To evaluate the incidence of diarrheal disease (DD) and acute respiratory infection (ARI) in children undergoing supplementation of zinc and other micronutrients through the use of sprinkles, as well as their acceptance by these patients.
MethodThis was a randomized double-blinded clinical trial of 143 healthy institutionalized children, aged 6 to 48 months. They were randomized into two groups and received daily zinc and micronutrients – test group (sprinkles), or micronutrients without zinc - control group. Children were supplemented for 90 days and followed regarding the outcomes of DD and ARI.
ResultsOf the randomized children, 52.45% belonged to the test and 47.55% to the control group. The incidence of DD in the test group was 14.7% and was 19.1% in the control group. The test group showed a lower risk of developing DD when compared to controls, but this finding was not statistically significant (RR=0.77 [0.37 to 1.6], p=0.5088). ARI had high incidence in both groups, 60% in the test group and 48.5% in the control group, with an increased risk of developing the disease in the test group, but with no statistical significance (RR=1.24 [0.91 to 1.68], p=0.1825). Regarding acceptance, the mean percentage of consumption, in days, of the entire content of the sachets containing sprinkles was 95.72% (SD=4.9) and 96.4% (SD=6.2) for the test and control groups, respectively.
ConclusionsZinc supplementation through the use of sprinkles did not reduce the incidence of DD or ARI among the evaluated children. The sprinkles were well accepted by all study participants.
Avaliar a incidência de doenças diarreicas (DA) e infecção respiratória aguda (IRA) em crianças submetidas à suplementação de zinco e outros micronutrientes através dos sprinkles, bem como a aceitação destes pelos pacientes.
MétodoEnsaio clínico, duplo cego, randomizado, realizado com 143 crianças institucionalizadas, saudáveis, de seis a 48 meses. As mesmas foram randomizadas em dois grupos e receberam diariamente zinco+micronutrientes – grupo teste (sprinkles), ou apenas micronutrientes sem zinco – grupo controle. As crianças foram suplementadas por 90 dias e acompanhadas quanto aos desfechos de DA e IRA.
ResultadosDas crianças randomizadas, 52,45% pertenciam ao grupo teste e 47,55% ao controle. A incidência de DA no teste foi de 14,7%, e no controle, 19,1%. O grupo teste apresentou menor risco de desenvolver DA em relação ao controle, porém esse achado não foi estatisticamente significante (RR=0,77 [0,37-1,6]; p=0,5088). A IRA apresentou incidência elevada em ambos os grupos, sendo 60% no teste e 48,5% no controle, com risco maior de apresentar a doença no grupo teste, porém sem significância estatística (RR=1,24 [0,91-1,68]; p=0,1825). Quanto à aceitação, o percentual médio de consumo, em dias, de todo conteúdo dos sachês contendo sprinkles foi 95,72% (DP=4,9) e 96,4% (DP=6,2), para o teste e controle, respectivamente.
ConclusõesA suplementação de zinco através dos sprinkles não reduziu a incidência de DA ou IRA entre as crianças avaliadas. Os sprinkles foram bem aceitos por todos os participantes do estudo.
