To evaluate the possible association between hyperuricemia and cardiorespiratory fitness levels/nutritional profile, grouped into a single variable, in schoolchildren.
MethodCross-sectional study of 2335 students from Elementary schools, aged 7–17 years of both genders, stratified by conglomerates of a municipality in Southern Brazil. Body mass index (BMI) was calculated and cardiorespiratory fitness (CRF) was assessed by the 6-minute run/walk test. The BMI and CRF were grouped into a single variable, considering: (1) low and normal weight/fit; (2) low and normal weight/unfit; (3) overweight-obesity/fit; (4) overweight-obesity/unfit. The Poisson regression (prevalence ratio, PR) was used for the association between hyperuricemia and BMI/CRF ratio with 95% confidence intervals and differences were considered significant when p<0.05.
ResultsThere is an association, although subtle, between the presence of hyperuricemia with low levels of CRF and the presence of excess weight, when grouped into a single variable. Boys and girls with this condition have higher prevalence of hyperuricemia (PR: 1.07; p=0.007 for boys; PR: 1.10; p<0.001 for girls).
ConclusionTogether, excess weight and low levels of cardiorespiratory fitness are associated with the presence of hyperuricemia in schoolchildren.
Avaliar a possível relação entre hiperuricemia com aptidão cardiorrespiratória e o estado nutricional, agrupados, em escolares.
MétodoEstudo transversal com 2335 escolares da educação básica de 7 a 17 anos, de ambos os sexos, estratificados por conglomerados de um munícipio do sul do Brasil. Foi calculado o índice de massa corporal (IMC) e a aptidão cardiorrespiratória (APCR) foi avaliada pelo teste de corrida/caminhada de 6 minutos. O IMC e a APCR foram agrupados em uma única variável, considerando: 1) baixo peso-normal/apto; 2) baixo peso-normal/inapto; 3) sobrepeso-obesidade/apto; 4) sobrepeso-obesidade/inapto. A regressão de Poisson (razão de prevalência; RP) foi utilizada para associação entre hiperuricemia e a relação APCR/IMC com intervalos de confiança de 95% e diferenças significativas consideradas para p<0,05.
ResultadosObserva-se associação, embora sutil, entre a presença de hiperuricemia com baixos níveis de APCR e a presença de excesso de peso, de forma agrupada. Meninos e meninas, com esta condição, têm maior prevalência de hiperuricemia (RP: 1,07; p=0,007; RP: 1,10; p<0,001, respectivamente), em comparação aos seus pares com bons níveis de APCR e estado nutricional adequado.
ConclusãoDe forma conjunta, o excesso de peso e os baixos níveis de aptidão cardiorrespiratória estão associados com a presença de hiperuricemia em escolares.
Cardiorespiratory fitness (CRF) is a strong predictor of mortality in adults. Its levels in adolescence are related to CRF in adulthood.1 Additionally, the incidence of low levels of CRF in children and adolescents has increased.2
Currently, CRF has been associated with the presence of metabolic syndrome in schoolchildren.3 It is known that low levels of CRF in childhood are predictors of metabolic risk; therefore, maintaining adequate levels of CRF since childhood is critical to reduce the incidence of metabolic syndrome in adulthood.4
Low levels of CRF have been associated with the classic parameters of metabolic syndrome in schoolchildren.5 Other non-traditional metabolic risk markers, such as uric acid, have also been associated with low levels of CRF, demonstrating that children and adolescents with low CRF levels have higher levels of this marker.6
However, the literature is scarce on the association between hyperuricemia, CRF levels, and the presence of overweight/obesity in children and adolescents, especially in Brazil. The identification of a nontraditional marker, such as uric acid, associated with low levels of CRF and excess weight in childhood and adolescence, may help pediatricians to make an early diagnosis, as well as other health professionals, particularly physical education and nutrition professionals, to create interventions aimed at preventing the occurrence of metabolic risk in adulthood. Moreover, the present study brings a new approach, as it jointly evaluates the levels of CRF and BMI of schoolchildren. The scarcity of information and the importance of the subject justified the development of the present study, which aimed to evaluate a possible association between hyperuricemia and cardiorespiratory fitness levels with nutritional status, grouped into a single variable, in schoolchildren.
