To investigate the differential expression of the thymic stromal lymphopoietin isoforms, short and long, and discern their biological implications under eosinophilic gastroenteritis.
MethodsThe expression of thymic stromal lymphopoietin and its two isoforms in tissues was assessed by quantitative RT-PCR in healthy controls (n=24) and patients with eosinophilic gastroenteritis (n=17).
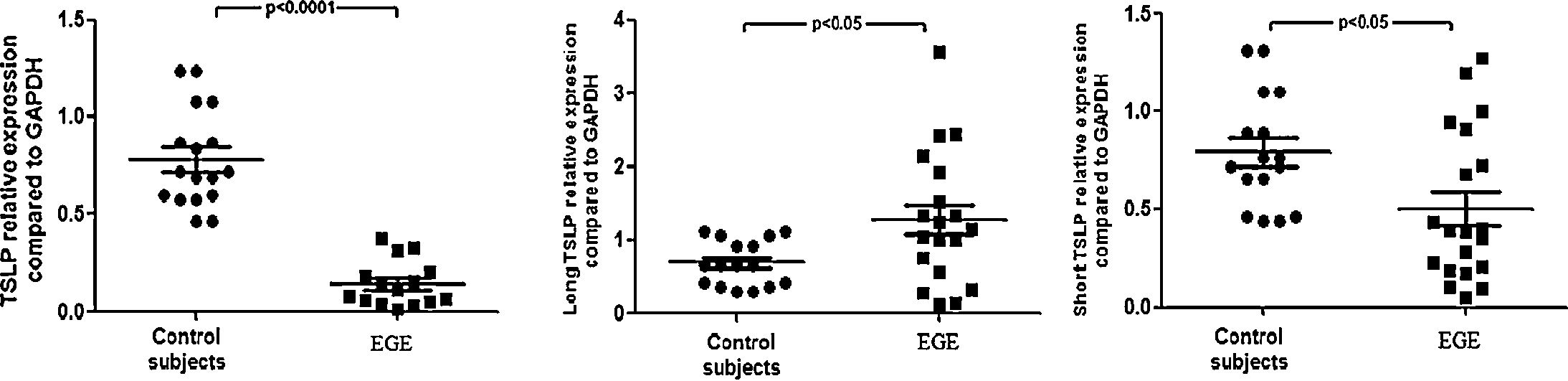
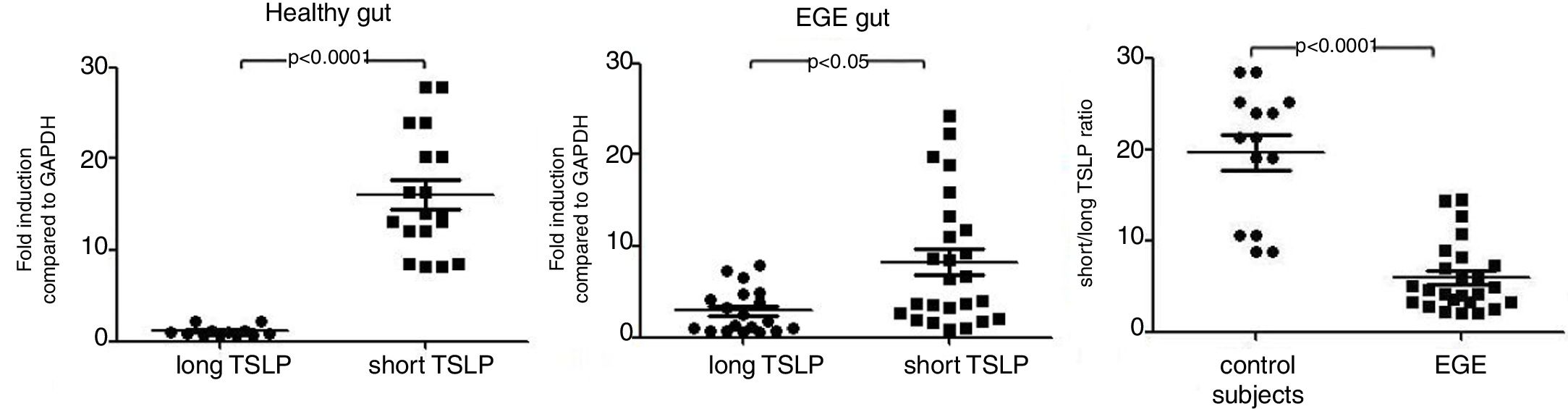
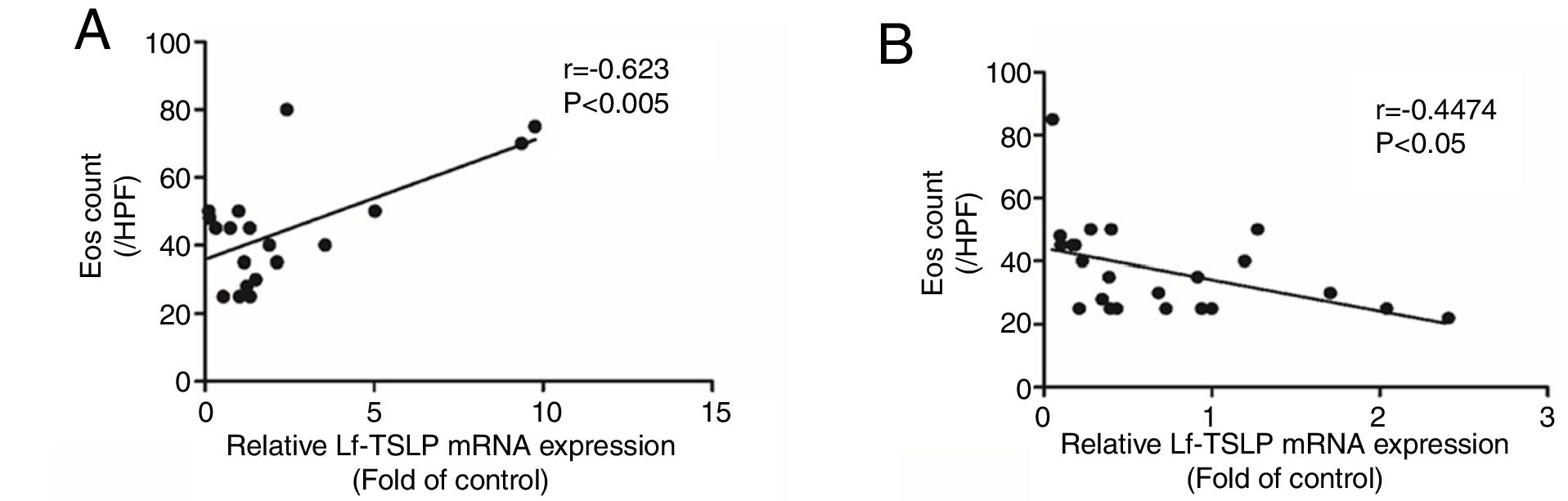
ResultsThymic stromal lymphopoietin mRNA was significantly reduced in eosinophilic gastroenteritis when compared with healthy controls (p<0.0001). A significantly lower amount of short thymic stromal lymphopoietin mRNA was observed in eosinophilic gastroenteritis when compared with controls (p<0.05), while a significantly higher amount of long thymic stromal lymphopoietin mRNA was observed in eosinophilic gastroenteritis when compared with controls (p<0.05). Peak eosinophilic count is significantly positively correlated with the expression of long thymic stromal lymphopoietin mRNA in the gastrointestinal mucosal of patients with eosinophilic gastroenteritis (rs=0.623, p<0.005), while peak eosinophilic count is significantly negatively correlated with the expression of short thymic stromal lymphopoietin mRNA in the gastrointestinal mucosal of patients with eosinophilic gastroenteritis (rs=−0.4474, p<0.05).
ConclusionsAbnormal mucosal thymic stromal lymphopoietin expression may contribute to gastrointestinal mucosa damage in eosinophilic gastroenteritis.
Investigar a expressão diferencial das isoformas da linfopoietina estromal tímica, curta e longa, e discernir suas implicações biológicas na gastroenterite eosinofílica.
MétodosAvaliamos a expressão das isoformas da linfopoietina estromal tímica e suas duas isoformas através da técnica RT-PCR quantitativa em tecidos de controles saudáveis (n=24) e pacientes com gastroenterite eosinofílica (n=17).
