The aim of this review was to address advances in management and treatment of acute viral bronchiolitis in infants.
SourcesA systematic review search was made including all articles published in English between 2010 and 2017, and available in the electronic databases PubMed and Cochrane Central Register of Controlled Trials (CENTRAL) and specialized register of the Acute Respiratory Infections Group (Cochrane review group). The following MESH terms in English were included, using different Boolean operators for the search strategy: “bronchiolitis, viral,” “diagnosis,” “epidemiology,” “etiology,” “therapy,” “virology,” “prevention and control,” “respiratory syncytial virus, human.” Additional filters were used.
Summary of findingsFew effective interventions are recommended for the management of RSV bronchiolitis in young infants. The main goal is to ensure an adequate oxygen supplementation and fluid balance whenever deemed necessary. Hypertonic saline nebulization is helpful only for hospitalized infants. Numerous antiviral drugs and specific vaccines for RSV are under evaluation and foretell advances in disease management in the near future.
ConclusionA number of promising new technologies are advancing in the field. Until new interventions became feasible, early detection and modification of preventable risk factors is essential to improve outcomes.
O objetivo desta análise é abordar avanços no manejo e no tratamento de bronquiolite viral aguda em neonatos.
FontesUma pesquisa de análise sistemática foi realizada e incluiu todos os artigos publicados em inglês entre 2010 e 2017 e disponíveis nas bases de dados eletrônicas PubMed, no Registro Central de Ensaios Controlados (CENTRAL) da Cochrane e no registro especializado do Grupo de Infecções Respiratórias Agudas (grupo de revisão Cochrane). Os seguintes termos MESH em inglês foram incluídos na abordagem utilizando diferentes operadores booleanos para a estratégia de pesquisa: “bronquiolite, viral”, “diagnóstico”, “epidemiologia”, “etiologia”, “terapia”, “virologia”, “prevenção e controle”, “vírus sincicial respiratório, humano”. Foram utilizados filtros adicionais.
Resumo dos achadosPoucas intervenções efetivas são recomendadas para o manejo da bronquiolite por VSR em neonatos jovens. O principal objetivo é garantir uma suplementação de oxigênio adequada e equilíbrio de fluidos sempre que considerado necessário. A nebulização de solução salina hipertônica ajuda apenas em casos de neonatos hospitalizados. Vários medicamentos antivirais e vacinas específicas contra VSR estão em fase de avaliação e predizem avanços no manejo da doença no futuro próximo.
ConclusãoVárias novas tecnologias promissoras estão avançando no campo. Até que as novas intervenções se tornem viáveis, a detecção precoce e a modificação de fatores de risco de prevenção são fundamentais para melhorar os resultados.
Respiratory syncytial virus (RSV) bronchiolitis is the most frequent cause of lower respiratory tract illness (LRTI) and hospitalization in young infants worldwide.1,2 This disease is associated with up to 199,000 deaths every year in children under the age of 5 years, and approximately million hospitalizations annually.1–4 Of these deaths, 99% occur in developing countries.1 In developed countries, RSV deaths are infrequent and associated with chronic lung disease, neuromuscular disorders, heart disease, Down's syndrome, and preterm birth.5 By the age of 2 years, over 95% of the children have been infected by the virus.6
Acute RSV bronchiolitis is a seasonal disease, which often starts every year between fall and spring, and peaks in winter. The tropics are the exception, and there is no specific seasonality in these regions, although some epidemics are hypothesized to be associated with the rainy season.2 RSV infection is typically mild and begins with upper respiratory tract signs, mimicking a common cold.7,8 After a few days, some patients will progress to experience disease affecting the distal bronchioles, with clinical signs of tachypnea, wheezing, crackles, rhonchus, and chest retractions.7,9 Approximately 1–3% of infected children develop feeding difficulties, apnea, or are unable to maintain adequate oxygen saturation (SpO2), requiring hospital admission for supportive therapy.2,4,10 A small number of infants, especially those with co-morbidities, will progress to respiratory failure or death.1,2,5 There are several studies suggesting an association between severe bronchiolitis by RSV and recurrent wheezing, an association that disappears by the end of the first decade of life.11–13 With greater frequency than RSV, rhinoviruses, when combined with early life atopic sensitization, are associated with asthma.14 In 2009, the total cost for hospitalizations due to bronchiolitis in the United States was close to two billion dollars. Although trends in hospitalizations rates in the US have declined between 2000 and 2009, costs have raised at the expense of increased use of intensive care for high-risk patients.15 Despite its high morbidity, the economic expenses, concerning mortality rates in developing countries, and the association of RSV with transient lung sequelae (e.g., recurrent wheezing), treatment of RSV LRTI is still symptomatic and has significant gaps. Moreover, over fifty years after its discovery, no licensed vaccine against RSV is available. Palivizumab, an effective humanized monoclonal antibody (mAb) against the RSV fusion (F) protein, is available for preterm infants, infants with BPD, and infants with cyanotic congenital heart disease.16 Even though palivizumab significantly reduces severe RSV LRTI, the drug is expensive and requires several doses, limiting its use in industrialized and developing countries. Therefore, safe and inexpensive vaccines and treatments are urgently needed to decrease the impact of RSV in children.