MethodsA total of 2335 schoolchildren of both genders, stratified by conglomerates, participated in the study, of whom 80.3% lived in the urban area. Age ranged from 7 to 17 years (mean=11.5 years, standard deviation=2.8 years). All of them were elementary school students in the municipality of Santa Cruz do Sul, Rio Grande do Sul, Brazil, which has a total of 52 elementary and high schools, of which 19 are state, 29 are municipal, and four are private schools, with a population of 20,380 students. These data were obtained from the official municipal government agencies.
The present study is part of a larger research project entitled “Schoolchildren's Health – Phase III”, approved by the Research Ethics Committee (REC) of Universidade de Santa Cruz do Sul (UNISC) under CAAE No. 31576714.6.0000.5343, according to Resolution 466/12 of the National Health Council. All evaluations were conducted in the University grounds, by professionals qualified for each function. The schoolchildren were enrolled in the study only after their parents or guardians signed the informed consent form, and children older than 12 years also agreed to collaborate through a term of assent; the study only included those who had no restrictions on blood collection.
To calculate sample size, the G*Power 3.1 program (Heinrich-Heine-Universität – Düsseldorf, Germany) was used, considering Poisson regression as a statistical test (presence versus absence of hyperuricemia as a dependent variable). A test power (1−β)=0.95, significance level of α=0.05, and an effect size of 0.30, as indicated by Faul et al.,7 were considered. Therefore, a minimum sample of 655 students was estimated to comprise the sample.
In total, 25 schools public and private schools, both rural and urban, participated in the study. The assessed schools were selected by drawing lots, following the proportionality of the schoolchildren population density, according to the city region (Center, North, South, East, and West) and urban and rural area. Subsequently, the lists of students were obtained from the selected schools, and the students were drawn by lots for the signing of the informed consent form.
Schoolchildren enrolled in the selected schools, of both genders, aged between 7 and 17 years and who returned the signed consent form and term of assent were included in the study, totaling 2502 students. Of these, 167 subjects were excluded due to the inability to have blood collected and undergo the CRF test, or due to removal of discrepant data (outliers) from the database, after verification in the exploratory analysis.
The BMI was calculated through the formula BMI=weight/height2 (kg/m2). To assess weight and height, the students were measured/weighed barefoot and wearing light clothing. The BMI was classified according to the percentile curves by age and gender.8 The values obtained were reclassified into two categories: low-normal weight and overweight-obesity. CRF was evaluated by the 6-minute walk/run test. The students were previously advised to wear appropriate footwear and light clothing to undergo the test. The results were obtained in meters covered by the schoolchild and classified according to the tables of the Brazilian Sports Project (Projeto Esporte Brasil [PROESP-BR]) for gender and age, as in the healthy range (good CRF levels, fit) or in the risk range (low CRF; unfit).9
The nutritional status/CRF variable was categorized using the BMI and CRF data, obtained separately. The results were classified into four categories: (1) low-normal weight/fit; (2) low-normal weight/unfit; (3) overweight-obese/fit and (4) overweight-obese/unfit.
Pubertal stage was classified based on pubic hair in five categories as Tanner stage I (prepubertal), stage II (initial development), stage III (continuing maturation 1), stage IV (continuing maturation 2), or stage V (matured).10 A survey card with figures it was presented to the schoolchildren, where the subjects indicated the one that most closely resembled their stage of development.
