, including illicit drug or alcohol intoxication at the time of the interview (patient or parent/legal guardian). A negative pregnancy test was required before immunization for participants of reproductive age. All participants received one dose (0.5mL intramuscular deltoid injection) of MCC oligosaccharide-CRM197, a toxoid of Corynebacterium diphtheria (Chiron/Novartis Vaccines – Siena, Italy) between February 2011 and December 2012. For HIVI volunteers, the vaccine was provided by the Brazilian National Ministry of Health; for HIVU, it was funded by the research grant.
Blood samples for serogroup C Neisseria meningitidis antibodies with hSBA, virological, and imunological measurements, as well as standardized questionnaires, including clinical, laboratory, and socioeconomic data were collected at the entry, one to two, and 12–18 months post-immunization visits. Demographic, clinical, virology, and immunologic data were obtained from medical records (Table 1). Viral load (VL) and CD4 count results before, at beginning, and after cART, as well as nadir and zenith data, were recorded.
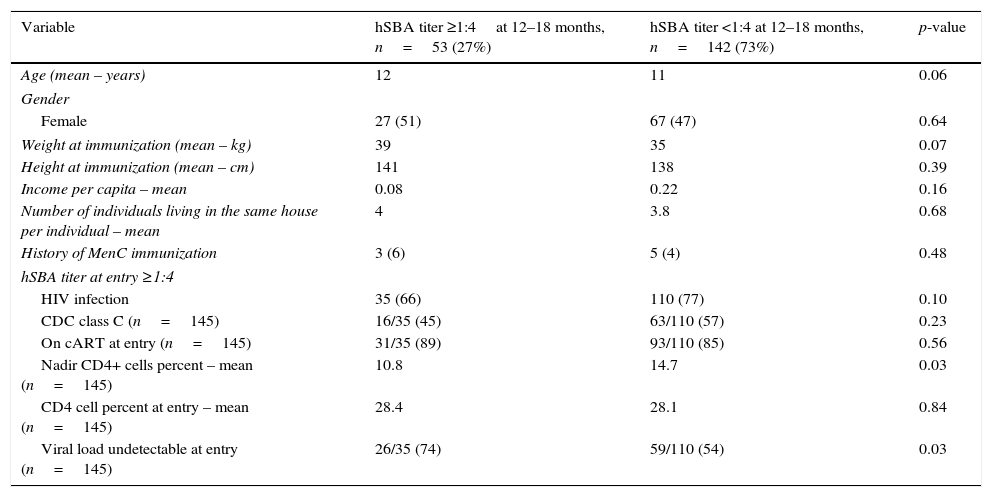
Difference between patients with hSBA titer ≥ or <1:4at 12–18 months.
| Variable | hSBA titer ≥1:4at 12–18 months, n=53 (27%) | hSBA titer <1:4 at 12–18 months, n=142 (73%) | p-value |
|---|---|---|---|
| Age (mean – years) | 12 | 11 | 0.06 |
| Gender | |||
| Female | 27 (51) | 67 (47) | 0.64 |
| Weight at immunization (mean – kg) | 39 | 35 | 0.07 |
| Height at immunization (mean – cm) | 141 | 138 | 0.39 |
| Income per capita – mean | 0.08 | 0.22 | 0.16 |
| Number of individuals living in the same house per individual – mean | 4 | 3.8 | 0.68 |
| History of MenC immunization | 3 (6) | 5 (4) | 0.48 |
| hSBA titer at entry ≥1:4 | |||
| HIV infection | 35 (66) | 110 (77) | 0.10 |
| CDC class C (n=145) | 16/35 (45) | 63/110 (57) | 0.23 |
| On cART at entry (n=145) | 31/35 (89) | 93/110 (85) | 0.56 |
| Nadir CD4+ cells percent – mean (n=145) | 10.8 | 14.7 | 0.03 |
| CD4 cell percent at entry – mean (n=145) | 28.4 | 28.1 | 0.84 |
| Viral load undetectable at entry (n=145) | 26/35 (74) | 59/110 (54) | 0.03 |
hSBA, human serum bactericidal assay; MenC, polysaccharide non-conjugate meningococcal C vaccine; HIV, human immunodeficiency virus; CDC, Centers for Diseases Control and Prevention – HIV clinical classification; cART, combined antiretroviral therapy.