Diarrheal diseases (DD) and acute respiratory infections (ARI) are a serious health problem in developing countries. They are the leading causes of morbidity and mortality in children younger than 5 years.1,2 Estimates published by the World Health Organization (WHO) in 2008 showed that respiratory infections affected 17% of children in this age group.3 Diarrheal diseases, in turn, are the cause of death of 2.5 million children/year.4
In Brazil, in 2009, respiratory infections killed 2,733 children younger than 5 years, which corresponds to 5.46% of deaths in this age group. The data also showed that ARI mortality (around 4.5% to 5.0%) was not much different in proportion among the regions of the country, although it was higher in the North (7.51%) and Midwest (6.47%) Regions.5
Between 1998 and 2008, 33,363 deaths related to diarrhea were recorded in the country in individuals younger than five years; of these, 82% were younger than one year.6 These data differ according to region. While in the Southeast the number of episodes/child/year is 1.04, in the Northeast, it increases to 5.55.7,8
It has been observed that even after the advent of oral rehydration solution and vaccination against rotavirus, both very effective methods to fight diarrheal diseases, their incidence still remains high.6,9,10
With the goal of reducing infant mortality caused by DD, in 2001, the WHO analyzed 12 studies involving children aged 1 months to 5 years who had diarrhea to verify the effect of zinc on the disease. The results showed that this mineral supplementation was associated with a reduction in the duration of episodes of DD by 25% and decreased progression to persistent diarrhea. The studies also showed reduced incidence of diarrhea by two to three months after supplementation.11
Based on these results, since 2006, the WHO and the United Nations Children's Fund (UNICEF) recommend zinc supplementation to treat and prevent future episodes of diarrhea,11,12 considering this is an essential micronutrient whose deficiency may increase the risk of infectious diseases.13
Zinc has also been shown to be effective in preventing infectious diseases of the respiratory tract. A meta-analysis conducted by the International Zinc Nutrition Consultative Group (IZiNCG) in 1999 found a 41% reduction in the incidence of pneumonia among the supplemented individuals. Several studies have identified the benefits of zinc are not limited to specific groups, and that intervention should include all children at risk, mainly those living in developing countries with high rates of morbidity and mortality from infectious diseases.14In Canada, in the 1990s, with the objective of preventing micronutrient deficiencies in children, sprinkles were developed as a strategy for home fortification of foods. The sprinkles are sachets containing dried and predetermined micronutrients encapsulated by a thin lipid layer, which prevents interaction with other nutrients and confers an almost imperceptible level of food modification regarding color, flavor, and texture, facilitating their acceptance by children.15–17
This study aimed to evaluate the incidence of DD and ARI in children receiving zinc supplementation combined with other micronutrients through the use of sprinkles, as well as their acceptance.
MethodsStudy design and settingBetween August and November of 2009, a randomized, controlled, double-blinded study was conducted in a non-profit day care center located in a lower socioeconomic class neighborhood of Salvador, capital of the state of Bahia, Brazil. Data were collected by the staff of Centro de Pesquisa Fima Lifishitz – Universidade Federal da Bahia. The sprinkles were donated by Emory University, Atlanta, USA.
Sample size calculationA sample was calculated to detect a 20% reduction in the occurrence of diarrheal episodes and respiratory infections compared to commonly observed rates over a period of three months in the children attending the studied day care center. Considering a beta error of 0.80 and an alpha error of 0.05, it was necessary to recruit at least 60 subjects per group.
EligibilityThe inclusion criteria were: healthy children aged 6 to 48 months, of both genders, whose parents or legal guardians consented to participation by signing an informed consent, who agreed not to offer any vitamin and/or mineral supplement during the study period, except for the sprinkles, which were sent home on weekends and holidays.
The exclusion criteria were: participants with severe malnutrition (z-score W/H<-3), severe anemia (Hb<9.0mg/dL); any active severe illness requiring hospitalization, including DD or ARI; and history of underlying disease that could eventually interfere in the evaluation.
RandomizationChildren enrolled in the institution considered eligible for the study were randomized into two groups: group A (test) and group B (control). Randomization was performed by rooms and nurseries, according to a computer-generated sequence. The study's medical team was responsible for identifying eligible subjects, collecting the medical history, and performing the physical examination. Finally, blood was collected for complete blood count analysis, to eliminate subjects with severe anemia from the analysis.
Nutritional assessmentThe nutritional assessment and diagnosis were made at baseline and at the end of the intervention. Weight was measured using a digital scale, appropriately calibrated and suitable for every age group. Length measurement in children younger than 2 years was performed using an infantometer; a stadiometer was used for the older children. The z-scores of the weight/height (W/H), height/age (H/A), and weight/age (W/A) indicators were calculated using the Anthro program, available from the WHO. The nutritional diagnosis was made following the WHO criteria.18
Before the intervention, all meals served to the children (four per day) were calculated using food composition tables19,20 and product labels, in order to quantify the macro and micronutrients present in a serving of 100g, followed by estimates of daily consumption. To quantify the consumption, the preparations were weighed before and after ingestion of each meal using a scale accurate to 1g. All records of consumption and anthropometry were made by the study dietitians.