Blood for uric acid measurement was collected from the brachial vein after a 12-hour fasting; the collection was made at the UNISC Exercise Biochemistry Laboratory by trained professionals (pharmacists and nursing technicians of the University), following biosafety standards. Uric acid levels in the serum of each patient were determined by the photometric enzymatic method, using a conventional reagent Kovalent and the Miura 200 automated system (I.S.E., Rome, Italy). Uric acid levels<5.5mg/dL were considered normal; levels ≥5.5mg/dL were considered hyperuricemia.
The data were analyzed using the statistical program SPSS (IBM Corp. Released 2013. IBM SPSS Statistics for Windows, Version 23.0, NY, USA). First, the Shapiro–Wilks test was used to test the normality of the continuous data. Descriptive analyses were expressed as frequencies and percentages. The chi-squared test was used to compare the descriptive characteristics of the students by gender. The association between the outcome variable (hyperuricemia) and the categorized variable nutritional status/CRF was tested using Poisson regression, using prevalence ratios (PR) and 95% confidence intervals (CI). The analyses were stratified by gender, being adjusted for the age group and pubertal stage. Differences were considered significant at p<0.05.
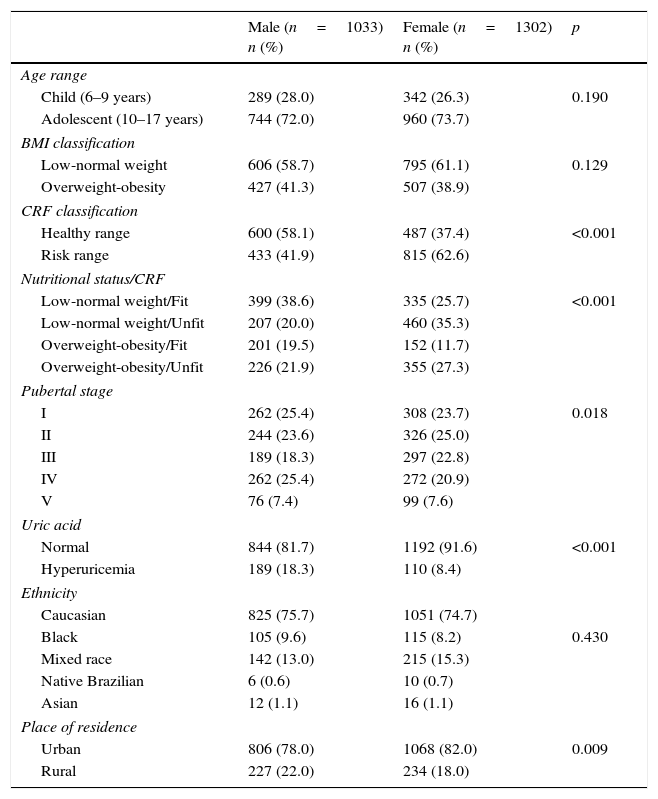
ResultsA total of 2335 students were evaluated. Regarding the association between the nutritional status and CRF, 21.9% of the boys and 27.3% of the girls were classified in the group overweight-obesity/unfit (Table 1).
Sample description. Santa Cruz do Sul, Brazil, 2014–2015.
