To describe the current recommendations on the best management of pediatric patients with acute diarrheal disease.
Data sourcePubMed, Scopus, Google Scholar.
Data summaryThere has been little progress in the use of oral rehydration salts (ORS) in recent decades, despite being widely reported by international guidelines. Several studies have been performed to improve the effectiveness of ORS. Intravenous hydration with isotonic saline solution, quickly infused, should be given in cases of severe dehydration. Nutrition should be ensured after the dehydration resolution, and is essential for intestinal and immune health. Dietary restrictions are usually not beneficial and may be harmful. Symptomatic medications have limited indication and antibiotics are indicated in specific cases, such as cholera and moderate to severe shigellosis.
ConclusionsHydration and nutrition are the interventions with the greatest impact on the course of acute diarrhea.
descrever as recomendações atuais sobre a melhor maneira de conduzir o paciente pediátrico com doença diarreica aguda.
Fonte dos dadosPubMed, Scopus, Scholar Google.
Síntese dos dadosHouve pouco avanço na utilização dos sais de reidratação oral (SRO) nas últimas décadas apesar de ser amplamente divulgado através de diretrizes internacionais. Vários estudos vêm sendo realizados na tentativa de melhorar a eficácia do SRO. Hidratação venosa com solução salina isotônica, infundida de forma rápida, deve ser indicada em casos de desidratação grave. A nutrição deve ser assegurada logo após a resolução da desidratação, sendo primordial para a saúde intestinal e imunológica. Restrições alimentares usualmente não são benéficas e podem ser prejudiciais. As medicações sintomáticas têm indicação restrita e antibióticos são indicados em casos específicos, cólera e shiguelose moderada a grave.
Conclusõesa hidratação e a nutrição continuam sendo as intervenções com melhor impacto sobre o curso da diarreia aguda.
Acute diarrheal disease (ADD) is a public health problem in many regions of the world, especially where poverty prevails. A model that aims to explain the incidence or mortality associated with the ADD involves a large number of variables (biological, environmental, socio-cultural) and is very complex. Conversely, a reductionist approach contributes little to the understanding and solution of the problem.1,2
The scientific community, over the past four decades, established a consensus on the most effective measures to reduce the incidence, morbidity, and mortality of ADD. Some measures aimed at reducing the incidence of diarrheal disease constitute interventions that are beyond the medical approach of the problem and are based on environmental condition improvement: water supply, adequate treatment of human waste, education, and food safety. Exclusive breastfeeding for at least 6 months and supplemented up to 2 years of age has a significant impact in reducing the disease incidence and severity. In the field of biomedicine, the development of a vaccine against rotavirus and universal vaccine coverage are important contributions that have an impact on ADD incidence, by decreasing the severe forms and the number of hospitalizations, thus reducing the risk of death.3,4
Regarding mortality, the therapeutic management with emphasis on oral rehydration therapy (ORT) and intravenous rehydration therapy (IRT), recommended since the 1970s, are milestones of twentieth-century medicine. In 1994, Ruxin5 wrote an article commemorating the 25th anniversary of the ORT implementation and concluded (by observation, and expressing some pessimism): “the formidable and persistent ignorance of the western medical establishment, which continues over twenty-five years after the discovery of ORT, is phenomenal.”
The 21st century has arrived, and despite several published articles showing the efficiency and effectiveness of ORT and IRT, it can be observed that ADD management is still being performed in ignorance of scientific evidence.6,7
In a recent article, Walker and Walker2 presented a model, The Lives Saved Tool (LiST), and analyzed the impact of using oral rehydration salts (ORS), zinc, and antibiotics for dysentery on ADD mortality reduction. Low-osmolality ORS, the use of zinc in risk groups for persistent diarrhea, and use of antibiotics only in selected cases of dysentery all demonstrated a positive impact on the assessed outcomes.