InterventionDuring 90 days, the subjects in group A received one sachet of sprinkles with added zinc and micronutrients daily, whereas group B received the same micronutrients, without zinc (Table 1).
Sprinkle composition.
| Components | Sachet (1 g) |
|---|---|
| Vitamin A (vitamin A acetate) | 375 mcg |
| Vitamin B1 (thiamine mononitrate) | 0.5 mg |
| Vitamin B2 (riboflavin) | 0.5 mg |
| Vitamin B6 (pyridoxine) | 0.5 mg |
| Vitamin B12 (cyanocobalamin) | 0.9 mcg |
| Vitamin C (ascorbic acid) | 35 mg |
| Vitamin D3 (cholecalciferol) | 5.0 mcg |
| Vitamin E (vitamin E acetate) | 6.0 mg |
| Niacin (niacinamide) | 6.0 mg |
| Copper (copper sulfate) | 0.6 mg |
| Iodine (potassium iodide) | 50 mcg |
| Iron (ferrous fumarate) | 12.5 mg |
| Zinc (zinc gluconate)a | 5.0 mg |
| Maltodextrin (carrier) | Q.S. |
| Silicon dioxide (carrier) | Q.S. |
The sprinkles were produced by Hexagon Nutrition Pvt Ltd – India, under license by SGHI, Canada.
The supplements were mixed in a small portion of the meal, always at the same time. Due to the nutritional composition and day-care routine, the most adequate meal for addition of supplements and the one best accepted by the children was the afternoon snack (silver banana). The supplements were only opened and added to food immediately before serving, by dietitians not blinded to the study, as the sachets were identified for the presence of zinc. The snacks were monitored by the team members (physicians and dietitians blinded to the study) to prevent exchange of plates or loss of the food serving that contained the supplements, as well as to identify those who did not ingest it.
Based on the assessment of what had been eaten at the afternoon snack, it was recorded in a form whether the child had ingested all the supplement (the entire snack), at least half of it (half the snack), or none of it. Based on this information, acceptance of the supplement was assessed.
On weekends, holidays, and planned absences, to ensure the continuity of the intervention process, parents or guardians were given enough supplements for consumption during the period. They were instructed to offer them once per day, in a meal similar to that served at the daycare.
Diagnosis of DD and ARIDD was defined as the presence of three or more liquid or semi-liquid stools in 24hours, lasting less than 14 days. The Brazilian Ministry of Health criteria was used for the diagnosis of ARI.21 Daily, prior to the start of the day at the daycare, the parents/guardians were questioned by the nursing staff, which were properly trained, as to the health status of the children. If any child was identified as having one of the outcomes (DD and/or ARI), he or she was referred for evaluation by the physicians of this project. The children were assessed on the first day of their return after the weekends, holidays, and absences, and parents or tutors were asked about the children's health status. The medical team performed daily rounds to identify events of interest. All physicians involved were blinded to the study.
Ethics committeeThe project was registered at the National Commission on Research Ethics (CONEP) on 10/28/2008, and approved by the Research Ethics Committee of the Hospital Universitário Professor Edgard Santos, under protocol No. 042/2008, on November 6, 2008.
Data analysisThe analyses were performed considering only the data collected at the daycare. To assess the main variables (presence of DD and ARI), measures of occurrence (incidence) and of association (relative risk - RR) were calculated, as well as the relative risk reduction (RRR) and the respective confidence intervals (CI). Fisher's exact test was used to compare characteristics between groups at baseline. The parametric Student's t-test was used to analyze the continuous variables (anthropometric indicators) with normal distribution. A Poisson regression model was used to identify interactions and confounding factors for DD and ARI. The Kaplan-Meier test was used to assess duration of DD episodes. The overall significance level was set at 5%. The database was built using the EpiData software, release 3.3.1, and the analyses were performed using R.