ResultadosDemonstramos que o RNAm das isoformas da linfopoietina estromal tímica estava significativamente reduzido na gastroenterite eosinofílica em comparação com os controles saudáveis (p<0,0001). Também descobrimos uma quantidade significativamente menor de RNAm das isoformas da linfopoietina estromal tímica curta na gastroenterite eosinofílica em comparação com os controles (p<0,05) e uma quantidade significativamente maior de RNAm das isoformas da linfopoietina estromal tímica longa na gastroenterite eosinofílica em comparação com os controles (p<0,05). O pico da contagem eosinofílica está correlacionado positiva e significativamente com a expressão do RNAm das isoformas da linfopoietina estromal tímica longa na mucosa gastrointestinal de pacientes com gastroenterite eosinofílica (rs=0,623, p<0,005), enquanto o pico de contagem eosinofílica está negativa e significativamente correlacionado com a expressão do RNAm das isoformas da linfopoietina estromal tímica curta na mucosa gastrointestinal de pacientes com gastroenterite eosinofílica (rs=−0.4474, p<0,05).
ConclusõesA expressão anormal das isoformas da linfopoietina estromal tímica na mucosa pode contribuir para o dano da mucosa gastrointestinal na gastroenterite eosinofílica.
Thymic stromal lymphopoietin (TSLP) is a cytokine produced mainly by epithelial cells and expressed in the skin, lungs, thymus, and intestinal mucosa.1 Recently, two human transcript variants of TSLP have been identified, namely the long and short TSLP isoforms. Short TSLP is the main TSLP isoform expressed under steady-state conditions, and has anti-inflammatory and antimicrobial properties. In turn, long TSLP is expressed at low/undetectable level at steady state and upregulated during inflammation in several tissues.2 The receptor for the long isoform of TSLP, referred to as TSLPR, is well characterized. The long isoform exerts its biological activities by binding to TSLPR, leading to the phosphorylation and activation of signal transducer and activator of transcription (STAT5).3 However, the receptor for the short isoform of TSLP induces phosphorylation of p38α, extracellular signal-regulated kinase 1/2, and Lyn, but has no effect on STAT5 phosphorylation.
Eosinophilic gastroenteritis (EGE) is a rare inflammatory disorder characterized by eosinophilic infiltration of the gastrointestinal wall. Both immunoglobulin E (IgE) dependent and delayed Th2 cell-mediated allergic mechanisms have been demonstrated to be involved in the pathogenesis of EGE.4,5 The signaling pathway triggered by TSLP has been extensively studied, and upregulation of the cytokine itself is linked to the pathogenesis of numerous Th2-related diseases, including atopic dermatitis, asthma, and allergic responses.6,7 The presence of a marked upregulation of the related cytokine observed in the lamina propria of patients with EGE led to the hypothesis that a dysregulation of TSLP expression could be implicated in this condition. Therefore, the present study explored mucosal TSLP expression and function in EGE.
MethodsPatients and tissuesA biopsy was performed on mucosa collected from the intestines or colons of patients with EGE (n=17, mean age 7.4±0.97 years, range 1–13 years) at the time of routine endoscopy. Biopsy specimens included intestinal mucosa (n=15) and colonic mucosa (n=13). As a control, colonic mucosa samples (n=24) were obtained from healthy tissue (at least 7cm away from the polyp) of patients (n=24, mean age 5.2±0.67 years, range: 2–12 years) undergoing endoscopy for colic polyps. Diagnosis was based on three criteria, namely: (1) presence of GI symptoms; (2) histological evidence of eosinophilic infiltration (EOS>20/HPF) in one or more areas of the GI tract; and (3) exclusion of other causes of tissue eosinophilia. All tissues were obtained from patients who signed an informed consent form approved by the institutional review board allowing material not required for diagnosis to be used for research purposes.
RNA extraction and analysis of mRNA expression by quantitative RT-PCRTotal RNA was isolated from biopsy using the RNA Trizol reagent (TaKaRa, Japan). cDNA synthesis was performed using HiScript QRT SuperMix+gDNA wiper (Vazyme Biotech, China). Real-time quantitative PCR was done on the ABI 7500 Real-Time PCR Detection System, using ChamQ SYBR qPCR Master Mix (Vazyme Biotech, China). Primer sequences were as follows: TSLP forward, 5′-CCCAGGCTATTCGGAAACTCAG-3′, and reverse, 5′-CGCCACAATCCTTGTAATTGTG-3′ (these primers do not distinguish between the two TSLP isoforms); long TSLP forward, 5′-CACCGTCTCTTGTAGCAATCG-3′, and reverse, 5′-TAGCCTGGGCACCAGATAGC-3′; short TSLP forward, 5′-CCGCCTATGAGCAGCCAC-3′, and reverse, 5′-CCTGAGTAGCATTTATCTGAG-3′. Typically, 40 cycles of 20s at 95°C and 20s at 60°C, followed by the thermal dissociation protocol for Fast SYBR green detection. PCR reactions were normalized by expression analysis of GAPDH with the following primers: GAPDH forward, 5′-GCACCGTCAAGGCTGAGAAC-3′ and reverse, 5′-ATGGTGGTGAAGACGCCAGT-3′. The amount of each mRNA was normalized to the amount of GAPDH in the same sample. Relative increases in mRNA expression were calculated using the 2−ΔΔCT method.