Short-term immunogenicity and safety results after first dose MCC immunization were previously reported.13 The hSBA assay was performed as previously described.13,14 Seroprotection was defined as an hSBA titer ≥1:4 and individuals were classified as hSBA persistence according to the presence of titer ≥1:4 12–18 months post-immunization.
Statistical analysisContinuous and categorical variables were compared between HIVI and HIVU using Mann–Whitney and Fisher's exact tests, respectively. Continuous and categorical variables were compared between groups according to antibody persistence using logistic regression analysis. Variables with p-values <0.15 in univariate analysis were included in a logistic regression model. The same strategy was used to study variables inherent to HIV infection to assess a model just for HIVI. Statistical significance was defined as p≤0.05.
All analyses were performed by using STATA software (version 13.0; Stata Corp LP – College Station, TX, USA).
Human subjectsEthical approval was obtained from the IPPMG Institutional Review Board (IRB) and the Brazilian Ministry of Health Ethics Commission (Comissão Nacional de Ética em Pesquisa [CONEP]). Participants and/or their parents/guardians signed an informed consent form before participation; assent for children aged 7 years or older was obtained, as required by the local IRB.
ResultsA total of 154 HIVI and 50 HIVU participants were enrolled in the study. Nine individuals in the HIVI group were excluded at the 12–18 month visit (six were lost to follow-up, one was transferred to another state, and one was on prolonged use of antibiotics); data was available for 195 participants. Females comprised 52% of the participants, with no differences between groups (p=0.11). The median age at enrollment was 11 years: 12 years for HIVI and 10 years for HIVU participants (p=0.02; Table 1).
There were no differences in median body mass index Z-scores between HIVI and HIVU, 0.39 and −0.14, respectively (p=0.17). The HIVU group had a higher median number of individuals living in the same household (five vs. four; p<0.01). Although the median family income in both groups was less than one Brazilian minimum wage, when the distribution of this variable was considered, the HIVU group had a significantly lower family income (p=0.01). A total of 89% of HIVI participants were receiving cART, 124 of whom started cART before entry and five, after entry. The median time of cART use was 7.5 years (IQ range=5.9–9.9 years). A total of 59 (41%) had a history of Centers for Diseases Control and Prevention (CDC) HIV clinical classification class C conditions and 85 (44%) had undetectable VL at entry.
Overall, 14.9% (29/195) had baseline (before immunization) hSBA >1:4, with a higher proportion among the HIVU (30% vs. 9.7%; p=0.01). Among those with baseline hSBA >1:4, the mean family income was 0.17 Brazilian minimum wage.
At 12–18 months post-immunization, 53 (27.2%) retained seroprotective SBA titers: 24.1% among HIVI and 36% among HIVU (p=0.14). Of the 29 individuals who had baseline immunity, 21 (72%) retained protective hSBA titer at 12–18 months visit. Although the baseline immunity rate was higher among HIVU, more HIVI remained with hSBA >1:4, if they had previous immunity at entry (8/15 for HIVU vs.13/14 for HIVI group, p=0.04).
There was no difference in the seroprotective rate by age at entry (p=0.08) or length of time between vaccination and 12–18 months visits (p=0.57).