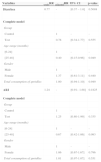
ResultsOf the 150 children enrolled in the study, seven were excluded: three for age less than six months and four for no longer attending the daycare. Thus, 143 were randomly assigned to the two groups. Of these, 52.45% (n=75) were assigned to group A (test) and 47.55% (n=68) to group B (control). All children completed the study. The groups were similar regarding admission characteristics – gender, age, and nutritional status (Table 2). Severe anemia was not identified in any of the participants.
Characteristics at admission of groups A (test) and B (control).
| Characteristics | Group An (%) | Group Bn (%) | p-valuea |
|---|---|---|---|
| n | 75 | 68 | |
| Gender | |||
| Male | 48 (64) | 35 (51.5) | 0.1745 |
| Female | 27 (36) | 33 (48.5) | |
| Age (months) | 0.8675 | ||
| 6-24 | 38 (50.7) | 33 (48.5) | |
| 24-48 | 37 (49.3) | 35 (51.5) | |
| Mean ageb(months) | 25.2 | 26.11 | |
| Nutritional status (W/H) | |||
| Malnourished | 02 (2.7) | 01 (1.5) | 0.596 |
| Normal weight | 62 (82.7) | 55 (80.9) | |
| Risk of overweight | 08 (10.7) | 11 (16.2) | |
| Overweight | 03 (4.0) | 01 (1.5) | |
| Obesity | 0 (0) | 0 (0) | |
| Nutritional status (H/A) | |||
| Adequate stature | 53 (70.7) | 55 (80.9) | |
| Low stature | 18 (24.0) | 8 (11.8) | 0.1604 |
| Severe low stature | 4 (5.3) | 5 (7.4) | |
H/A, height/age; W/H, weight/height.
The anthropometric indicators W/H, W/A and H/A were also similar between groups at baseline (Table 3). However, after the intervention (p-values not shown in Table), there was an increase in mean z-scores of W/H and W/A in both groups, whereas in controls this improvement was statistically significant for both W/H (p=0.033) and W/A (p=0.005). The same result was not observed for the H/A ratio: in the test group, it maintained the same mean (p=0.9634) and in controls, it showed a slight decrease (p=0.007).
Medians (Md) and interquartile intervals (IqI) of z-scores of anthropometric indicators W/H, H/A, and W/A in the test (Group A) and control (Group B) groups before and after the intervention.
| Anthropometric indicators | Group A | Group B | p-value* | ||
|---|---|---|---|---|---|
| Md | IqI | Md | IqI | ||
| Before intervention | |||||
| W/H | 0.10 | 1.18 | 0.22 | 1.59 | 0.6334 |
| H/A | -0.52 | 1.09 | -0.33 | 1.22 | 0.1577 |
| W/A | -0.15 | 1.24 | 0.06 | 1.71 | 0.4582 |
| After intervention | |||||
| W/H | 0.12 | 1.15 | 0.32 | 1.45 | 0.4177 |
| H/A | -0.57 | 1.10 | -0.38 | 1.32 | 0.7726 |
| W/A | -0.1 | 1.14 | 0.08 | 1.39 | 0.3937 |
H/A, height/age; W/A, weight/age; W/H, weight/height.
The calculation of ingestion, including the addition of sprinkles, showed no difference in the mean consumption of energy (A: 72.09kcal/kg vs. B: 72.96kcal/kg; p=0,728), fibers (A: 6.7g vs. B: 6.83g; p=0.564), proteins (A: 2.08g/kg vs. B: 2.11g/kg; p=0.654), carbohydrates (A: 9.31g/kg vs. B: 9.48g/kg; p=0.602) and lipids (A: 2.95g/kg vs. B: 2.95g/kg; p=0.949) between the groups. Only zinc levels were different between the groups, due to the supplementation in the test group (A: 7.16mg vs. B: 2.3mg; p<0.001).
It can be observed that calorie consumption accounted for over 80% of the recommended for the age range. Macronutrients were within the recommended levels by DRIs, except for proteins, which exceeded nearly 50% of the recommendations. Fibers accounted for 1/3 of those proposed for the age range.