The accumulated scientific knowledge on the best management of patients with ADD is extensive; however, researchers have observed physicians’ poor adherence to the recommendations provided by international health organizations, as well as by medical societies, which periodically publish guidelines on the subject.1,8–10
Why – in spite of broad scientific evidence – do physicians choose to treat ADD based on obsolete conduct? This is the reason for the performance of this review. Even at present, the inappropriate use of ORT/IRT can be observed, as well as dietary guidelines that are almost iatrogenic, and even the indication of medications without any scientific basis.4 Therefore, this review aimed to carry out a synthesis of the current knowledge on ADD management by focusing on ORT/IRT, diet during the acute diarrheal process, the judicious use of symptomatic medications, probiotics, zinc, and antibiotics.
ADD managementThere is no consensus on the concept of ADD, but some basic aspects have been covered in several publications.8,9,11 In this review, ADD is considered as a diarrheal episode that has the following characteristics: abrupt onset, presumably infectious etiology, potentially self-limited, with a course of less than 14 days, increased volume and/or frequency of stool, and fecal loss of nutrients (mainly water and electrolytes). Its major complications can thus be inferred (hydroelectrolytic disorders, nutritional deficits), providing the basis for its management.
From a clinical point of view, ADD can be classified as: watery diarrhea syndrome (which constitutes the vast majority of infectious diarrheal diseases), bloody diarrhea syndrome, and persistent diarrhea (when the episode lasts more than 14 days). Regardless of the causative agent, in the majority of diarrheal episodes of infectious etiology, therapeutic management is based on hydration maintenance and nutritional status.1,4,9,12
Regarding severity, ADD is classified as mild, moderate, or severe: mild when signs of dehydration are not observed; moderate when there are mild or moderate signs of dehydration and rehydration can be performed orally; and severe when it results in more intense dehydration with or without electrolyte disturbances, and requires intravenous therapy.9,13
Most ADD cases show mild or moderate severity and are not treated at health services, hence the importance of home treatment guidelines for diarrheal disease in order to prevent dehydration. Hospitals receive cases with more exuberant symptoms and dehydrated patients or those at risk for dehydration; clinical pictures secondary to severe vomiting or high-output diarrhea.9,13
From a physiopathological point of view, there are two basic mechanisms involved: osmotic and secretory. Secondary to these mechanisms, alterations in intestinal motility can also occur. The osmotic mechanism is observed when there is an increase in luminal osmolality, as it occurs in diarrhea associated with rotavirus, in which damage occurs in the proximal small bowel mucosa, resulting in increasing undigested lactose in the intestinal lumen. The excess of lactose when fermented by bacteria that are part of the colonic microflora, originate short-chain fatty acids, acid radicals that explain the distension and abdominal pain, and in some cases, perianal hyperemia. The diarrhea is watery and explosive. The secretory mechanism occurs when there is a stimulation of secretion mediators by the exotoxins produced by bacterial pathogens (Vibrio cholerae, enterotoxigenic Escherichia coli) or by inflammation mediators, such as in diarrhea associated with Shigella strains. From the viewpoint of fecal losses, what essentially differentiates the two mechanisms is the loss of sodium, which is higher in the secretory form and may be greater than 70mEq of sodium per liter of stool.14
In more severe forms of ADD, in which high-output diarrhea occurs, it is important to characterize the type of mechanism involved so that the losses can be appropriately replaced. However, most ADD pictures in childhood, even those that lead to dehydration and require hospital treatment, show good response to standard management,13 which will be discussed elsewhere in this article.
The digestive-absorptive functions are maintained in almost all children affected by ADD, and thus, if an adequate caloric intake is offered, there is minimum risk of malnourishment or aggravation of a pre-existing malnutrition status. There are few situations where diet restrictions or changes are necessary. The nutritional approach will be reviewed in another item.
