This study aimed to investigate human adenovirus 36 (Adv36) as an associated factor for adiposity in children and adolescents aged 9–12 years.
MethodsThis was a case-control study comparing overweight (cases) and eutrophic (controls) children and adolescents aged 9–12 years based on their body mass index in relation to human adenovirus 36 serology. Human adenovirus 36-specific neutralizing antibodies were assessed using the serum neutralization assay, and a questionnaire regarding the subjects’ personal backgrounds, breastfeed history, age of starting daycare, and eating and exercise habits was also applied.
ResultsA total of 101 (51, eutrophic; 50, overweight) children were included in the study. The Adv36 seropositivity rate was of 15.8%, which increased the chance of being overweight by 3.17 times (p=0.049). Enrollment in a full-time daycare center before the age of 24 months increased the chance of being overweight by 2.78 times (p=0.027). Metabolic parameters (total cholesterol and blood glucose) were insignificantly different among children who were seropositive or seronegative for human adenovirus 36.
ConclusionThis study concluded that excessive weight was positively associated with seropositivity for human adenovirus 36. Early enrollment in a full-time daycare was also an associated factor for obesity. Such data, confirmed in new studies, reinforces the role of human adenovirus 36 in the increase of childhood adiposity.
Childhood obesity has been increasing worldwide.1–3 Data from the Nutritional Surveillance System in 2016 emphasized that 17.53% of Brazilian adolescents are overweight, 6.85% are obese, and 1.48% suffer from severe obesity.4 The increase in the prevalence of childhood obesity results in a higher occurrence of comorbidities not only in adulthood but also in children, which include systemic arterial hypertension, early atherosclerosis, type 2 diabetes mellitus, and sleep disorders.5 Children with obesity are at high risk of acquiring chronic diseases early in life, which subsequently increases the rates of complications in the medium- and long term.6
Obesity is a multifactorial disease, representing an interaction between genetic characteristics, metabolism, lifestyle, and environmental factors.7,8 Despite the existing factors for obesity, the exponential increase in obesity and its occurrence in individuals who apparently do not have these determinants seem to be influenced by external factors, regardless of eating habits, which can be transmitted from person to person, such as infection of human adenovirus 36 (Adv36).9
In addition to the factors already known to be associated with obesity, Adv36 has been shown to be a factor also involved in the genesis of this disease.10 The first study that evaluated the prevalence of Adv36 infection in humans indicated a seropositivity for this virus in 30% and 11% of obese and nonobese patients, respectively.9
Data from different countries have already demonstrated the association between Adv36 and obesity and overweight.11–14 However, no studies assessed the association between Adv36 and childhood obesity and overweight in Brazil. This study aimed to evaluate Adv36 as an associated factor for childhood adiposity and to determine some factors known to be associated with this disease.
MethodsThis was a case-control study with a sample selected through the nutritional classification of children aged 9–12 years from elementary schools of Southern Brazil in 2018. For the sample calculation, two-sided/two-sample test of proportions, which were projected to provide 80% power, were used. The authors aimed to enroll a minimum of 50 children with excessive weight (obesity and overweight) to detect a 33% seroprevalence rate in children with excessive weight, while the seroprevalence rate of eutrophic children was assumed to be in the range of 1%–10%.15
The selected age follows the guidelines of the American Academy of Pediatrics for the start of metabolic screening without risk factors, in order to avoid blood collection only for the purposes of the study. Exclusion criteria were factors that might lead to the misclassification of a child’s weight status, i.e., an acute or chronic illness affecting weight, genetic conditions associated with obesity or failure to thrive, and/or use of medications associated with weight gain or weight loss. Children with Tanner’s stage greater than II were excluded, as they could have already started the process of growth spurt, which could determine significantly large differences in body composition. An informed consent was signed by the parents/guardians of all subjects. Written assent was obtained from all children. This study was approved by the Research Ethics Committee of the Universidade do Sul de Santa Catarina, following the ethical standards of Ethics Resolution 466 of 2012, under report number 2,051,799 on May 8, 2017.
