To assess the prevalence of acute kidney injury in pediatric intensive care unit according to diagnostic criteria – pediatric risk, injury, failure, loss, end-stage renal disease, Acute Kidney Injury Network and Acute Kidney Injury Work Group, or Kidney Disease: Improving Global Outcomes –, and determining factors associated with acute kidney injury as well as its outcome.
MethodologyThis was a cross-sectional monocentric observational study, including patients aged between 29 days and 17 years who were admitted to the pediatric intensive care unit between January 1, 2012 and December 31, 2016. To evaluate the association between the study variables and acute kidney injury, the log-binomial generalized univariate and multivariate linear models were adjusted.
ResultsThe study included 1131 patients, with prevalence of acute kidney injury according to the Acute Kidney Injury Network and Kidney Disease: Improving Global Outcomes criteria of 12.6% and of 12.9% according to the pediatric risk, injury, failure, loss, end-stage renal disease. In the multivariate analysis of older children (PR 1.007, 95% CI: 1.005–1.009), sepsis (PR 1.641, 95% CI: 1.128–2.387), demand for ventilatory support (PR 1.547, 95% CI: 1.095–2.186), and use of vasoactive amines (PR 2.298, 95% CI: 1.681–3.142) constituted factors associated with statistical significance to the development of acute kidney injury. The mortality rate among those with acute kidney injury was 28.7%.
ConclusionOlder children, diagnosis of sepsis, demand for ventilatory support, and use of vasoactive amines were correlated with a higher risk of developing acute kidney injury. The mortality associated with acute kidney injury was elevated; it is crucial that all measures that ensure adequate renal perfusion are taken for patients with risk factors, to avoid the installation of the disease.
Acute kidney injury (AKI) is a disorder of multiple causes, with manifestations ranging from minimal increase in serum creatinine to kidney failure. It is a common complication in patients admitted to an intensive care unit (ICU), and its incidence varies according to the population assessed and criteria used. Studies demonstrate that up to one-third of the children hospitalized1,2 and between 5% and 89% of the children hospitalized in pediatric intensive care unit (PICU) have some degree of AKI.3–7
AKI is associated with long hospital stay, prolonged ICU stay, and need for mechanical ventilation (MV), with mortality between 8% and 89%.2,8,9 Additionally, children who survive AKI have a great propensity to develop residual kidney abnormalities. Proteinuria, hypertension, and reduced glomerular filtration rate (GFR) may persist in up to 60% of the survivors.2
The recognition of the factors associated with AKI and its early diagnosis through a validated criterion, as well as the understanding of its severity and its possible consequences, are extremely important to elaborate preventive and therapeutic strategies.