HydrationDehydration is the main complication of acute diarrhea, and hydration status assessment should be one of the first actions to be taken regarding the management of a child with diarrhea. Acute weight loss during the diarrheal episode is considered the best parameter to assess dehydration. According to the loss, dehydration is classified as mild (<5% weight loss), moderate (5–10%), or severe (>10%); dehydration severity classification is essential for the treatment.9 Due to the difficulty in obtaining information on the previous weight (to estimate weight loss), this parameter has limited practical usefulness, and other clinical variables should be used.
Clinical evaluation is usually used to define the hydration status; however, it may show interpersonal variations and, thus, validated clinical signs capable of being evaluated in a simple and objective way should be used. The best signs related to moderate/severe dehydration are slowed capillary filling, decreased skin turgor, and changes in breathing pattern. Clinical presentation of the disease can also alert to the risk of dehydration, and a child with high-output diarrhea associated with vomiting has a higher risk of dehydration.9
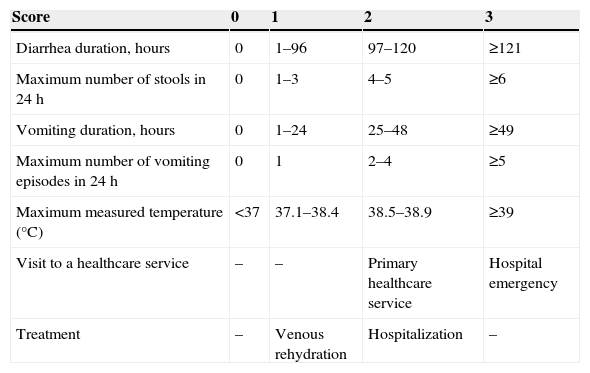
The use of scoring systems to determine the hydration status and disease severity is considered useful in the management of children with diarrhea. The clinical dehydration scale (CDS; Table 1), developed in 2008 for children aged 1–36 months with ADD treated in emergency rooms, has been validated in several studies.15 The CDS considers four clinical items (overall appearance, eyes, mucosa, and tears) to classify the child as “no dehydration,” “some dehydration,” or “moderate/severe dehydration.” The disease severity score provides a more comprehensive measure of ADD impact on the child's health. The Vesikari severity score (Table 2) is a classic score that has been recently validated in a modified version; it has demonstrated good applicability in different services and populations.16 It does not assess hydration status, but rather the impact of ADD in different populations (mild, moderate, and severe) and the response to interventions.17
Clinical dehydration scale (adapted from Freedman et al.23).
| Characteristics | 0 | 1 | 2 |
|---|---|---|---|
| General appearance | Normal | Thirsty, restless, or lethargic, but irritable when touched | Drowsy, limp, cold, sweaty; comatose or not |
| Eyes | Normal | Slightly sunken | Very sunken |
| Mucous membranes (tongue) | Moist | Sticky | Dry |
| Tears | Present | Decreased tears | Absent tears |
Score=0, no dehydration; Score=1–4, some dehydration; Score=5–8, moderate to severe dehydration.
Vesicari modified severity score (adapted from Carmo et al.29).
| Score | 0 | 1 | 2 | 3 |
|---|---|---|---|---|
| Diarrhea duration, hours | 0 | 1–96 | 97–120 | ≥121 |
| Maximum number of stools in 24h | 0 | 1–3 | 4–5 | ≥6 |
| Vomiting duration, hours | 0 | 1–24 | 25–48 | ≥49 |
| Maximum number of vomiting episodes in 24h | 0 | 1 | 2–4 | ≥5 |
| Maximum measured temperature (°C) | <37 | 37.1–38.4 | 38.5–38.9 | ≥39 |
| Visit to a healthcare service | – | – | Primary healthcare service | Hospital emergency |
| Treatment | – | Venous rehydration | Hospitalization | – |
Mild, 0–8; Moderate, 9–10; Severe, ≥11.