Statistical analysisThe data were statistically analyzed using the SPSS version 17.0 software (SPSS, Inc. – Chicago, USA) using the non-parametric Mann–Whitney U-test (for comparison of the in vivo data obtained in different groups of patients for non-normally distributed data). The values were reported as medians and ranges. For the comparison of multi-group, the Kruskal–Wallis test was used to analyze non-normally distributed data, respectively, and p-values were adjusted with the Bonferroni correction for multiple comparisons. Correlation coefficients were obtained by Spearman's rank-order method with correction for tied values. Categorical data were summarized using numbers (percentage), and were compared using the chi-squared test. For all tests, p<0.05 was considered significant.
ResultsThe clinical manifestation and endoscopic findings of patients with EGESeventeen patients (ten male, seven female) were diagnosed as EGE according to the aforementioned criteria. The most common symptoms and the endoscopic findings in our series are show in Table 1. The endoscopic findings were nonspecific, with most patients having only hyperemic and edematous mucosa.
Clinical features of patients with EGE (n=17) and healthy controls (n=24).
| EGE (n=17) | Controls (n=24) | p | |
|---|---|---|---|
| Age (mean, years) | 7.40±0.97 | 5.24±0.67 | 0.06 |
| Gender (M:F) | 10/7 | 18/6 | 0.273 |
| Food allergy (n, percentage) | 4 (23.53) | 0 (0) | 0.012 |
| Peripheral blood eosinophilic count (109/L) | 0.87±0.49 | 0.25±0.05 | 0.05 |
| Clinical manifestationAbdominal pain (n, percentage) | 13 (76.47%) | ||
| Diarrhea (n, percentage) | 4 (23.53%) | ||
| Vomiting (n, percentage) | 4 (23.53%) | ||
| Rectal bleeding (n, percentage)Endoscopic findings | 2 (11.76%) | ||
| Hyperemic and edematous mucosa | 15 (88.24%) | ||
| Friable mucosa | 4 (23.53%) | ||
| Bleeding | 3 (17.65%) | ||
| Nodular mucosa | 2 (11.76%) | ||
| Erosion | 1 (5.88%) |
Twelve patients with mild mucosal disease were successfully treated with an elimination diet. Five patients were treated with prednisolone, 0.5–1mg/day initially, which was then gradually tapered over four to six weeks. Of these five, four had relief of symptoms within one week, while one patient with abdominal pain improved within two weeks. Four patients presented a food allergy history, and no patients presented bronchial asthma or eosinophilic esophagitis.
Eosinophilic infiltrates of patients with EGEIn the present study, the definitive diagnosis was established by endoscopic biopsy in 17 patients. A total of 28 biopsy specimens were collected, including 15 from the proximal ileum and 13 from the colon. Eosinophilic infiltrates are usually patchy in distribution, and multiple deep biopsies may be necessary to establish the diagnosis. The peak eosinophilic count was above 50/high-power field (HPF) in five biopsy specimens, including three biopsy specimens from intestine and two biopsy specimens from colon (supplemental material).