This study aimed to evaluate the prevalence of AKI among the population admitted to the PICU of a tertiary university hospital, according to the diagnostic criteria available in the literature, comparing the accuracy of each criteria for early diagnosis, and to determine which factors are associated with kidney injury, as well as its outcome. Finally, it should be noted that a large proportion of patients had been diagnosed with a complex chronic condition (86%), a variable not evaluated in the studies found in the literature on AKI in pediatrics.
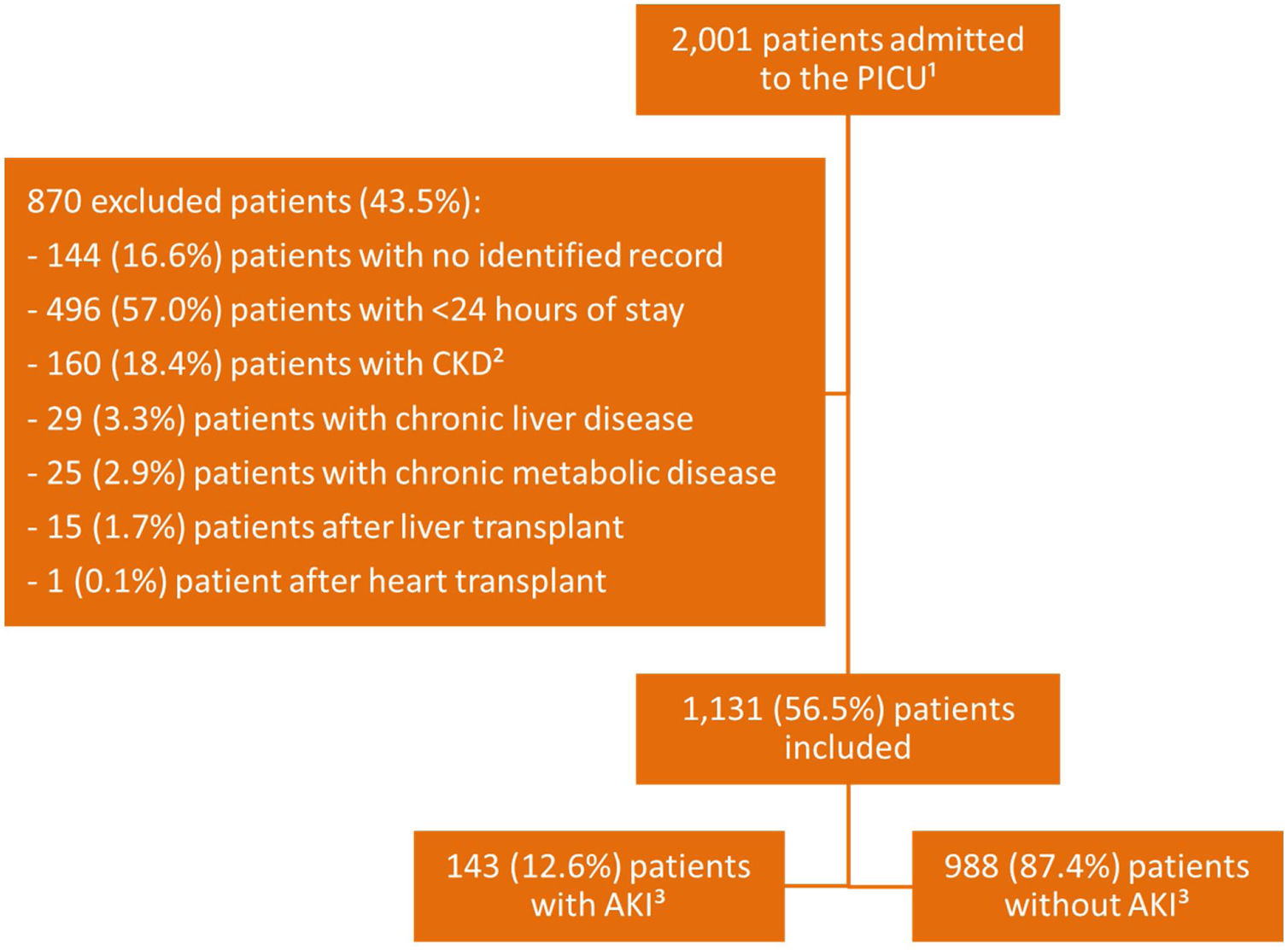
MethodsThis was a cross-sectional monocentric observational study, in which patients aged between 29 days and 17 years were admitted to the PICU between January 1, 2012 and December 31, 2016. The following groups were not considered for this study: newborns; patients diagnosed with chronic kidney disease (CKD) or chronic liver disease, due to the possibility of renal involvement pre-established by the evolution of the underlying disease; patients diagnosed with severe metabolic disease with previous kidney injury and postoperative renal transplantation, liver and heart disease, due to the possibility of previous renal involvement; patients without record of serum creatinine dosage; patients whose data recorded in the medical record throughout the follow-up lacked the necessary information for the study; and/or those who remained hospitalized for less than 24h.
During the characterization of patients, the following variables were evaluated: age; gender; type of admission (clinical or surgical); presence of onco-hematologic disease and/or complex chronic condition, recorded when the patient presented any condition in which the duration of the pathology was expected to be at least 12 months (except when the patient progressed to death) and that had compromised any organ system or organ sufficiently to require the care of a pediatric specialty10; risk of tumor lysis; Pediatric Index of Mortality 3 (PIM3); sepsis11; use and duration of nephrotoxic medications (divided into group 1: antibiotics=aminoglycoside, vancomycin, polymyxin, sulfonamide, rifampicin, beta-lactams, cephalosporin, and quinolone; group 2: antifungals=conventional amphotericin, amphotericin lipid complex, liposomal amphotericin, voriconazole, fluconazole, micafungin, and caspofungin; group 3: antivirals=acyclovir, ganciclovir, and oseltamivir; group 4: chemotherapy=cyclosporine, cyclophosphamide, tacrolimus, methotrexate, mercaptopurine, cytarabine, doxorubicin, tretinoin, daunoblastin, and mesna; and group 5: anti-inflammatory=ketoprofen and acetylsalicylic acid); use and duration of vasoactive drugs (vasopressors: epinephrine at dose above 0.3 micrograms/kg/min and norepinephrine; and inotropics: epinephrine at dose below 0.3 micrograms/kg/min, dopamine, dobutamine, and milrinone); and use and duration of MV.