| Male (n=1033) n (%) | Female (n=1302) n (%) | p | |
|---|---|---|---|
| Age range | |||
| Child (6–9 years) | 289 (28.0) | 342 (26.3) | 0.190 |
| Adolescent (10–17 years) | 744 (72.0) | 960 (73.7) | |
| BMI classification | |||
| Low-normal weight | 606 (58.7) | 795 (61.1) | 0.129 |
| Overweight-obesity | 427 (41.3) | 507 (38.9) | |
| CRF classification | |||
| Healthy range | 600 (58.1) | 487 (37.4) | <0.001 |
| Risk range | 433 (41.9) | 815 (62.6) | |
| Nutritional status/CRF | |||
| Low-normal weight/Fit | 399 (38.6) | 335 (25.7) | <0.001 |
| Low-normal weight/Unfit | 207 (20.0) | 460 (35.3) | |
| Overweight-obesity/Fit | 201 (19.5) | 152 (11.7) | |
| Overweight-obesity/Unfit | 226 (21.9) | 355 (27.3) | |
| Pubertal stage | |||
| I | 262 (25.4) | 308 (23.7) | 0.018 |
| II | 244 (23.6) | 326 (25.0) | |
| III | 189 (18.3) | 297 (22.8) | |
| IV | 262 (25.4) | 272 (20.9) | |
| V | 76 (7.4) | 99 (7.6) | |
| Uric acid | |||
| Normal | 844 (81.7) | 1192 (91.6) | <0.001 |
| Hyperuricemia | 189 (18.3) | 110 (8.4) | |
| Ethnicity | |||
| Caucasian | 825 (75.7) | 1051 (74.7) | |
| Black | 105 (9.6) | 115 (8.2) | 0.430 |
| Mixed race | 142 (13.0) | 215 (15.3) | |
| Native Brazilian | 6 (0.6) | 10 (0.7) | |
| Asian | 12 (1.1) | 16 (1.1) | |
| Place of residence | |||
| Urban | 806 (78.0) | 1068 (82.0) | 0.009 |
| Rural | 227 (22.0) | 234 (18.0) | |
BMI, body mass index; CRF, cardiorespiratory fitness; chi-squared test, considering the comparison between genders.
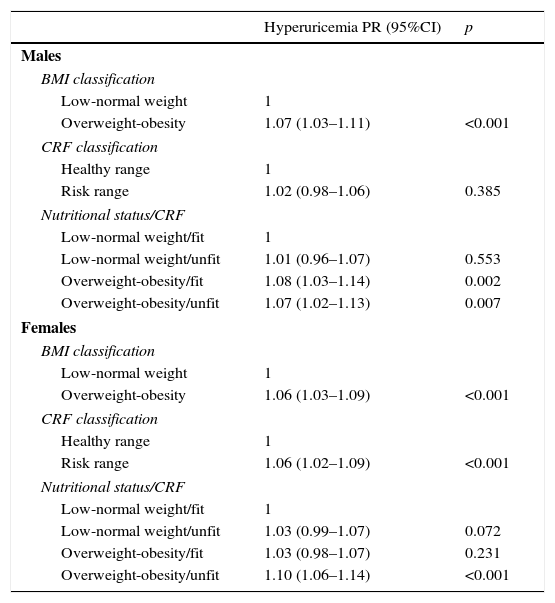
When hyperuricemia was separately associated with nutritional status, it was observed that overweight or obese male students had a higher prevalence of hyperuricemia when compared was students with low or normal weight (PR: 1.07, p<0.001). This association was also verified among girls, this (PR: 1.06, p<0.001). When associated with CRF, unfit girls had higher prevalence of hyperuricemia than fit girls (PR: 1.06, p<0.001; Table 2).
Association between hyperuricemia and CRF with BMI.
| Hyperuricemia PR (95%CI) | p | |
|---|---|---|
| Males | ||
| BMI classification | ||
| Low-normal weight | 1 | |
| Overweight-obesity | 1.07 (1.03–1.11) | <0.001 |
| CRF classification | ||
| Healthy range | 1 | |
| Risk range | 1.02 (0.98–1.06) | 0.385 |
| Nutritional status/CRF | ||
| Low-normal weight/fit | 1 | |
| Low-normal weight/unfit | 1.01 (0.96–1.07) | 0.553 |
| Overweight-obesity/fit | 1.08 (1.03–1.14) | 0.002 |
| Overweight-obesity/unfit | 1.07 (1.02–1.13) | 0.007 |
| Females | ||
| BMI classification | ||
| Low-normal weight | 1 | |
| Overweight-obesity | 1.06 (1.03–1.09) | <0.001 |
| CRF classification | ||
| Healthy range | 1 | |
| Risk range | 1.06 (1.02–1.09) | <0.001 |
| Nutritional status/CRF | ||
| Low-normal weight/fit | 1 | |
| Low-normal weight/unfit | 1.03 (0.99–1.07) | 0.072 |
| Overweight-obesity/fit | 1.03 (0.98–1.07) | 0.231 |
| Overweight-obesity/unfit | 1.10 (1.06–1.14) | <0.001 |
PR, prevalence ratio; CI, confidence interval; analysis adjusted for age and pubertal stage; BMI, body mass index; CRF, cardiorespiratory fitness.