Laboratory tests are not indicated in the routine assessment of children with ADD, but can help determine dehydration severity, with low levels of serum bicarbonate (<15mEq/L) and increase in urea levels (>10nmol/L) showing a good positive predictive value for moderate to severe dehydration.18
In a dehydrated child, electrolyte treatment consists of rehydration and loss replacement. ORT should be preferably used for rehydration, whereas IRT should be used only in cases of ORT failure or severe dehydration. A systematic review that compared the use of ORT and IRT in children with different degrees of dehydration concluded that there was no difference regarding the risk of metabolic disorder, mean duration of diarrheal episode, and need for fluids in relation to the type of therapy used. The hospital length of stay was lower in the group using ORT. Regarding the unfavorable outcomes, there was more phlebitis in the group that received IRT, and higher incidence of paralytic ileus in the group that received ORT. The ORT failure rate was 1:25, i.e., for every 25 children that received ORT, one required IRT.19
Intravenous hydration has been used for more than a century, but the logistics required for its implementation and the associated complications have shown that it is of little use when it is necessary to hydrate a large number of individuals during infectious diarrhea epidemics. Around 1970, ORS was developed in order to correct dehydration caused by severe infectious diarrhea, particularly cholera-related diarrhea. ORS was initially developed as an isotonic solution, i.e., osmolality of 311mOsm/kg H2O and sodium concentration of 90mEq/L, thus becoming the standard solution of the World Health Organization (WHO).20
In spite of the initial success, there was a change in the world scenario, characterized by a lower occurrence of cholera-related diarrhea and higher incidence of viral diarrhea. In this context, there was a concern regarding the sodium concentration of the standard WHO solution, which would be very high in relation to losses in viral diarrhea cases. Approximately a decade ago, studies confirmed the benefit of using hypotonic solutions with osmolality of 245mOsm/kg H2O and sodium concentrations of 60–75mEq/L in non-cholera-related diarrhea. It has been shown that children who used hypotonic solutions had less vomiting, lower fecal losses, shorter duration of disease, and less need for intravenous hydration when compared with those who used the solution previously recommended by the WHO. Hypotonic solutions also contain lower glucose concentrations, which ensure the adequate ratio for the coupled transport of sodium ions and water by the intestinal mucosa.21
To promote its acceptance, the oral hydration solution should be given in fractionated, small portions. However, the high volume required for rehydration may not be tolerated by the child, and solution intake refusal or even vomiting may occur. A nasogastric tube (NGT) is indicated in such circumstances, as well as in situations where intravenous or intraosseous hydration is impossible, with advantages such as: hyper-hydration prevention, non-invasiveness, rapid treatment onset, and lower cost. It has been demonstrated that hydration via NGT is as effective as intravenous hydration in cases of moderate dehydration.22 Nevertheless, healthcare workers are more familiar with the use of intravenous hydration than with NGT hydration.23
The effectiveness of ORS in reducing morbidity and mortality from acute diarrhea episodes is undeniable, but its use does not meet the goals and has not made any progress in the last 30 years. A possible explanation for the lack of progress regarding the use of ORS would be the fact that, initially, there was a large investment in educational programs for the use of ORS, but with the emergence of several other educational efforts for ADD prevention and treatment (vaccination, breastfeeding campaigns, nutrition, and hygiene), ORT has lost priority. The need to maintain educational campaigns for priority use of ORS should be emphasized, so that new mothers can be educated about its use.4
Other possible explanations for the inadequate use of ORS include children's refusal to drink it (possibly related to the flavor) and the fact that the oral solution does not reduce diarrheal losses. Considering this fact, a way to improve this scenario has been sought. Flavored ORS, present in some commercial products, increases its palatability, but it does not appear to modify the consumed volume.24 The addition of zinc, prebiotics, amino acids, disaccharides, and glucose polymers has resulted in only a modest improvement in ORS effectiveness.9
The addition of the substrate that leads to the production of short chain fatty acids (SCFAs) has aroused interest, as SCFAs are readily absorbed by colonocytes and stimulate the absorption of fluids and sodium. Studies have suggested a benefit of adding a resistant starch (substrate that leads to the formation of SCFAs in the colon) to the ORS. In a systematic Cochrane review, the authors found that the use of ORS added to a resistant starch was associated with a reduced need for intravenous infusion and lower losses from diarrhea.25 Despite the possible benefits, some technical problems are yet to be solved, as an opaque solution is formed, which rapidly precipitates; the ideal suspension to solve this problem has not been identified yet.