A questionnaire about birth and neonatal history, mother’s gestational history, daycare starting age, and immunization status was given to all parents of children enrolled in study. An interview was conducted with the participating children, using the validated Physical Activity List (PAL) questionnaire. The PAL questionnaire estimates the total time in physical activity (minutes), total time in sedentary activity (minutes), total metabolic cost, and weighted metabolic cost. The values of adequate physical activity duration (60min daily) and adequate duration of screen use (less than 2h a day) were established in accordance with those indicated by the World Health Organization (WHO).
Height was measured to the nearest 1mm by using a wall-mounted stadiometer, and weight was measured to the nearest 0.1kg by using a scale. With the subject standing erect with their arms at the side and feet together, waist circumference was measured with a non-stretchable tape midway between the inferior costal margin and the superior border of the iliac crest. Hip circumference was measured at the point yielding the maximal circumference over the buttocks. The neck circumference was measured at the midpoint of the neck height.
Body mass index (BMI) was calculated as weight (in kg) divided by height (in m) squared. The WHO Z-scores growth curves of BMI according to age and sex were used for the nutritional classification of children. Cases were children with BMI greater than Z-score +1, considered with weight excess (Z-score from +1 to +2 were classified as overweight and greater than +2, as obese), and control were children with BMI from Z-score −2 to +1, considered eutrophic.
The studied children were sent to the laboratory to undergo several examinations. Fasting blood was collected to assess total cholesterol, high-density lipoprotein fraction, triglyceride, and blood glucose levels. An aliquot of the serum was centrifuged, frozen and stored at −80°C to perform the serology for Adv36.
The test for Adv36 detection was conducted in partnership with Texas Tech University using the technique developed by the researchers.16 It comprised the use of samples of human Adv36 (ATCC– Manassas, VA, United States) that grow on A549 cells (human lung carcinoma cell line). Initially, a purified stock of Adv36 was prepared to perform the neutralizing antibody test in the serum. The test serum was inactivated in water at 56°C for 30min and diluted serially from 1:2 to 1:512 in 96-well plates. The virus particles were added to each well in a TCID50 amount (50% of the infectious dose in tissue culture). After 1h of incubation at 37°C, A549 cells were added to the wells and tested in duplicate. For each test, a serum control (patient serum and cells), cell control (cells only), and virus control (cells and viruses) were available. The 96-well plates were subsequently incubated at 37°C for another 11 days, and the presence of a cytopathic effect (CPE) was observed. The absence of CPE at 1:8 dilutions of serum or greater was considered as a positive sample for the presence of neutralizing antibodies for Adv36. In the samples sent, toxicities of the serum to cells and in the serum control until 1:16 were observed (an unexplained phenomenon that is experienced in some sample sets). Thus, the laboratory increased the dilution to be positive, as lack of CPE in serum dilution of 1:32 or higher, in both replicates, making the test more stringent. The entire set of 101 samples was tested at least twice (each with two technical replicates) for the presence of neutralizing antibodies. Both sets yielded identical results.
In the descriptive analysis, quantitative and qualitative variables were described. The Shapiro–Wilk normality test was used to verify whether the following data were normally distributed: body weight (kg), body height (cm) and BMI Z-score (kg/(E[m])2 Case and control groups were compared through mean, standard deviation, and Student’s t-test for independent samples when the distribution was considered normal with significance level of p<0.05.
Binomial logistic regression was also performed, estimating the isolated effect of age, sex, Adv36 serology status, daycare starting age, and duration of exclusive and total breastfeeding in actual nutritional status. From binomial regression, gross and adjusted odds ratios and 95% confidence intervals (95% CI) were obtained. A p-value <0.05 was considered significant. All statistical analyses were performed using the statistical software package SPSS, version 21.0.