The variables for the calculation of urinary output (diuresis volume, weight, and measurement time) and GFR were recorded using the bedside Schwartz formula (height and serum creatinine).12 Regarding the urinary output and serum creatinine or GFR, patients were diagnosed with AKI according to each diagnostic criterion, on admission and during hospitalization – pediatric risk, injury, failure, loss, end-stage renal disease (pRIFLE),13 Acute Kidney Injury Network (AKIN),14 and Acute Kidney Injury Work Group, the Kidney Disease: Improving Global Outcomes (KDIGO).15
The outcome variables assessed were: diagnosis of AKI; indication of renal replacement therapy (RRT); PICU exit event (discharge or death); and length of stay. In patients submitted to RRT, the method used to perform dialysis was recorded: hemodialysis or peritoneal dialysis; time elapsed from admission to diagnosis of AKI, in days; and duration of RRT, in days.
The SPSS software version 20.0 from IBM was used for statistical analysis. The quantitative variables with non-normal distribution verified by the Shapiro Wilk test were described as medians and interquartile ranges (Q1; Q3). Categorical variables were described as absolute frequencies and percentages. Comparison of the AKI outcome, defined by the KDIGO criterion, with the study variables was performed by the asymptotic Pearson’s chi-squared test (20% of the expected value less than 5 and 80% of the expected value greater than 5) and exact Pearson’s chi-squared test (more than 20% of the expected value less than 5). Comparison of quantitative variables age, PIM3, length of hospital stay, and MV time was performed using the Mann-Whitney test. The significance level adopted was 5%.
In order to evaluate the association between the study variables and AKI in the PICU population, log-binomial generalized linear models were adjusted, as this was a cross-sectional study. The measure of association used was prevalence ratio (PR). First, univariate models of the study variables and the AKI response variable were adjusted. All variables that resulted in p-values less than or equal to 0.20 were the candidate variables for the multivariate model. Multivariate models were adjusted so that, at each stage, the variable with the highest p-value was removed until reaching the final model, in which all variables were statistically significant (p<0.05). The fit quality of the univariate and multivariate linear generalized log binomial models was evaluated by two tests: deviance and Pearson’s chi-squared; for any value above 0.05, it can be said that the model was well adjusted.
The study was approved by the Research Ethics Committee (CAAE - 47210215.8.0000.5149).
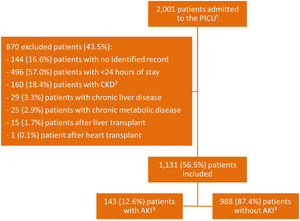
ResultsThere were 2001 admissions to the PICU during the study period, of which 1131 (56.5%) were included (Fig. 1).
Median and interquartile range of the patients' age was 3.58 years (Q1; Q3 – 0.67; 10.33), with 609 (53.8%) males. The study population had the following age distribution: age less than 1year, 345 (30.5%); age between 1 and 3 years, 179 (15.8%); age between 3 and 6 years, 151 (13.4%); age between 6 and 10 years, 164 (14.5%); age between 10 and 14 years, 158 (13.9%); and age between 14 and 18 years, 134 (11.8%).
Clinical patients corresponded to 595 (52.7%), while 536 (47.4%) were admitted after a surgical procedure. The presence of a complex clinical condition was detected in 973 (86.0%) patients, and 165 (14.6%) had an onco-hematological pathology. Out of all patients, 705 (62.4%) presented sepsis.
Nephrotoxic medications were administered to 756 (66.8%) patients. Most patients (490 [64.9%]), used only one group of medications: 453 (59.9%) used antibiotics; 11 (1.5%), anti-fungal; 12 (1.6%), chemotherapy; three (0.4%), anti-viral; and 11 (1.5%), anti-inflammatory. The other 266 (35.2%) patients used more than one group of medications: 216 (28.6%) used two groups and 50 (6.6%) used three or more groups.
The use of vasoactive drugs was indicated in 279 (24.7%) patients, with median time of use of 4.00 (Q1; Q3 – 2.00; 8.00) days. In this group, 117 (41.9%) used vasopressors only and 41 (14.7%) used inotropics only. A total of 121 (43.4%) patients used both groups in the same hospitalization.
A total of 550 (48.6%) patients required MV. Median time of MV use was 3.00 (Q1; Q3 - 2.00; 8.00) days.
Using the AKIN and KDIGO criteria, the same results were observed: 143 (12.6%) patients received the diagnosis of AKI, with 72 (50.3%) in stage 1, seven (4.9%) in stage 2, and 64 (44.8%) in stage 3. In these groups, 50 (35%) were surgical and 93 (65%) were clinical patients. According to the pRIFLE criterion, 146 (12.9%) patients were identified with AKI, of whom 65 (44.5%) were diagnosed in stage R (1), 24 (16.4%) in stage I (2), 54 (37%) in stage F (3), and three (2.1%) in stage L (4). Comparing the results found by the three criteria, pRIFLE identified more patients in stage 2 than the other criteria, with statistical significance at the level of 5% (Pearson’s chi-squared test). No statistically significant difference was observed in the other stages. The standardized adjusted residue analysis test was applied to determine at which stages the difference presented a significance.