Although preferably ORT should be used, intravenous hydration is necessary and crucial in severe dehydration cases. Possible controversies about what represents the best procedure to implement intravenous hydration are related to the type of fluid, the volume, and rate of infusion. Regarding the type of solution, there is evidence that the isotonic saline solution (0.9% saline) is preferable to the hypotonic solution (0.45% saline), preventing the occurrence of hyponatremia without causing hypernatremia.26
As for the infusion volume and velocity, studies comparing the infusion of 20mL/kg (fast) vs. 60mL/kg (ultrafast) of 0.9% saline solution, for one hour, in children with intravenous hydration indication due to ORT failure, showed that children submitted to ultra-fast infusion had a higher frequency of hypernatremia and later hospital discharge than those submitted to rapid infusion, with no difference in rehydration rate. Therefore, the current evidence does not justify the use of ultra-fast rehydration.27
According to WHO recommendations, loss replacement should be carried out, whenever possible, through the oral route, and it should be started during intravenous rehydration.13 Intravenous hydration should be suspended as soon as the child is hydrated and alert, ensuring the child's hydration through ORT. As a guideline, the WHO recommends a volume of ¼ cup (50–100mL) for children younger than two years, ½ cup (100–200mL) for children aged 2–10 years, and free volume for those aged >10 years. The solution to be used for diarrheal loss replacement should be the hypotonic ORS, but if it cannot be used, the WHO advises using other salinized fluids, such as rice water, vegetable broth, and homemade oral hydration solution. Breast milk can be used as replacement fluid in a nursing child. However, fluids such as energy drinks, soft drinks, and juices high in sorbitol should not be used as replacement fluids due to low sodium content and high osmolality.
The use of homemade ORS, a solution prepared by hand at home by adding salt and sugar to water, is included in the Child Health Handbook of the Brazilian Ministry of Health28 (Caderneta de Saúde da Criança do Ministério da Saúde do Brasil), which teaches how to prepare the solution by using the “pinch and scoop” method (a handful of sugar and three pinches of salt in 200mL of water). The ORS can also be prepared by using a measuring spoon and a teaspoon/tablespoon. The WHO, in its 2005 document on acute diarrhea treatment, makes a brief comment on the possibility of its use (by using a teaspoon/tablespoon), reporting that, while potentially effective, it is not recommended due to its inadequate preparation and consumption.
A study carried out in Ouro Preto, Brazil, which assessed the concentration of sodium and glucose in ORS solutions prepared by health workers in the region, found a high percentage (71.1–96.1%) of inadequate preparation, varying according to the preparation method used (lower inadequacy was observed with the pinch and scoop method). When the health agents were asked about the ORS preparation method they taught to the families, about 30% reported they indicated the use of the measuring spoon, followed by the teaspoon/tablespoon (19%), and finally the pinch and scoop method (6%). Conversely, only 17% of health workers reported the availability of the measuring spoon in the Basic Health Units (Unidades Básicas de Saúde [UBS]) of the region. In that study, the authors point to the fact that inadequate concentration of solutes and the balance between salt and glucose impairs the hydration potential of the homemade ORS, putting children at risk; the main message of the study was the lack of qualification of the health workers to teach the population with regard to homemade ORS.29
In a systematic review on the effect of ORS on mortality from diarrhea, it was concluded that there is clear evidence that the WHO ORS is effective in reducing mortality; however, there is no evidence on the effectiveness of other homemade solutions (including the homemade ORS) in combating child death from dehydration.30 Despite the lack of evidence and possible risks associated with ORS replacement by the homemade ORS, the National Demographic Research on Women's and Children's Health (Pesquisa Nacional de Demografia e Saúde da Criança e da Mulher [PNDS]) of 2006 found an increased use of homemade ORS when compared to that observed in the 1996 PNDS (16% vs. 37%) and consequent decrease in the use of ORS in the same period (44% vs. 19%).31
Consistent with this problem, Munos et al.,30 in the previously mentioned systematic review, found that advising on the use of both ORS and homemade solutions confuses the population, decreasing the effectiveness of the strategy to combat mortality from diarrhea, and they recommend that priority should be given to ORS, by making it available to the entire population.