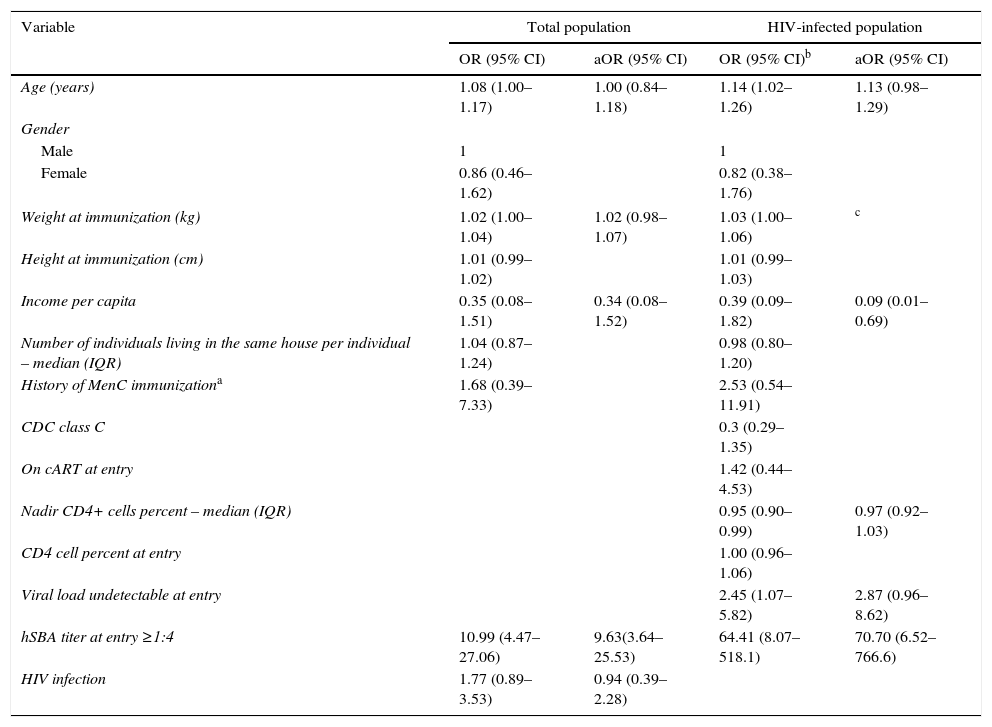
The main variable associated with hSBA persistence 12–18 months after MCC for the total population, as well as for the HIVI, was baseline hSBA >1:4; however, older age and higher body mass index were also associated with hSBA persistence in the total population. Undetectable VL at entry and income were also important among HIVI group (Table 2). On the logistic regression model, baseline hSBA >1:4 was the main significant variable on the model of the total population; the same was observed on the model of the HIVI group. In the latter group, undetectable VL at entry (at the immunization) also presented a trend of significance (Table 2).
Unadjusted and adjusted odds ratio (OR and aOR), and 95% confidence interval (95% CI) for hSBA ≥1:4 after 12–18 months of immunization for total population (n=195) and specifically for HIV-infected individuals (n=145).
| Variable | Total population | HIV-infected population | ||
|---|---|---|---|---|
| OR (95% CI) | aOR (95% CI) | OR (95% CI)b | aOR (95% CI) | |
| Age (years) | 1.08 (1.00–1.17) | 1.00 (0.84–1.18) | 1.14 (1.02–1.26) | 1.13 (0.98–1.29) |
| Gender | ||||
| Male | 1 | 1 | ||
| Female | 0.86 (0.46–1.62) | 0.82 (0.38–1.76) | ||
| Weight at immunization (kg) | 1.02 (1.00–1.04) | 1.02 (0.98–1.07) | 1.03 (1.00–1.06) | c |
| Height at immunization (cm) | 1.01 (0.99–1.02) | 1.01 (0.99–1.03) | ||
| Income per capita | 0.35 (0.08–1.51) | 0.34 (0.08–1.52) | 0.39 (0.09–1.82) | 0.09 (0.01–0.69) |
| Number of individuals living in the same house per individual – median (IQR) | 1.04 (0.87–1.24) | 0.98 (0.80–1.20) | ||
| History of MenC immunizationa | 1.68 (0.39–7.33) | 2.53 (0.54–11.91) | ||
| CDC class C | 0.3 (0.29–1.35) | |||
| On cART at entry | 1.42 (0.44–4.53) | |||
| Nadir CD4+ cells percent – median (IQR) | 0.95 (0.90–0.99) | 0.97 (0.92–1.03) | ||
| CD4 cell percent at entry | 1.00 (0.96–1.06) | |||
| Viral load undetectable at entry | 2.45 (1.07–5.82) | 2.87 (0.96–8.62) | ||
| hSBA titer at entry ≥1:4 | 10.99 (4.47–27.06) | 9.63(3.64–25.53) | 64.41 (8.07–518.1) | 70.70 (6.52–766.6) |
| HIV infection | 1.77 (0.89–3.53) | 0.94 (0.39–2.28) | ||
hSBA, human serum bactericidal assay; HIV, human immunodeficiency virus; IQR, interquartile range; MenC, polysaccharide non-conjugate meningococcal C vaccine; CDC, Centers for Diseases Control and Prevention – HIV clinical classification; cART, combined antiretroviral therapy.