The median of time elapsed between admission to the PICU and alteration in creatinine clearance among patients with AKI was 2.00 (Q1; Q3 – 1.50; 6.00) days.
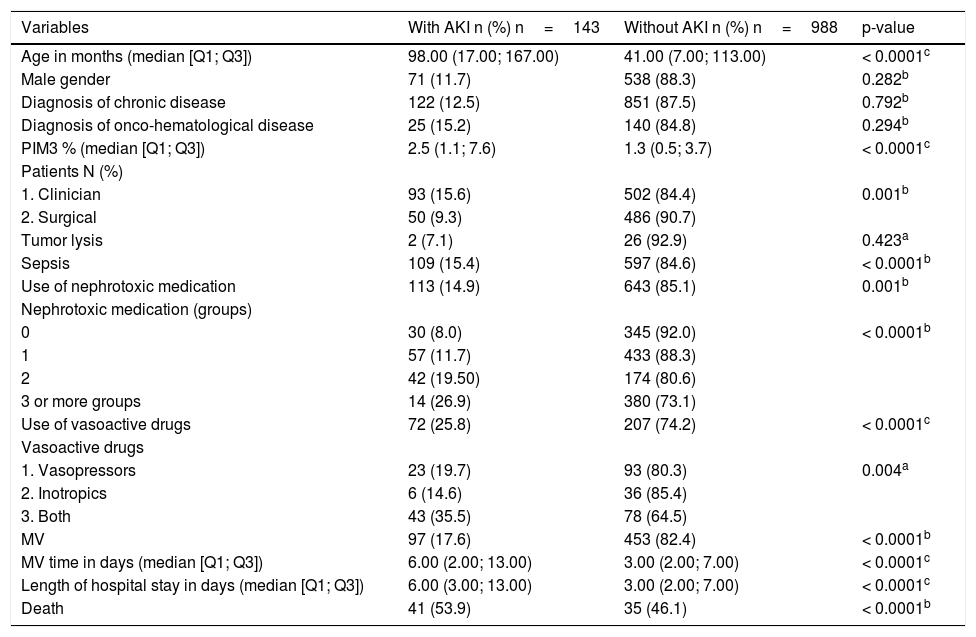
Table 1 presents the results of the comparative analysis of the associated factors evaluated among patients who developed AKI and those who did not. The variables age, PIM3, clinical admission, sepsis, use of nephrotoxic medication and groups of nephrotoxins used, use of vasoactive amine and type of drug, use and time of MV, length of hospital stay, and progress to death were statistically significance at the level of 5%.
Result of the comparison of variables - patients with AKI versus patients without AKI.
| Variables | With AKI n (%) n=143 | Without AKI n (%) n=988 | p-value |
|---|---|---|---|
| Age in months (median [Q1; Q3]) | 98.00 (17.00; 167.00) | 41.00 (7.00; 113.00) | < 0.0001c |
| Male gender | 71 (11.7) | 538 (88.3) | 0.282b |
| Diagnosis of chronic disease | 122 (12.5) | 851 (87.5) | 0.792b |
| Diagnosis of onco-hematological disease | 25 (15.2) | 140 (84.8) | 0.294b |
| PIM3 % (median [Q1; Q3]) | 2.5 (1.1; 7.6) | 1.3 (0.5; 3.7) | < 0.0001c |
| Patients N (%) | |||
| 1. Clinician | 93 (15.6) | 502 (84.4) | 0.001b |
| 2. Surgical | 50 (9.3) | 486 (90.7) | |
| Tumor lysis | 2 (7.1) | 26 (92.9) | 0.423a |
| Sepsis | 109 (15.4) | 597 (84.6) | < 0.0001b |
| Use of nephrotoxic medication | 113 (14.9) | 643 (85.1) | 0.001b |
| Nephrotoxic medication (groups) | |||
| 0 | 30 (8.0) | 345 (92.0) | < 0.0001b |
| 1 | 57 (11.7) | 433 (88.3) | |
| 2 | 42 (19.50) | 174 (80.6) | |
| 3 or more groups | 14 (26.9) | 380 (73.1) | |
| Use of vasoactive drugs | 72 (25.8) | 207 (74.2) | < 0.0001c |
| Vasoactive drugs | |||
| 1. Vasopressors | 23 (19.7) | 93 (80.3) | 0.004a |
| 2. Inotropics | 6 (14.6) | 36 (85.4) | |
| 3. Both | 43 (35.5) | 78 (64.5) | |
| MV | 97 (17.6) | 453 (82.4) | < 0.0001b |
| MV time in days (median [Q1; Q3]) | 6.00 (2.00; 13.00) | 3.00 (2.00; 7.00) | < 0.0001c |
| Length of hospital stay in days (median [Q1; Q3]) | 6.00 (3.00; 13.00) | 3.00 (2.00; 7.00) | < 0.0001c |
| Death | 41 (53.9) | 35 (46.1) | < 0.0001b |
AKI, acute kidney injury; PIM3, Paediatric Index of Mortality 3; MV, mechanical ventilation.