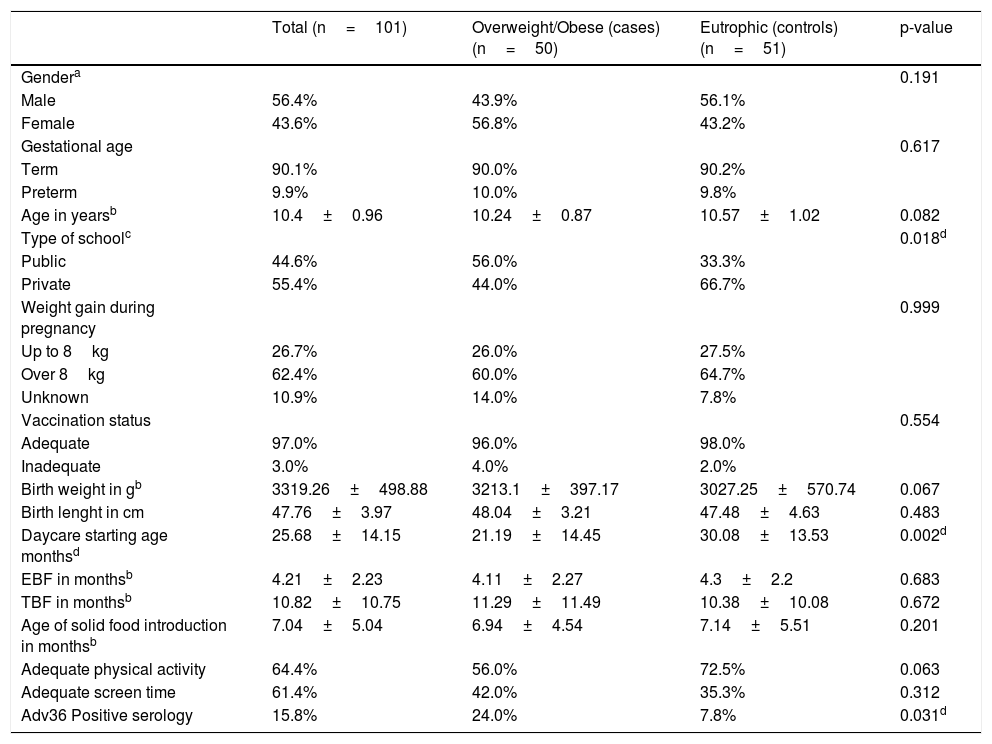
ResultsThe total sample included 101 children. The prevalence rate of positive serology for Adv36 in the sample was 15.8%. The seropositivity prevalence for Adv36 was significantly higher in children with excessive weight (24%) than that in eutrophic children (7.8%; p=0.02). The demographic and background characteristics of the sample are presented in Table 1.
Characteristics of the study population.
| Total (n=101) | Overweight/Obese (cases) (n=50) | Eutrophic (controls) (n=51) | p-value | |
|---|---|---|---|---|
| Gendera | 0.191 | |||
| Male | 56.4% | 43.9% | 56.1% | |
| Female | 43.6% | 56.8% | 43.2% | |
| Gestational age | 0.617 | |||
| Term | 90.1% | 90.0% | 90.2% | |
| Preterm | 9.9% | 10.0% | 9.8% | |
| Age in yearsb | 10.4±0.96 | 10.24±0.87 | 10.57±1.02 | 0.082 |
| Type of schoolc | 0.018d | |||
| Public | 44.6% | 56.0% | 33.3% | |
| Private | 55.4% | 44.0% | 66.7% | |
| Weight gain during pregnancy | 0.999 | |||
| Up to 8kg | 26.7% | 26.0% | 27.5% | |
| Over 8kg | 62.4% | 60.0% | 64.7% | |
| Unknown | 10.9% | 14.0% | 7.8% | |
| Vaccination status | 0.554 | |||
| Adequate | 97.0% | 96.0% | 98.0% | |
| Inadequate | 3.0% | 4.0% | 2.0% | |
| Birth weight in gb | 3319.26±498.88 | 3213.1±397.17 | 3027.25±570.74 | 0.067 |
| Birth lenght in cm | 47.76±3.97 | 48.04±3.21 | 47.48±4.63 | 0.483 |
| Daycare starting age monthsd | 25.68±14.15 | 21.19±14.45 | 30.08±13.53 | 0.002d |
| EBF in monthsb | 4.21±2.23 | 4.11±2.27 | 4.3±2.2 | 0.683 |
| TBF in monthsb | 10.82±10.75 | 11.29±11.49 | 10.38±10.08 | 0.672 |
| Age of solid food introduction in monthsb | 7.04±5.04 | 6.94±4.54 | 7.14±5.51 | 0.201 |
| Adequate physical activity | 64.4% | 56.0% | 72.5% | 0.063 |
| Adequate screen time | 61.4% | 42.0% | 35.3% | 0.312 |
| Adv36 Positive serology | 15.8% | 24.0% | 7.8% | 0.031d |
EBF, exclusive breastfeeding; TBF, total breastfeeding.