Despite all considerations about rehydration, the goal to be achieved is the initial prevention of dehydration. Thus, it is necessary, according to the strategy proposed by the WHO, to start ORT at home, at the start of the diarrheal picture, in order to replace the losses.13 The families should be educated about the early onset of oral hydration and evidence of its failure, such as vomiting and signs of dehydration. In a study that assessed the mother's knowledge on ADD management in the city of Recife, it was found that most mothers did not have adequate knowledge of the usefulness of ORS in preventing or treating dehydration. The authors made the following considerations: “The results of this study show that, even with the improvement in maternal knowledge about ORT for more than a decade, a greater effort is necessary by health professionals to create strategies to transmit the information to the mothers in a more efficient manner.”32
DietAlthough the maintenance of an adequate diet for the child's age is a priority for intestinal mucosa regeneration, inadequate feeding practices are still observed in the management of children with acute diarrhea. Enterocytes obtain their nutrients primarily from the intestinal lumen content; thus fasting or dietary restrictions can slow down the renewal process of the cells damaged by infectious process.33 Intestinal malabsorption, of higher or lower severity, may occur in ADD depending on the damage caused by the pathogen; however, good nutrition must be ensured and dietary restrictions should not be implemented with the justification of decreasing diarrheal losses. The usual diet must be maintained when the child is hydrated. In case of mild to moderate dehydration, food should be offered four to five hours after the onset of rehydration.9 The maintenance of breastfeeding during diarrheal episode, even in children with mild to moderate dehydration, is a consensus.34
In a systematic review on dietary management of diarrhea in low- and middle-income countries, a few points about the use of lactose in the diet were analyzed. The normal amount of lactose can be maintained safely in most children with diarrhea; however, the transient lactase deficiency and the consequent poor digestion of lactose can worsen the diarrheal picture in a small group of children. Lactose restriction would be beneficial in selected cases, with reduced losses and shorter time of diarrheal episode after the restriction is observed. The children most likely to benefit from lactose restriction would be those who develop severe dehydration and the malnourished. Lactose restriction by decreasing milk supply, associated with the maintenance of the rest of the homemade diet, would be related to a better weight gain compared to the predominant use of formulas without lactose.34
It is suggested that, for children not yet exposed to cow's milk-based formula, this first exposure should be avoided during or shortly after the ADD episode, to avoid sensitization to cow's milk protein.35 However, there is no evidence that switching to soy or hypoallergenic formula would be beneficial for the child.