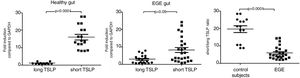
The gastrointestinal mucosal TSLPs expression of patients with EGEThere are three transcript variants (RefSeq database), but only two give rise to coding RNAs: the canonical TSLP transcript variant 1 (NM_033035_hg19 160 chr5:110407589-110411772) and a transcript variant 2 (NM_138551_hg19 64chr5:110409281-110411772). The two coding transcripts code for the long isoform of TSLP of 159amino acids (variant 1) and for short TSLP (variant 2), which encompasses the last 63 residues of long TSLP and is identical to its C-terminal portion. The authors evaluated mucosal TSLP transcripts (total: short+long TSLP isoforms) by qRT-PCR (Fig. 1). Upon normalization for GAPDH, TSLP mRNA was significantly reduced in EGE when compared with healthy controls (p<0.0001). The transcripts of long and short isoforms of TSLP were also measured in the gastrointestinal mucosa of EGE and controls; a significantly lower amount of short TSLP mRNA was observed in EGE when compared with controls (p<0.05), while a significantly higher amount of long TSLP mRNA was observed in EGE when compared with controls (p<0.05). In addition, mucosal TSLP transcripts were assessed among EGE patients with food allergy history (n=4) and those without food allergy history (n=13), with no statistically significant difference between two groups (p>0.05, data not shown). Moreover, mucosal TSLP transcripts were assessed among different clinical manifestation groups (abdominal pain, diarrhea, vomiting, and rectal bleeding group) and no statistically significant difference was observed (p>0.05, data not shown). The authors further evaluated mucosal TSLP transcripts between EGE patients treated with prednisolone (n=5) and those treated with an elimination diet (n=12), with no statistically significant difference (p>0.05, data not shown).
TSLP, short TSLP, and long TSLP expression in EGE mucosa. TSLP transcripts, quantified by qRT-PCR, in the intestines or colons of 17 patients with EGE and 24 controls. Horizontal bars are mean values. Statistical significance was calculated using the Mann–Whitney test. The horizontal bars are medians with ranges.
As the long TSLP is not expressed under steady state conditions, it is now known that the reduction in TSLP expression in the gastrointestinal mucosa of EGE compared with controls was attributable to the lack of short TSLP expression, although a significantly higher expression of long TSLP RNA was observed in EGE patients when compared with controls. The authors analyzed the short/long TSLP ratios in the gastrointestinal mucosa from patients with EGE and from patients with healthy controls. It was observed that the short/long TSLP ratios were significantly reduced in tissue from patients with EGE when compared with the results observed in healthy controls (Fig. 2).
Correlation between TSLPs expression and eosinophilic count in the gastrointestinal mucosal of EGE patientsThe mean peak eosinophilic count in the gastrointestinal mucosal of patients with EGE and healthy control was 42.79±3.50/HPF and 4.33±0.57/HPF, respectively. Peak eosinophilic count was significantly positively correlated with the expression of long TSLP mRNA in the gastrointestinal mucosal of patients with EGE (rs=0.623, p<0.005, Fig. 3A). Conversely, peak eosinophilic count was significantly negatively correlated with the expression of short TSLP mRNA in the gastrointestinal mucosal of patients with EGE (rs=−0.4474, p<0.05, Fig. 3B).
DiscussionEosinophilic gastrointestinal disorder was first described by Kaijer in 1937; it is characterized by infiltration of eosinophils into different layers of the gastrointestinal tract in the absence of secondary causes. According to previous studies, the incidence of EGE is estimated to be approximately 1–30/100,000. Recent studies and case reports have demonstrated that this incidence has been increasing.8 TSLP, an immunoregulatory cytokine constitutively expressed by enterocytes, plays an important role in preserving mucosal tolerance during homeostasis in the gut. In 2009, Harada et al.9 described the existence of two different variants for TSLP in humans, the long and short TSLP isoforms. As the sequence of the 63 amino acids that make up short TSLP is completely homologous to the C-terminus of the long form, short form-specific antibodies are not available.2,10 Hence, up to that point, the differential expression of the two isoforms had only been studied by quantitative polymerase chain reaction analyses, with primer pairs specifically targeting one or the other transcript variant. In the present study, the authors measured the mRNA level of the long and short TSLP isoforms in active patients with EGE and compared with healthy controls. It was observed that TSLP mucosal transcripts are reduced in active patients with EGE when compared with healthy controls. When analyzing the expression of TSLP isoforms in EGE, an even more complex picture was observed. As expected from the literature, the present study indicated an upregulation of the long TSLP isoform in lesional biopsy specimens of EGE, as opposed to healthy controls; unexpectedly, it was observed that short TSLP was significantly down regulated in lesional biopsy specimens of EGE. This indicates an imbalance of the two isoforms in patients with EGE, given the concomitant down regulation of short TSLP and upregulation of long TSLP.