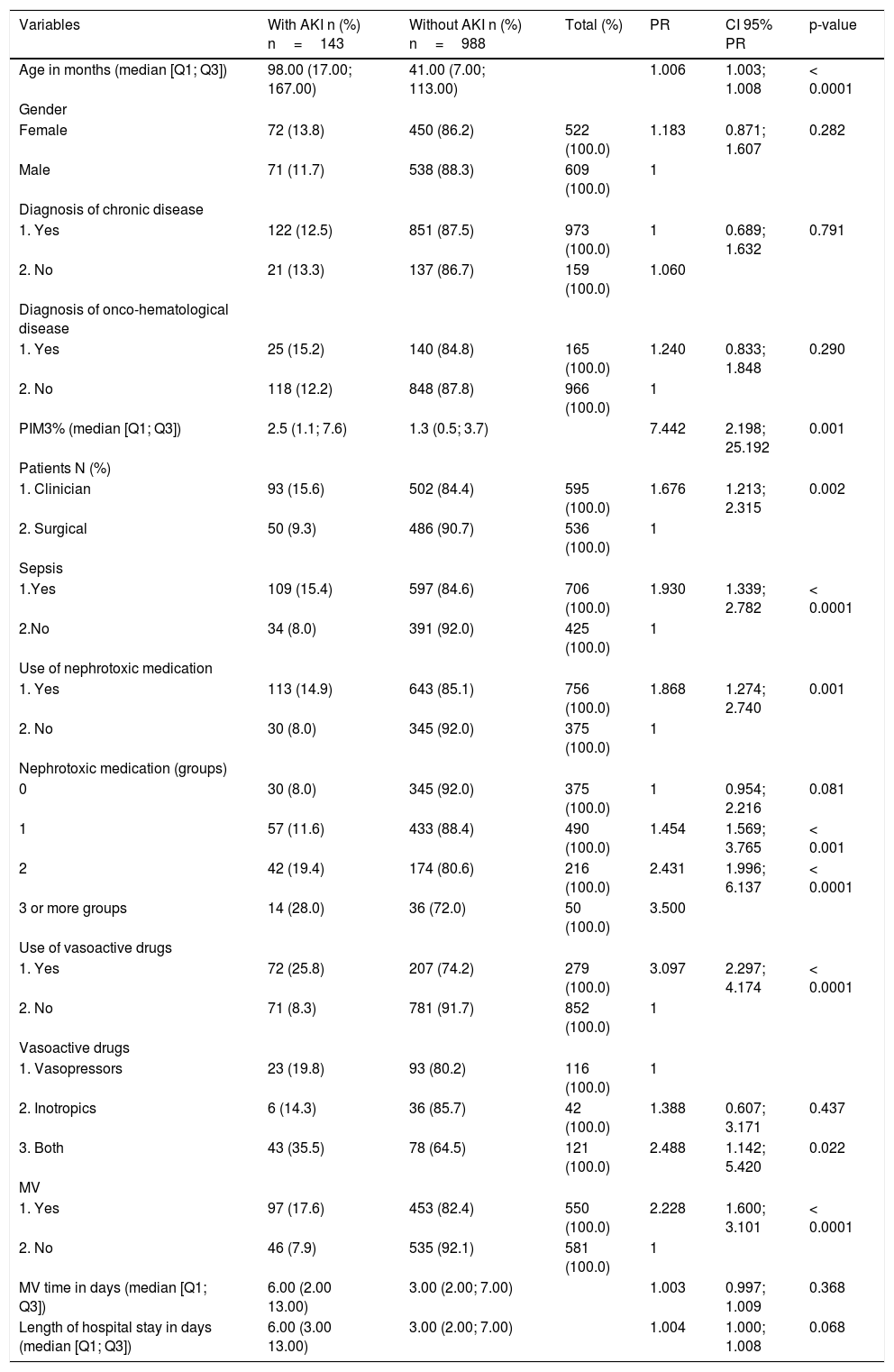
Table 2 presents the results of the univariate adjustment, of the log-binomial generalized linear model, with the candidate variables to the multivariate model, considering the patients with AKI (p-value: 0.20).