In children that have started a solid food diet, it must have an adequate caloric content, as well as macro- and micronutrients. In hospitalized children with diarrhea, higher energy intake was associated with shorter duration of the episode and, consequently, to a better outcome.36 An adequate diet during the diarrheal picture can reduce the occurrence of new episodes. Inadequate nutritional approach during the diarrheal period can lead to malnutrition, as well as installation of the vicious cycle of malnutrition, reduced resistance to new enteropathogens, recurrence of diarrheal episodes, and more malnutrition.37
Regarding the use of handmade or processed food in the diet during the diarrheal episode, no evidence was found on the superiority of industrial formulas compared to adequate homemade diet. Juices with high fructose, sucrose, and sorbitol contents should be avoided, because their high osmolality can exacerbate diarrheal losses.35
The child should be offered a usual diet, including foods with fiber and fat. Diet supplementation with vegetable oil is a WHO recommendation to increase the caloric density of foods, preventing malnutrition. Studies carried out in the 1990s suggested that fiber intake can decrease the time of liquid stools.38
Anorexia can affect children with acute diarrhea, a fact commonly found in the acute phase of the disease, which is more severe in the event of dehydration, acidosis, and hypokalemia. The disorders must be corrected and food should be offered in small portions, often respecting the child's wishes. The lack of appetite is transient and the appropriate food should be available to promote nutritional recovery at the earliest opportunity.38
Drug management of ADDSymptomatic: pain and feverFever is absent in most cases of ADD. Dehydration may lead to an increase in body temperature in young children and it can be an important symptom in ADD with blood in the stool. Fever should be treated when >39°C or when the temperature increase is associated with symptoms that cause discomfort to the infant. The antipyretic drugs most frequently used are acetaminophen and metamizole.1,4,8
Cramp-like abdominal pain is a common symptom of osmotic diarrhea (excess of intestinal gas), and tenesmus is observed when there is a significant inflammatory component, usually in ADD associated with Shigella. In the first case, a reduction in the diet supply of dairy products alleviates the symptoms; in the second case, the indication of drugs with analgesic effect – acetaminophen and metamizole – benefits the patient.1,4,8 Antispasmodic drugs (scopolamine) and antiphysetics agents (simethicone) should not be indicated.
Antiemetic drugsVomiting is frequent in ADD, and antiemetics are excessively prescribed without considering the intensity of vomiting. In most cases, the vomiting ceases when the child is hydrated, as dehydration, even when subclinical, can cause vomiting.
When vomiting is sporadic, there is no indication for antiemetic use; when vomiting is intense, there is an increased risk of dehydration and hospitalization, and these drugs benefit the patients. It is important to remember that the risk of side effects increases when antiemetics are used in dehydrated patients or those with electrolyte disturbances.39
Among the most commonly used drugs are: H1-Histamine receptor blockers (promethazine, dimenhydrinate), dopamine receptor antagonists (metoclopramide), and serotonin-5HT (ondansetron).
The literature does not have good scientific evidence that supports the use of metoclopramide and dimenhydrinate in ADD.39 Regarding ondansetron, several studies have shown that it reduces the risk of dehydration and hospitalization in the subset of patients with a high frequency of vomiting.40
Antidiarrheal drugsThe search for drugs that act by reducing the volume of stool and/or the time of diarrheal episode has been the subject of a constant search. Studies with adsorbents, aluminum silicate, and diosmectite are found in the literature, but without encouraging results. Loperamide, an antimotility drug, was banned from pediatric prescription since its toxic effects were identified as associated with the central nervous system, in addition to the risk of causing paralytic ileus.41
Among the drugs classified as adsorbents, kaolin–pectin was used in the past, but its use was discontinued, as its effectiveness was not demonstrated. Its cosmetic effect of making the stool semi-solid, without changing the fluid volume, could give the impression of an improved clinical picture and could reduce diarrheal surveillance in relation to fluid supply. Another drug, diosmectite, a natural product based on aluminum silicate and magnesium that is not commercialized in Brazil, has been the object of studies, but its effectiveness has not been demonstrated.41
The international guidelines are unanimous in stating that there is no indication for the use of these drugs in ADD.1,4,8,9