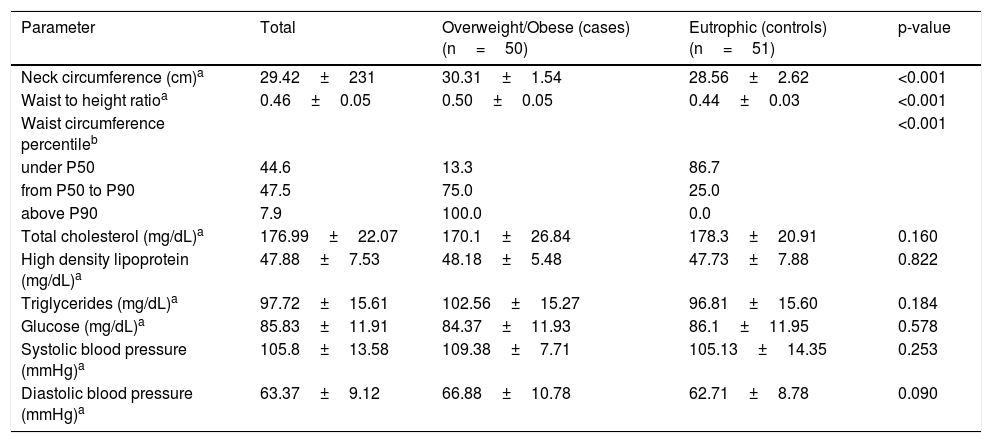
Regarding cardiovascular risk parameters, neck circumference, waist-to-height ratio, and abdominal circumference were higher in overweight children than in eutrophic children. Other cardiovascular risk parameters of the sample are presented in Table 2.
Cardiovascular risk factors among children enrolled in the study.
| Parameter | Total | Overweight/Obese (cases) (n=50) | Eutrophic (controls) (n=51) | p-value |
|---|---|---|---|---|
| Neck circumference (cm)a | 29.42±231 | 30.31±1.54 | 28.56±2.62 | <0.001 |
| Waist to height ratioa | 0.46±0.05 | 0.50±0.05 | 0.44±0.03 | <0.001 |
| Waist circumference percentileb | <0.001 | |||
| under P50 | 44.6 | 13.3 | 86.7 | |
| from P50 to P90 | 47.5 | 75.0 | 25.0 | |
| above P90 | 7.9 | 100.0 | 0.0 | |
| Total cholesterol (mg/dL)a | 176.99±22.07 | 170.1±26.84 | 178.3±20.91 | 0.160 |
| High density lipoprotein (mg/dL)a | 47.88±7.53 | 48.18±5.48 | 47.73±7.88 | 0.822 |
| Triglycerides (mg/dL)a | 97.72±15.61 | 102.56±15.27 | 96.81±15.60 | 0.184 |
| Glucose (mg/dL)a | 85.83±11.91 | 84.37±11.93 | 86.1±11.95 | 0.578 |
| Systolic blood pressure (mmHg)a | 105.8±13.58 | 109.38±7.71 | 105.13±14.35 | 0.253 |
| Diastolic blood pressure (mmHg)a | 63.37±9.12 | 66.88±10.78 | 62.71±8.78 | 0.090 |
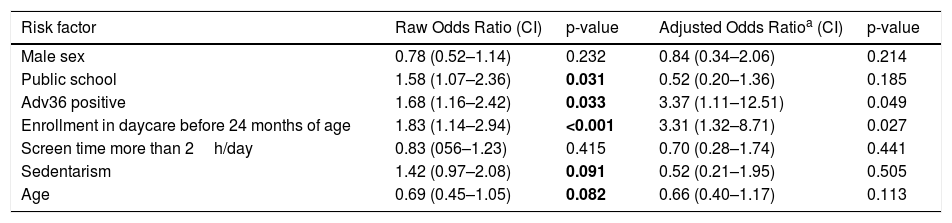
The analysis of daycare starting age was shown to be significant, with a higher mean age in overweight children than in eutrophic children. Two cutoff points were considered for the age at which the child started attending daycare, one after 24 months and the other after 36 months. The risk analysis performed using a binary logistic regression test showed that children who started attending daycare before 24 months were 2.8 times more likely to be overweight at 9–11 years old compared to children who did so after 24 months (95% confidence interval [CI], 1.12–6.92). When the 36-month cutoff point was used, this risk was 1.2 times greater (95% CI, 0.51–4.79).