An association, albeit small, was observed between hyperuricemia and nutritional status/CRF grouped into a single variable, adjusted for age and pubertal stage (Table 2). Unfit male schoolchildren with excess weight had a higher prevalence of hyperuricemia when compared with fit individuals with normal weight (PR: 1.07, p=0.007). Similar values were found for boys with excess weight, but who had good CRF (PR: 1.08, p=0.002). Among the assessed girls, significant differences were found only when comparing overweight and poor CRF with normal weight and good CRF levels (PR: 1.10, p<0.001).
DiscussionThe present study demonstrated, albeit subtly, that the presence of hyperuricemia was more prevalent in overweight and obese schoolchildren, in both boys and girls, as well as in girls with poor CRF. Moreover, a higher prevalence of hyperuricemia was observed in schoolchildren of both genders who had both overweight/obesity and low levels of CRF.
It is noteworthy that, although this association is small, it is relevant, since the sample comprised children and adolescents and changes in uric acid levels are more frequently observed in the adult population. It is known that, in these individuals, serum uric acid levels are associated with several cardiometabolic risk factors, and hyperuricemia is predictive of obesity development.11 Although uric acid has an antioxidant function in the extracellular environment, it has detrimental effects when it enters the cells. Its detrimental impact includes endothelial function inhibition, platelet aggregation induction, and chronic systemic inflammation, among others.12,13
Based on the results found, it a higher prevalence of hyperuricemia was observed in schoolchildren of both genders with excess weight, corroborating the current literature. Lee et al.,14 when assessing 2284 children aged 6–12 years in 104 schools in 13 cities in Taiwan, found that hyperuricemia is associated with metabolic syndrome, mainly with obesity and abdominal fat. In a study of 2614 school-aged children (4–18 years) in the city of Bogalusa, United States, Sun et al.13 evaluated the association between uric acid and the individual components of the metabolic syndrome; elevated levels of uric acid showed a stronger association with BMI.
One explanation for the association between body fat and hyperuricemia would be the increase in the production of keto acids during the night in individuals with more body fat, which would cause competition between the acids for renal excretion, in addition to a possible genetic predisposition to lower excretion of uric acid in some ethnic groups.14 Nishida et al. explained the association of excess weight with hyperuricemia due to increased production of uric acid together with the synthesis of triglycerides and decreased excretion of uric acid as a result of hyperinsulinemia, which accompanies obesity.12
The present study showed that only girls who are in the CRF risk zone had a higher prevalence of hyperuricemia. No significant associations were found in the male gender, which corroborates a study carried out with individuals of all ages treated at the Cardiovascular Healthare Program of Universidade Federal de Viçosa, Minas Gerais, Brazil, which indicated differences between the genders when uric acid alterations are associated with biomarkers of cardiometabolic risk, such as excess weight and sedentary lifestyle.15
These alterations may be associated with the fact that boys and girls differ in several parameters, such as insulin resistance, inflammatory markers, and amino acid catabolism products.16 Additionally, girls have a higher percentage of body fat, lower levels of CRF, and are less active,17 which may influence the obtained results.