Antisecretory drugsIn ADD pictures in which the secretory mechanism is involved and diarrheal losses are important, the use of racecadotril can benefit patients. By reducing fecal loss and disease duration (it affects the secretory process by inhibiting the enkephalinase), it facilitates the hydration status maintenance and, therefore, reduces the chance of hospitalization. In these cases, ORT has been recommended as adjuvant therapy; there is no evidence that its use reduces the need for IRT.42
ZincIn 2004, the WHO and UNICEF brought attention to the impact of zinc in reducing the severity of the diarrheal episode and the number of subsequent ADD episodes in children younger than 5 years. The explanation for this effect would be the modulation of the immune system and also because it has an antisecretory property.43
Most studies were conducted in poor regions and recruited children at higher risk of developing more severe diarrheal episodes, including persistent diarrhea. At that moment, the recommendation was to use zinc associated with ORT for all children younger than 5 years old. Later studies in developed regions, which recruited children at low risk for severe and/or persistent diarrhea, showed no additional benefit of the use of zinc. Currently, the indication is restricted to children belonging to risk groups that originate mainly from the poorest regions: malnourished children younger than 5 years of age and those with history of previous episodes of ADD or hospitalization.44
ProbioticsOnly some strains of probiotics have been studied in the ADD context. Such studies should be carefully analyzed regarding the evaluated outcomes and assessed strains, because there are different mechanisms of action; what is assessed in relation to a strain cannot simply be transferred to another. Lactobacillus GG and Saccharomyces boulardi are the most often scientifically tested.
The action of probiotics occurs mainly through antagonism, immunomodulation, or pathogen exclusion. The antagonism and/or exclusion can have a short-term effect on ADD.45
Most studies were carried out in developed countries. They analyzed the following variables as outcome: duration of diarrheal episode, reduction of fecal losses, and hospitalization, and found a beneficial effect. It is necessary to conduct studies to analyze the cost-benefit of using probiotics as an adjunct therapy to ORT/IRT in underdeveloped and developing countries.8,12
AntibioticsAntibiotics are not indicated in most ADD episodes, even when the cause is bacterial. Almost all cases have a self-limited and benign course, as long as the patient remains hydrated. Even in the most severe diarrheal episodes, the use of antimicrobials is a conduct of exception.
The main issue to be highlighted is that there is no effective antibiotic therapy for most agents associated with ADD. Furthermore, the indiscriminate use may bring harm to the patient due to the devastating effect in the intestinal microbiota, an important mechanism of protection.
The WHO recommends the use of antimicrobial drugs in severe cases of ADD associated with Shigella (ciprofloxacin, ceftriaxone) and cholera (tetracycline, erythromycin). When the causative agent is a protozoan, the etiological treatment is rarely indicated, except in immunodepressed patients.1,8,9
Final considerationsIn 2005, the World Health Organization revised13 the guidelines for ADD treatment, and defined the treatment goals: prevent/treat dehydration, prevent nutritional aggravation, and reduce the duration and severity of diarrheal episode. These goals can be achieved through the proper use of ORT/IRT, maintenance of adequate food intake and, in some cases, judicious use of symptomatic medication (antipyretic, analgesic, and anti-emetic drugs), zinc, antisecretory drugs, probiotics, and antibiotics. These recommendations remain unaltered and almost all international guidelines published since then corroborate them.
Despite the scientific evidence supporting this conduct, why are children not being adequately treated in practice? Why do pediatricians not adhere to the guidelines?
The explanation is not simple and involves several aspects: the fact that the families expect medical care to offer an intervention that will result in a rapid disappearance of symptoms; the belief that, for each disease, there is a medication that will immediately terminate the pathological process; and the difficulty physicians have in building a trusting relationship during consultations that often last only a few minutes.
Nevertheless, researchers worldwide have been evaluating intervention studies on ADD and assessing which conducts actually have a scientific basis. The consensus is that the maintenance of hydration status and proper nutrition are the recommended interventions for almost all children with ADD.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Brandt KG, de Castro Antunes MM, da Silva GA. Acute diarrhea: evidence-based management. J Pediatr (Rio J). 2015;91:S36–43.