Despite clear evidence of a dichotomy for the two TSLP isoforms in humans, the role of the short isoform was poorly understood. In vivo, long TSLP is upregulated in conditions such as atopic dermatitis, asthma, ulcerative colitis, and smokeless tobacco-exposed oral mucosa, while it is absent in healthy tissues.9,11,12 In contrast to long TSLP, short TSLP is the predominant form of TSLP constitutively expressed in healthy tissue. Tsilingiri et al.10 revealed immunomodulatory activity for short TSLP. They found that, in presence of short TSLP, dendritic cells (DCs) respond more mildly to bacterial infection. Two recent reports also have shown an antimicrobial activity of peptides within the short TSLP sequence, indicating a possible additional homeostatic activity of short TSLP in vivo.11,12 Under inflammatory conditions, short TSLP appears to be downregulated as exposure to S. typhimurium in Caco-2 cells.12 Interestingly, a significant downregulation of the short TSLP transcript in untreated celiac disease patients was observed,13 which is in line with the previous observations for reduced short TSLP expression in another intestinal pathology, Crohn's disease.14,15 This contributes to chronic inflammation due to the consequent impairment in tolerogenic dendritic cell differentiation. By contrast, when long TSLP is upregulated, such as in patients with atopic dermatitis and ulcerative colitis (presumably through NF-κB activation, which is a hallmark of inflammation in both inflammatory bowel disease and atopic dermatitis),16–18 a Th2 component is induced. Co-culture of long TSLP-stimulated DCs with allogeneic CD4+T cells results in the generation of inflammatory Th2 cells, producing classical Th2 cytokines, including IL-4, IL-5, and IL-13. The expression pattern for the two translated TSLP variants in patients with EGE reveals that the long form is induced by gastrointestinal inflammation, while the short form appears to be downregulated by gastrointestinal inflammation.
During eosinophilia, eosinophils are regulated by chemokines and cytokines. TSLP promotes eosinophilia by induction of Th2 cytokine, including IL-4, IL-5, and IL-13, expression.19 Transgenic mice with TSLP expression present elevated serum IL-5 levels. Moreover, TSLP-induced chemotaxis of eosinophils increased after preincubation with IL-3 and tumor necrosis factor alpha (TNFα) when compared with eosinophils that were not preincubated with IL-3 and TNFα. Studies have shown that TSLP-induced phosphorylation of L-plastin and eosinophil migration may be mediated by the protein kinase C (PKC) signaling pathway. Based on these data, the authors the correlation between TSLP and the peak eosinophilic count in the gastrointestinal mucosal of patients with EGE. The present data showed that the expression of long TSLP mRNA is significantly positively correlated with peak eosinophilic count in the gastrointestinal mucosal of EGE patients. However, the expression of short TSLP mRNA is significantly negatively correlated with the peak eosinophilic count in the gastrointestinal mucosal of patients with EGE.
Taken together, there is a concomitant downregulation of short TSLP and upregulation of long TSLP in the gastrointestinal mucosa of patients with EGE. The abnormal TSLPs expression in the gastrointestinal mucosa of patients with EGE may be involved in the pathogenesis of EGE. The potent action demonstrated by TSLPs in EGE suggests that these two aspects should be taken into account. On the one hand, they might block the inflammatory potential of long TSLP when this is upregulated and, on the other hand, it might be possible to re-establish immune homeostasis through administration of short TSLP or drugs involved in its upregulation, when short TSLP is downregulated.
There were some limitations to this study. First, this was a single center study and the sample size was not large, thus restricting generalizabilty. However, the present data was representative enough to observe the alteration of mucosal TSLP expression with gastrointestinal mucosa damage in EGE. Further studies with larger numbers of patients are required to confirm the present findings. Second, the authors only investigated the changes in the mucosa of two locations from patients with EGE. Ideally, more samples should be taken from the gastrointestinal tissue, where the inflammatory events are occurring. However, samples obtained through gastrointestinal biopsies are limited by complexity, especially among pediatric patients.
FundingThis study was supported by the National Natural Science Foundation of China (81672020).
Conflicts of interestThe authors declare no conflicts of interest.
The authors would like to thank all patients who agreed to provide biopsies and tissue samples, without whom this study would not have been possible. YJ acknowledges the support of the National Natural Science Foundation of China(81672020).
Please cite this article as: Guo H, Ji X, Yang G, Jin Y. Abnormal thymic stromal lymphopoietin expression in the gastrointestinal mucosa of patients with eosinophilic gastroenteritis. J Pediatr (Rio J). 2020;96:350–5.