When associated with CRF and nutritional status, the prevalence of hyperuricemia is higher in individuals with excess weight combined with low levels of CRF. This was observed in both boys and girls. In the male gender, it was observed that students with excess weight and with good levels of CRF also have a higher prevalence of hyperuricemia. Thus, excess weight appears to carry a higher health risk behavior than adequate levels of CRF. Nonetheless, Barnett et al., in a study of 1045 children (aged 7–12 years) in New York, United States, associated inadequate levels of CRF with obesity, considering both as risk factors for cardiovascular diseases; moreover, CRF classified in the risk zone in adolescence was associated with high percentages of body fat in adulthood.18
In Spain, a study has shown that children with low levels of CRF have higher levels of uric acid.6 Notwithstanding, little is known about the influence of physical fitness, since there are few studies associating physical exercise and serum uric acid levels in children and adolescents.
Nishida et al.,12 in Nabeshima, Japan, verified the influence of physical activity intensity and aerobic fitness on uric acid concentration in 71 obese adult men. Moderate physical activity intensity was associated with lower levels of uric acid. Villegas et al.19 verified the prevalence and risk factors of hyperuricemia in 3978 adult men (40–74 years) in Shanghai, China. Physical activity was inversely associated with hyperuricemia prevalence, whereas BMI and weight gain were positively associated. Williams,20 in the United States, analyzed the distance covered per week and coronary risk factors in 8282 male runners (15–80 years). The data were obtained through a questionnaire, while the levels of biochemical data, including uric acid, were obtained from data provided by the runners’ physicians. Among the conclusions, it was observed that individuals who ran greater distances had lower levels of uric acid, which may be associated with better levels of CRF.
Studies with children and adolescents that associated CRF and uric acid levels are very scarce. The present study brings important contributions to the understanding of uric acid behavior in relation to clinical and metabolic variables. It is noteworthy the representative number of subjects, children and adolescents, whose data were scarcely available to date. The authors believe it is important to add the measurement of serum uric acid levels to the evaluation protocols in children and adolescents, as it may allow the assessment of possible cardiovascular complications in the beginning of their development. Barnett et al. also emphasize the importance of physical education classes at school as a way to increase the CRF levels in children and adolescents. This can be achieved by promoting not only their participation in physical activity classes, but also in activities that lead to CRF improvement, bringing benefits both in childhood and adolescence, as well as in adult life.18
The present study contributes with knowledge in the area, since the association of uric acid levels with CRF and excess weight in children and adolescents is still poorly established, especially in Brazil. The association between elevated uric acid levels with CRF and nutritional status may help healthcare professionals in the diagnosis, treatment, and prevention of metabolic risk conditions in childhood. Additionally, the study brings a new approach by grouping the CRF and BMI variables into a single one. Another strength of the study is the fact that it contains a representative sample of a population that lacks research.
However, the study has limitations. Due to its cross-sectional design, it is not possible to establish cause and effect associations, but only associations between variables, which may be influenced by intervening factors, such as the students’ food consumption and the presence of arterial hypertension, not assessed in the present study; these variables could indirectly influence serum uric acid levels. It is also known that obesity is a multifactorial condition, which can also be influenced by other inflammatory markers.
The use of BMI should also be considered, since it is not the best parameter to evaluate obesity. Although useful in studies with a representative sample, as in the present study, it does not differentiate between lean and fat body mass. Further studies that can correlate the intensity of exercise with changes in serum uric acid levels are suggested, so that it may be possible to explore the beneficial effects of an active lifestyle on hyperuricemia.
Hyperuricemia showed a slight association with the presence of excess weight and low levels of CRF, when assessed together. The measurement of uric acid in childhood may be a useful public healthcare measure for early intervention and prevention of future cardiovascular complications.
FundingUniversidade de Santa Cruz do Sul (UNISC).
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Reis LN, Renner JD, Reuter CP, Horta JA, Paiva DN, Valim AR, et al. Hyperuricemia is associated with low cardiorespiratory fitness levels and excess weight in schoolchildren. J Pediatr (Rio J). 2017;93:538–43.
Study carried out at Universidade de Santa Cruz do Sul (UNISC), Santa Cruz do Sul, RS, Brazil.