The persistence of MCC bactericidal antibodies 12–18 months after immunization in Brazilian children and adolescents with and without HIV infection was low (27.2%), and lower in HIVI individuals, although not statistically significant. The antibody persistence rate was in accordance with that observed on the literature (19–57%).12,15–17
The main variable associated with persistence of MCC antibodies was hSBA titer at entry ≥1:4. For individuals who had baseline immunity, the single dose vaccine was at least the second contact with this agent, and the prime immunization would be considered a booster.18
To the best of the authors’ knowledge, this is the first study of MCC antibody persistence in HIVI children and adolescents. Siberry et al. followed-up 45 children aged 2–10 years, up to 72 weeks after two doses of meningococcal ACWY immunization.19 They observed a 45% persistence rate of the N. meningitidis C antibodies, defined as rabbit SBA ≥1:128; Black patients had higher rates of long-term immunity. Indeed, in the multivariate analysis, a socioeconomic proxy variable, income, was associated with immune persistence. The authors hypothesize that patients with lower socioeconomic level were exposed to a crowded environment, and therefore had more chance to having been exposed to serogroup C N. meningitidis. Nonetheless, genetic factors must also play a role on the association of lower socioeconomic level and decreased immune persistence.
Based on the present results, protective SBA titer gradually waned over time, and at least two doses of MCC are necessary to maintain protection against N. meningitidis in participants aged 2–18 years. Furthermore, optimal immune response to MCC among HIVI may coincide with undetectable VL, which would have implications for the timing of immunization in this group.
In other pediatric studies, antibody persistence was higher among older the patients at immunization.12,16,17,20–24 This phenomenon was not observed in the present study, following adjustment for other variables, such as baseline immunity. However, HIV infection was not significantly associated with lack of antibody response after 12–18 months of the immunization as expected. Considering that the HIVI group had a worse MCC immune response 1–2 months after immunization,13 and since the HIVI group was older than the HIVU group and had higher socioeconomic status, perhaps these variables played a role in determining the lack of difference between HIVI and HIVU groups or in age. Probably, the study sample size was not large enough to discern differences in some groups’ covariates.
In conclusion, it was demonstrated that one dose of MCC vaccine was not sufficient to maintain immune protection at 12–18 months among both HIVI and HIVU, even among HIVI with preserved or restored immunity. Studies that evaluate new approaches to vaccination must be pursued. In order to maintain immune response among the HIVI individuals, it is probably important to immunize these individuals with undetectable VL.
FundingThe study was funded by grants from Fogarty International Center of the National Institutes of Health to CBH (Grant No. 5R01 TW008397) and from Foundation for Research Support of the State of Rio de Janeiro (FAPERJ, Grant No. #E-26/112.645/2012) to LGM. This work was also supported in part by a grant from the Fogarty International Center Global Infectious Diseases Research Training Program, National Institutes of Health, to the University of Pittsburgh (Grant No. D43TW006592). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of interestThe authors declare no conflicts of interest.
The authors would like thank the parents, children, and adolescents who participated in this study.
Please cite this article as: Frota AC, Harrison LH, Ferreira B, Menna-Barreto D, Castro RB, Silva GP, et al. Antibody persistence following meningococcal C conjugate vaccination in children and adolescents infected with human immunodeficiency virus. J Pediatr (Rio J). 2017;93:532–7.