The positive serology for Adv36 determined a 3.17 times greater chance of being overweight at school age (95% CI, 1.85–11.79). Table 3 shows the raw and adjusted odds ratio according to binomial logistic regression of associated factors with obesity. Besides the fact that positive serology for Adv36 was more frequently among children who started attending daycare before 24 months of age, no other statistically significant association was observed (p=0.142).
Risk factors for excess of weight.
| Risk factor | Raw Odds Ratio (CI) | p-value | Adjusted Odds Ratioa (CI) | p-value |
|---|---|---|---|---|
| Male sex | 0.78 (0.52–1.14) | 0.232 | 0.84 (0.34–2.06) | 0.214 |
| Public school | 1.58 (1.07–2.36) | 0.031 | 0.52 (0.20–1.36) | 0.185 |
| Adv36 positive | 1.68 (1.16–2.42) | 0.033 | 3.37 (1.11–12.51) | 0.049 |
| Enrollment in daycare before 24 months of age | 1.83 (1.14–2.94) | <0.001 | 3.31 (1.32–8.71) | 0.027 |
| Screen time more than 2h/day | 0.83 (056–1.23) | 0.415 | 0.70 (0.28–1.74) | 0.441 |
| Sedentarism | 1.42 (0.97–2.08) | 0.091 | 0.52 (0.21–1.95) | 0.505 |
| Age | 0.69 (0.45–1.05) | 0.082 | 0.66 (0.40–1.17) | 0.113 |
CI, confidence interval. Binary logistic regression.
The analysis of serum lipid rates in overweight children according to their seropositivity for Adv36 is shown in Table 4. No statistically significant difference was observed between the groups.
Glucose and serum lipid in children with adiposity according to their seropositivity for Adv36.
Childhood obesity is a multifactorial disease.17 A simple imbalance in the energy balance is inconsistent with the available findings on the etiology, prevention, and treatment of childhood obesity. Thus, Adv36 has been studied as an infectious agent that can promote obesity in children.14,18–20 This is the first study in Brazil that analyzed the seropositivity rate of Adv36 and its association with childhood obesity.
The present study found that the seropositivity of Adv36 was 15.8%, in a sample comprising 50% of overweight children and 50% of eutrophic children. The same seropositivity prevalence as the current study was reported by Gabbert et al.,13 which confirmed a seropositivity of 15% in a sample of 50% of obese children and 50% of eutrophic children. A study by Dusatkova et al.12 showed a seropositivity rate of 30%, but this study comprised a sample of children with only obesity. Furthermore, in a study conducted in Korean children, the seropositivity rate was 35.6%, with a sample comprising 84% of children with obesity.21 The study that showed the highest prevalence of seropositivity for Adv36 (70%) was conducted in Hispanic adolescents, with a sample of only 40 subjects.14
When analyzing the association between seropositivity for Adv36 and nutritional status, the data in most studies are relatively similar to that found in the present study, with higher rates of seropositivity observed in obese children (27%–30%) than in non-obese children (8%–15%).10,13,22 The risk analysis showed that the positive Adv36 serology determined a 3.17 times greater chance of being overweight among individuals infected with Adv36. Although correlational, such data demonstrate several similarities in the distribution profile of Adv36 in studies conducted in several countries worldwide, and are consistent with the theory of an infectious cause of obesity. Here, the homogeneity between cases and controls is emphasized, except for the characteristics of seropositivity for adenovirus, daycare starting age, and type of school (whether public or private).