Univariate log-binomial generalized linear model - patients with AKI versus without AKI.
| Variables | With AKI n (%) n=143 | Without AKI n (%) n=988 | Total (%) | PR | CI 95% PR | p-value |
|---|---|---|---|---|---|---|
| Age in months (median [Q1; Q3]) | 98.00 (17.00; 167.00) | 41.00 (7.00; 113.00) | 1.006 | 1.003; 1.008 | < 0.0001 | |
| Gender | ||||||
| Female | 72 (13.8) | 450 (86.2) | 522 (100.0) | 1.183 | 0.871; 1.607 | 0.282 |
| Male | 71 (11.7) | 538 (88.3) | 609 (100.0) | 1 | ||
| Diagnosis of chronic disease | ||||||
| 1. Yes | 122 (12.5) | 851 (87.5) | 973 (100.0) | 1 | 0.689; 1.632 | 0.791 |
| 2. No | 21 (13.3) | 137 (86.7) | 159 (100.0) | 1.060 | ||
| Diagnosis of onco-hematological disease | ||||||
| 1. Yes | 25 (15.2) | 140 (84.8) | 165 (100.0) | 1.240 | 0.833; 1.848 | 0.290 |
| 2. No | 118 (12.2) | 848 (87.8) | 966 (100.0) | 1 | ||
| PIM3% (median [Q1; Q3]) | 2.5 (1.1; 7.6) | 1.3 (0.5; 3.7) | 7.442 | 2.198; 25.192 | 0.001 | |
| Patients N (%) | ||||||
| 1. Clinician | 93 (15.6) | 502 (84.4) | 595 (100.0) | 1.676 | 1.213; 2.315 | 0.002 |
| 2. Surgical | 50 (9.3) | 486 (90.7) | 536 (100.0) | 1 | ||
| Sepsis | ||||||
| 1.Yes | 109 (15.4) | 597 (84.6) | 706 (100.0) | 1.930 | 1.339; 2.782 | < 0.0001 |
| 2.No | 34 (8.0) | 391 (92.0) | 425 (100.0) | 1 | ||
| Use of nephrotoxic medication | ||||||
| 1. Yes | 113 (14.9) | 643 (85.1) | 756 (100.0) | 1.868 | 1.274; 2.740 | 0.001 |
| 2. No | 30 (8.0) | 345 (92.0) | 375 (100.0) | 1 | ||
| Nephrotoxic medication (groups) | ||||||
| 0 | 30 (8.0) | 345 (92.0) | 375 (100.0) | 1 | 0.954; 2.216 | 0.081 |
| 1 | 57 (11.6) | 433 (88.4) | 490 (100.0) | 1.454 | 1.569; 3.765 | < 0.001 |
| 2 | 42 (19.4) | 174 (80.6) | 216 (100.0) | 2.431 | 1.996; 6.137 | < 0.0001 |
| 3 or more groups | 14 (28.0) | 36 (72.0) | 50 (100.0) | 3.500 | ||
| Use of vasoactive drugs | ||||||
| 1. Yes | 72 (25.8) | 207 (74.2) | 279 (100.0) | 3.097 | 2.297; 4.174 | < 0.0001 |
| 2. No | 71 (8.3) | 781 (91.7) | 852 (100.0) | 1 | ||
| Vasoactive drugs | ||||||
| 1. Vasopressors | 23 (19.8) | 93 (80.2) | 116 (100.0) | 1 | ||
| 2. Inotropics | 6 (14.3) | 36 (85.7) | 42 (100.0) | 1.388 | 0.607; 3.171 | 0.437 |
| 3. Both | 43 (35.5) | 78 (64.5) | 121 (100.0) | 2.488 | 1.142; 5.420 | 0.022 |
| MV | ||||||
| 1. Yes | 97 (17.6) | 453 (82.4) | 550 (100.0) | 2.228 | 1.600; 3.101 | < 0.0001 |
| 2. No | 46 (7.9) | 535 (92.1) | 581 (100.0) | 1 | ||
| MV time in days (median [Q1; Q3]) | 6.00 (2.00 13.00) | 3.00 (2.00; 7.00) | 1.003 | 0.997; 1.009 | 0.368 | |
| Length of hospital stay in days (median [Q1; Q3]) | 6.00 (3.00 13.00) | 3.00 (2.00; 7.00) | 1.004 | 1.000; 1.008 | 0.068 |
AKI, acute kidney injury; PR, prevalence ratio; CI, confidence interval; PIM3, Paediatric Index of Mortality 3; MV, mechanical ventilation.