The incidence of DD in the test group was 14.7% (n=11), whereas in the control group it was 19.1% (n=13). Descriptively, the test group showed a lower risk of developing DD when compared to controls, but this finding was not statistically significant (RR [95% CI]=0.77 [0.37 to 1.6], p=0.5088). The RRR was 23.3%.
Variable adjustment (gender, age range, and total consumption of sprinkles) was made using the Poisson regression model (Table 4); none of them acted as confounders for the association with the test group. The incidence of DD, as well as the age range, was not an interaction variable (p=0.4219). There was, however, a lower risk of developing DD in those older than 24 months, regardless of the group (RR [95% CI]=0.41 [0.157 to 0.94], p=0.045).
Relative risks, 95% confidence intervals, and p-values for the association between type of supplementation, age range, gender, and total consumption of sprinkles adjusted by Poisson regression.
| Variables | crudeRR | adjustedRR | 95% CI | p-value |
|---|---|---|---|---|
| Diarrhea | 0.77 | - | [0.37 – 1.6] | 0.5088 |
| Complete model | ||||
| Group | ||||
| Control | 1 | - | - | |
| Test | 0.78 | [0.34-1.77] | 0.555 | |
| Age range (months) | ||||
| [6-24] | 1 | - | - | |
| [25-48] | 0.40 | [0.15-0.96] | 0.049 | |
| Gender | ||||
| Male | 1 | - | - | |
| Female | 1.37 | [0.61-3.11] | 0.440 | |
| Total consumption of sprinkles | 1.00 | [0.94-1.10] | 0.949 | |
| ARI | 1.24 | - | [0.91– 1.68] | 0.1825 |
| Complete model | ||||
| Group | ||||
| Control | 1 | - | - | |
| Test | 1.25 | [0.80-1.99] | 0.335 | |
| Age range (months) | ||||
| [6-24] | 1 | - | - | |
| [25-48] | 0.67 | [0.42-1.06] | 0.093 | |
| Gender | ||||
| Male | 1 | - | - | |
| Female | 1.06 | [0.67-1.67] | 0.796 | |
| Total consumption of sprinkles | 1.01 | [0.97-1.07] | 0.551 | |
Residual deviance (ARI): 89.807 at 138 degrees of freedom; AIC (Akaike's Information Criterion): 255.8.
Residual deviance (Diarrhea): 80.158 at 138 degrees of freedom; AIC: 138.2.
ARI, acute respiratory infection; RR, relative risk.
Regarding the duration of episodes, it was observed that most participants had only one day of DD in both groups; in the test group, six days was the maximum duration (n=1), and in the control group, five (n=1). There was no significant difference between the groups (p=0.846).
ARI had high incidence in both groups, 60% (n=45) in the test and 48.5% (n=33) in the control group; the test group showed a greater risk of having the disease, but without statistical significance (RR [95% CI]=1.24 [0.91 to 1.68], p=0.1825).
The Poisson regression model was used to adjust variables for ARI (Table 4), which did not identify any confounders for the association between the test group and the incidence of ARI. The age range was not shown to be an interaction variable (p=0.482). However, unlike what was observed for DD, there was a lower risk of developing ARI in children younger than 24 months, regardless of the group (RR [95% CI]=0.65 [0.48 to 0.89], p=0.007).
Sprinkle acceptance was evaluated through the consumption at the daycare. The mean percentage of days on which the test and control groups consumed the entire contents of the sachets was 95.72% (SD=4.9) and 96.4% (SD=6.2), respectively. Partial acceptance was 2.5% (SD=3.4) for the test and 1.5% (SD=2.5) for the control group.
DiscussionOver several years, researchers have been making efforts aimed at reducing the high rates of morbidity and mortality caused by ARI and DD. In this scenario, some studies have been published showing the positive results obtained with zinc supplementation in the treatment and prevention of these diseases.11,14 Despite the scientific evidence, the present study results showed no statistically significant differences regarding supplementation.