The association between adenoviruses and obesity has been studied since 1992, with the publication of the first article evaluating the association between obesity in chickens and SMAM-139, a virus similar in effect to the human Adv36. Since then, studies conducted in humans have strengthened the hypothesis of obesity of infectious cause; Adv36 has been studied both in the scope of obesity and in the glycemic and lipid control in individuals who are infected with Adv36.13,16,23
The mechanism by which Adv36 triggers obesity is not yet fully understood. Adv36 acts in different ways: it stimulates the conversion of glucose into fatty acids, increases fat transport in adipocytes and glucose uptake through glucose transporter type 1 (GLUT1) and glucose transporter type 4 (GLUT4),24 and activates the PPAR-gamma signaling pathway to promote stem cell differentiation into adipocytes.
The activation of the glucose transporters GLUT1 and GLUT4 appears to be an important mechanism in the decrease in glucose levels, mediated by the Adv4 E4orf1 gene.24 In in vitro studies, this gene has been shown to act as an activator of GLUT4, which determines greater glucose entry into the cells, independent of insulin. This effect, coupled with reduced insulin requirement, leads to a decrease in systemic glucose levels, but it can also lead to an increase in the accumulation of intracellular energy, leading to excess weight. Adv36 also increases the commitment and differentiation of adipose tissue-derived stromal vascular cells to adipogenic lineage and promotes lipid accumulation. It is conceivable that Adv36 promotes adipogenesis in children exposed to the virus.
The present study did not present the expected result regarding the levels of total cholesterol and glycemia. Lower levels of glucose and serum lipids in obese or eutrophic subjects were not associated with Adv36 positive serology, as demonstrated by Sapunar et al.19 This result is probably a reflection of the small number of Adv36-positive subjects.
In a recent review by Kim et al.,25 the role of Adv36 in the genesis of obesity was reaffirmed, and the mechanism involved remains unclear. The E4orf1 protein, derived from Adv36, is responsible for increasing glucose uptake due to the translocation of GLUT4, which is involved in ‘distal’ insulin signaling. Adv36 appears to determine a type of obesity that, even related to high levels of adiponectin and low leptin, reduces the levels of blood lipids and glucose. It may also determine high levels of interleukin-6 and adiponectin and low levels of leptin, through a mechanism not yet known.19,20
According to the result of the present study, children who started day care before 24 months were 2.8 times more likely to be overweight when compared with children who did so after 24 months, and this study result was proven to be relevant even after performing a logistic regression analysis to adjust for the confounding factors. Considering that adenoviral infections commonly happen in childhood, it would be possible that earlier start to preschool results in earlier exposure to Adv36 and hence, longer time for excess adiposity to develop by the school age of 5 years old, if indeed Adv36 promotes adiposity. A study conducted in Denmark with children attending day care from 0 to 6 years old showed that the elimination of viruses in feces in 1 year was 34%, and adenovirus wasobserved in 3.4% of children. Adenovirus was the most prevalent respiratory virus in children aged less than 2 years, and half of these children were asymptomatic.26
Based on the data analysis, the risk associated with starting attending daycare before 24 months requires special attention, even though the sample size was calculated based on Adv36 serology. The data on the increased risk of obesity in children who started daycare early (before the age of 3 years) were observed in the study by Black et al.; according to their study, the factors involved in the increased risk of obesity should be comprehensively assessed.27
When analyzed in isolation, attending public school was considered a risk factor for excess weight according to a logistic regression analysis. These data were demonstrated by a large study conducted in France with 7154 children aged 11–15 years. According to this study, socioeconomic status was inversely associated with obesity.28 In a study conducted in Italy with 77,113 children aged 11–15years, obesity was associated with parents’ lower educational level,29 an association not observed in the present study.
The main limitation of this study was the sample size; however, the minimum calculated sample has been successfully obtained. Different from the other studies, the association of Adv36 with lower total cholesterol or blood glucose levels was not demonstrated; further studies with larger sample sizes and conducted in different countries are required. Due to ethical reasons, humans cannot be infected with Adv36 to unequivocally determine the adipogenic effect of Adv36. It would appear that existing data sustain the relationship of Adv36 with human obesity in adults and children.
Conflicts of interestThe authors declare no conflicts of interest.