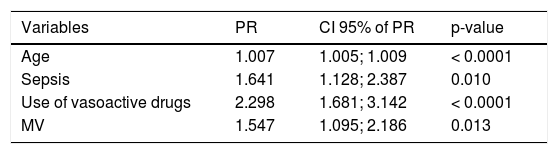
The generalized multivariate log binomial linear model was adjusted where the variables that remained associated with AKI were age, PIM 3, sepsis, use of nephrotoxic medication, use of vasoactive drugs, and MV (p < 0.05; Table 3). According to the values of deviance and Pearson’s chi-squared tests, the model was well-adjusted to the data.
Generalized multivariate log-binomial linear model of all patients with AKI.
| Variables | PR | CI 95% of PR | p-value |
|---|---|---|---|
| Age | 1.007 | 1.005; 1.009 | < 0.0001 |
| Sepsis | 1.641 | 1.128; 2.387 | 0.010 |
| Use of vasoactive drugs | 2.298 | 1.681; 3.142 | < 0.0001 |
| MV | 1.547 | 1.095; 2.186 | 0.013 |
MV, mechanical ventilation; PR, prevalence ratio; CI, confidence interval.
Deviance=0.390; Pearson=0.764.
A total of 42 (3.7%) patients presented indication for RRT. In this group, 20 (47.6%) were in anuria when the procedure was indicated, 13 (31%) had uremia, six (14.3%) had hyperkalemia, and in three (7.1%) RRT was indicated due to metabolic acidosis. In 35 (83.3%) patients, hemodialysis was chosen, and six (14.3%) underwent peritoneal dialysis; one (2.4%) required both types of RRT. The median RRT duration was 9.00 (Q1; Q3 – 5.00; 13.50) days.
Only two patients progressed to CKD until the time this study was completed. The mean length of stay in the PICU was 8.86 days and the median was 3.00 (Q1; Q3 – 2.00; 7.00), and the longest hospitalization was observed among patients who developed AKI (median of 6.00 [Q1; Q3 – 2.00; 13.00]).
The PICU mortality was 76 (6.7%) patients. Among those with AKI, 41 (28.7%) died: four of the surgical (8%) and 37 of the clinical patients (39.8%). Considering patients with AKI who underwent RRT, 17 (40.5%) of the 42 patients died. No difference was observed in the mortality rate among the diagnostic criteria of AKI – the three patients diagnosed only by pRIFLE did not progress to death.
DiscussionAKI is a serious public health problem. Studies show that AKI in pediatrics is rising, especially among critically ill patients.16 There is evidence that AKI is often underdiagnosed; delays in recognition and treatment can lead to death before diagnosis.
A large proportion of the study population had been previously diagnosed with a complex chronic condition (86%), a variable not evaluated in the studies found in the literature on AKI in pediatrics. Such findings are related to recent advances in medical care, especially in pediatric intensive care, which have improved survival rates and a consequent increase in patients with complex chronic conditions, who present a higher risk of PICU hospitalization than the general population.17 Another characteristic of this study was the predominance of children with onco-hematological diseases who underwent chemotherapy and immunosuppression, which may explain the high proportion of patients admitted with sepsis and septic shock, both recognized as risk factors for AKI.
Following the current trend in using KDIGO as a diagnostic criterion for AKI and the fact it is used in this service, it was chosen for this study. The prevalence of AKI observed in the PICU population (12.6%) was within the range observed in the literature (from 4% to 26.9%).3–5,18–20 Regarding staging, according to the KDIGO criteria, there was a higher prevalence of AKI in stages 1 and 3, a result similar to those found by Sutherland et al. and Li et al.1,21 Kaddourah et al. found predominance of children in stage 1 (56.9%), assuming early diagnosis in institutions where the study was conducted.6 Comparing the three criteria, a statistically significant difference was found only in stage 2, differing from the study of Sutherland et al., where the difference found was in stages 1 and 3.1 In a study with patients admitted to PICU in Turkey, Kavaz et al. observed that the pRIFLE appears to be more sensitive in pediatric patients.22 Although this was not the main objective, this study showed that the three criteria were similar for the diagnosis of AKI.
In the multivariate analysis, age was associated with the development of AKI, with higher prevalence in older children, differently from the reported in the literature, where a predominance is observed in children younger than 1year, excluding the neonatal population.1,19,23,24 The study by Yadav et al. was the only to observed a mean age similar close to that found in the present study (8.3 years).19 This variation can be explained by the different characteristics of the pediatric populations admitted to the various intensive care centers.