Concerning the nutritional status, improvement was observed in mean z-scores of weight indicators in both groups. However, due to the significant increase in the control group, the result cannot be solely attributed to zinc supplementation – other factors, such as the presence of other micronutrients, might be acting to improve these children's weight.
As for height, no alteration was observed in the test group. In the control group, the decrease in the mean z-score for H/A, although statistically significant, was not relevant from a clinical viewpoint, considering that this decrease did not cause alterations in the nutritional status.
In this respect, this study differs from another recently published study, which showed that zinc supplementation effectively contributed to the growth of children under the age of 5 years, and that the dose of 10mg/day, offered during 24 weeks, promoted best results for height increase.22
Although it is not possible to identify the reasons for the abovementioned discrepancy, both the short intervention period, perhaps insufficient to identify changes in anthropometry especially with regard to height, and the dose of zinc used, may have been limiting factors in this study.
Regarding DD, although the incidence was lower in the test group, the difference compared to the control group was not significant and, additionally, there was no difference in the duration of disease episodes between the groups. This result is similar to those of two other studies with children supplemented with zinc for 14 days, which showed no significant effects in reducing the incidence or prevalence of DD.23,24
The different result obtained in a study conducted in Brazil is noteworthy. In that study, zinc supplementation was proven to be effective in reducing the duration and number of stools in children aged 3 to 60 months who had DD.25
Contrary to what was described for DD, the test group showed a higher risk of developing ARI when compared to control group; however, these findings were not statistically significant. A meta-analysis published by Aggarwal et al. evaluated the performance of zinc supplementation and concluded that it reduced the incidence of ARI in children by 8%.26
A Brazilian study with children with low birth weight found a 33% reduction in the prevalence of coughing among the group supplemented with 5mg/day of zinc. No significant difference, however, was observed when other characteristic symptoms of respiratory infection (fever, increased respiratory rate, and fatigue) were analyzed.27
The fact that this study found no positive results regarding zinc supplementation on the occurrence of the assessed diseases may be due to the healthy status of the study population. Most published studies used as a sample children who already had DD or ARI,23,24 malnutrition, or impaired immune function, such as those with human immunodeficiency virus (HIV) infection.28
It is worth mentioning that several factors, such as low immunity, malnutrition, and poor hygiene10,29 are involved in the etiology of the diseases being assessed. The etiology of ARI, for instance, has multiple conditioning factors that are difficult to control, such as weather changes and pollution.30
The zinc dose used in this study is another important variable to consider, as it may have been insufficient to achieve the expected effect, as the recommended dose for treatment and prevention of DD and ARI is 10 to 20mg/day.12 Larger amounts of zinc were not offered as the objective was to evaluate the supplementation through the use of sprinkles with 5mg/zinc, whose recommended use is of one sachet/day.15 Therefore, in a healthy population, zinc supplementation with 5mg/day may not be as effective in the prevention of infectious diseases.
Regarding acceptance, sprinkles appear to be an efficient way of supplementing zinc intake, as well as other micronutrients. The data analyzed in this study reflect an acceptance>90%. It is noteworthy the fact that the sprinkles were added to the food that was best accepted by the participants and, as they provide almost no change in flavor or color of preparations, their consumption may be associated with the acceptance of the food offered to the children.
Other studies that assessed the acceptance of sprinkles16,17 also showed similar results, suggesting they are a good option to fight micronutrient deficiencies, especially because they do not alter the organoleptic characteristics of food.
Based on the present study, it can be concluded that zinc supplementation through the use of sprinkles had no impact in reducing the incidence of DD and ARI, nor influence on the nutritional status of the study population. However, the good acceptance of sprinkles offers a new form to administer supplemental micronutrients, representing an innovation in the management of children's nutritional deficiencies.
FundingThe sprinkles were donated by Emory University, Atlanta, GA, USA.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Sampaio DL, de Mattos AP, Ribeiro TC, Leite ME, Cole CR, Costa-Ribeiro Jr H. Zinc and other micronutrients supplementation through the use of sprinkles: impact on the occurrence of diarrhea and respiratory infections in institutionalized children. J Pediatr (Rio J). 2013;89:286–93.