In hospitalized children, exposure to nephrotoxic drugs is among the factors that most contribute to AKI, being responsible for approximately 16-25% of the cases, and associated with significant morbidity, increase in hospitalization time, and high cost.25 Among all patients with AKI in this study, 79% used nephrotoxic medication; however, in the multivariate analysis, no significant association strength was found between AKI in any of the groups, unlike Li et al. and Olowu et al., who found nephrotoxicity as the main risk factor for AKI.21,24 The authors believe the divergence in relation to other reports in the literature can be justified by the large number of cancer patients diagnosed with complex chronic disease in the service, where most patients are using a varied group of nephrotoxic medications, unlike other studies. Another limiting factor of this study was the impossibility of dividing the drugs more clearly from those less strongly associated with nephrotoxicity, given the wide variety of drugs used and because the fact that this classification is not very clear in the literature.
The association between sepsis and AKI was statistically significant, similar to observations in several studies.3,18,24 Many mechanisms appear to be involved in the etiology of AKI in septic children.6 Studies suggest that it results from interactions between microvascular alterations, dysregulation of inflammatory homeostasis, necrosis/apoptosis, and hypoxic-ischemic lesion.26
An association between MV and AKI, found in the literature, presented statistical significance in multivariate analysis. Miklaszewska et al. observed that, among patients with AKI, 100% of those under 1year of age required MV, while 78% of older children needed ventilatory support, with a mean of 92%.23 Al-jboor et al. found that 32.8% of all children admitted to the PICU and 36% of children with AKI needed MV.18 Although both studies found a greater prevalence of MV in patients with AKI, those authors did not evaluate if there was an association among the variables with statistical significance.
The use of vasoactive drugs presented significant association strength with AKI in multivariate analysis, similar to the results found by Al-jboor et al., who observed that 42% of the patients with AKI used some vasoactive amine.18 The use of vasopressors is important when reestablishing and maintaining hemodynamic stability and, therefore, its indication presupposes the impairment of circulation and consequent reduction of renal blood flow, with risk of evolution to hypoxic-ischemic AKI.
Among the patients with AKI, only 1.4% evolved into CKD and the literature data reveals a great variation in this outcome, with studies indicating a prevalence of 1.4% to 44%.6 In cohort conducted by Mammen et al., 10.3% developed CKD after 1–3 years of follow-up.27 Hui-Stickle et al. found that only 5% of the children needed RRT at the time of discharge.28 Askenazi et al. evaluated renal function 3–5years after AKI of Hui-Stickle et al. cohort patients and observed that more than half who returned for evaluation had evidence of CKD,29 while Ball and Kara observed that 40% had decreased GFR, proteinuria, or hypertension upon hospital discharge in New Zealand.30 Thus, survivors of pediatric AKI need long-term follow-up because of the risk of developing CKD.
The median PICU length of stay among the patients who developed AKI was double that of other patients, corroborating the literature findings about AKI and increased length of hospital stay.1,2,18,23
The mortality rates currently described in the PICU range from 5 to 10%3,7,9; therefore, the overall mortality observed in the present study (6.7%) is in the expected range. Among patients with AKI, mortality was 28.7%, similar to that found by Li et al. (30.8%)21 and slightly lower than those observed by Martin et al., Miklaszewska et al., and Olowu (40%, 44%, and 50.4%, respectively).3,23,24 Considering only children that have been through dialysis, mortality was even higher (40.5%), as observed by Sutherland et al. (27.1%) and Li et al. (64.3%).2,21 Although the findings of the present study are corroborated by data from the literature, the relationship between AKI and death in multivariate analysis did not present any association strength.
Finally, it can be concluded that the prevalence of AKI according to the KDIGO criterion found in the present study is similar to that described in the literature, although the population admitted to the PICU has unique characteristics in relation to other populations, highlighting the high prevalence of children with complex chronic diseases and onco-hematologic pathologies. Older children, sepsis, ventilatory support, and use of vasoactive amines were associated with a higher risk of developing AKI, corroborating data from the literature. Such findings are a warning that, in the face of patients with these variables, professionals who work in intensive care should be attentive, establishing measures to ensure adequate renal perfusion. The mortality associated with AKI is high, especially among patients submitted to RRT; therefore, it is essential that all measures are taken to avoid the installation of the disease. The follow-up of patients who develop AKI is essential, considering the possibility of progressing to CKD in the long term.
Conflicts of interestThe authors declare no conflicts of interest.
Study conducted at Universidade Federal de Minas Gerais, Hospital das Clínicas, Belo Horizonte, MG, Brazil.