SourcesA systematic review search was conducted, and it included articles published in English between 2010 and 2017, available in electronic databases PubMed, Cochrane Central Register of Controlled Trials (CENTRAL), and specialized register of the Acute Respiratory Infections Group (Cochrane review group). Recent guidelines reports were also searched. The following MESH terms in English were included in the approach using different Boolean operators in PubMed: “bronchiolitis, viral,” “diagnosis,” “epidemiology,” “etiology,” “therapy,” “virology,” “prevention and control,” “respiratory syncytial virus, human”. Additional filters were used: ages between 1 and 23 months, and study methodology (clinical trial, comparative study, controlled clinical trial, guideline, meta-analysis, practice guideline, randomized controlled trial, and systematic reviews). In addition, studies were searched in the Cochrane library, following specialized register in ‘Acute Respiratory Infections Group,’ topic ‘child health,’ ‘lung and airways, respiratory infections: bronchiolitis and respiratory syncytial virus.’ MESH terms for CENTRAL search were “respiratory syncytial virus, human” and “bronchiolitis, viral.”
A search was made in ClinicalTrials.gov to find new vaccines, antibodies, and antivirals technologies against RSV infection. For this aim, terms in English were included, such as “respiratory syncytial virus infections,” filtered by study status (active, recruiting, not yet recruiting, enrolling), eligibility criteria (child and pregnant adult), interventional study type, and study phase (1–3).
Summary of findingsDiagnosis and monitoringThere is no widely validated score for RSV LRTI severity. A thorough and physical exam is critical for the initial assessment of patients. Evidence of inadequate feeding or fluid intake, history of apnea, lethargy, or moderate to severe respiratory distress (nasal flaring, tachypnea, grunting, retractions or cyanosis), and/or an SpO2≤92% in room air (cutoffs for acceptable SpO2 vary per country), warrant hospitalization, ideally in a secondary care level hospital.8,17
The pathogenesis of acute respiratory failure in RSV bronchiolitis is characterized by obstruction of the small airways, increased airways resistance, alveolar atelectasis, muscle fatigue, and hypoxemia due to mismatch between ventilation and perfusion.18 Therefore, pediatric intensive care unit (PICU) admission should be considered in patients presenting with clinical signs of exhaustion, markers of acute respiratory failure (defined as PaO2/FiO2≤300mmHg), or signs of apnea.2,8,17,18
Oxygen saturationOne of the main concerns during severe RSV LRTI is an inadequate oxygen supply to the tissues (hypoxemia).8 The arterial oxygen content that is distributed through tissues can be measured through arterial oxygen saturation (SaO2), which represents a ratio between oxyhemoglobin concentration and total hemoglobin concentration.8 The most widely used tool to assess SaO2 is pulse oximetry (SpO2), since it is a noninvasive technique.8 Despite its frequent use, SpO2 is known to present a variability of ±2%.8,17,19 Monitoring oxygen saturation is not recommended in outpatients whose clinical and feeding status are adequate, because this intervention could potentially induce unnecessary hospital admissions. Since the cutoff criteria for SpO2 tend to differ between studies and between clinical practice guidelines, a good clinical evaluation is important in the decision process.8,19 The American Academy of Pediatrics (AAP) recommends a SpO2 of 90% as a limit for the administration of supplemental oxygen.19 In the absence of clear evidence about SpO2 levels to predict the progression of bronchiolitis, the Committee of the National Institute for Health and Care Excellence (NICE), determined a SpO2 of 92% as the cutoff for supplementation.8 Other factors, including a thorough clinical evaluation and an assessment of living conditions and social risk factors, should also contribute to the decision-making process.
Blood gas testing is not routinely indicated for hospitalized patients, and it is not helpful in the routine management of viral bronchiolitis. The exception is for patients with signs of respiratory exhaustion, apnea, and unable to maintain an adequate SpO2 levels despite supplemental oxygen use.8
Etiological testingEtiologic diagnosis is common during clinical practice at hospitals, and the norm in epidemiological studies.20 While virus-specific therapies are not yet available, virus identification may help reduce the use of antibiotics.17 Real-time protein chain reaction (qPCR) is the gold standard for diagnosis, although its costs, particularly in developing countries, hinder its routine use.17,19,21 Immunofluorescence is cheaper, with very good sensitivity for RSV in particular, but it is operator-dependent.17 While there is no good reason to obtain blood cultures or leukocyte counts in patients with acute bronchiolitis, bacterial infection should be investigated in those with signs of sepsis or pneumonia.2,19 Bacterial sepsis in young infants with viral bronchiolitis, particularly episodes triggered by Gram-positive cocci, has been associated with an increased risk of death in developing countries.2 Chest radiography could be considered in patients with impeding respiratory failure.8,19,22
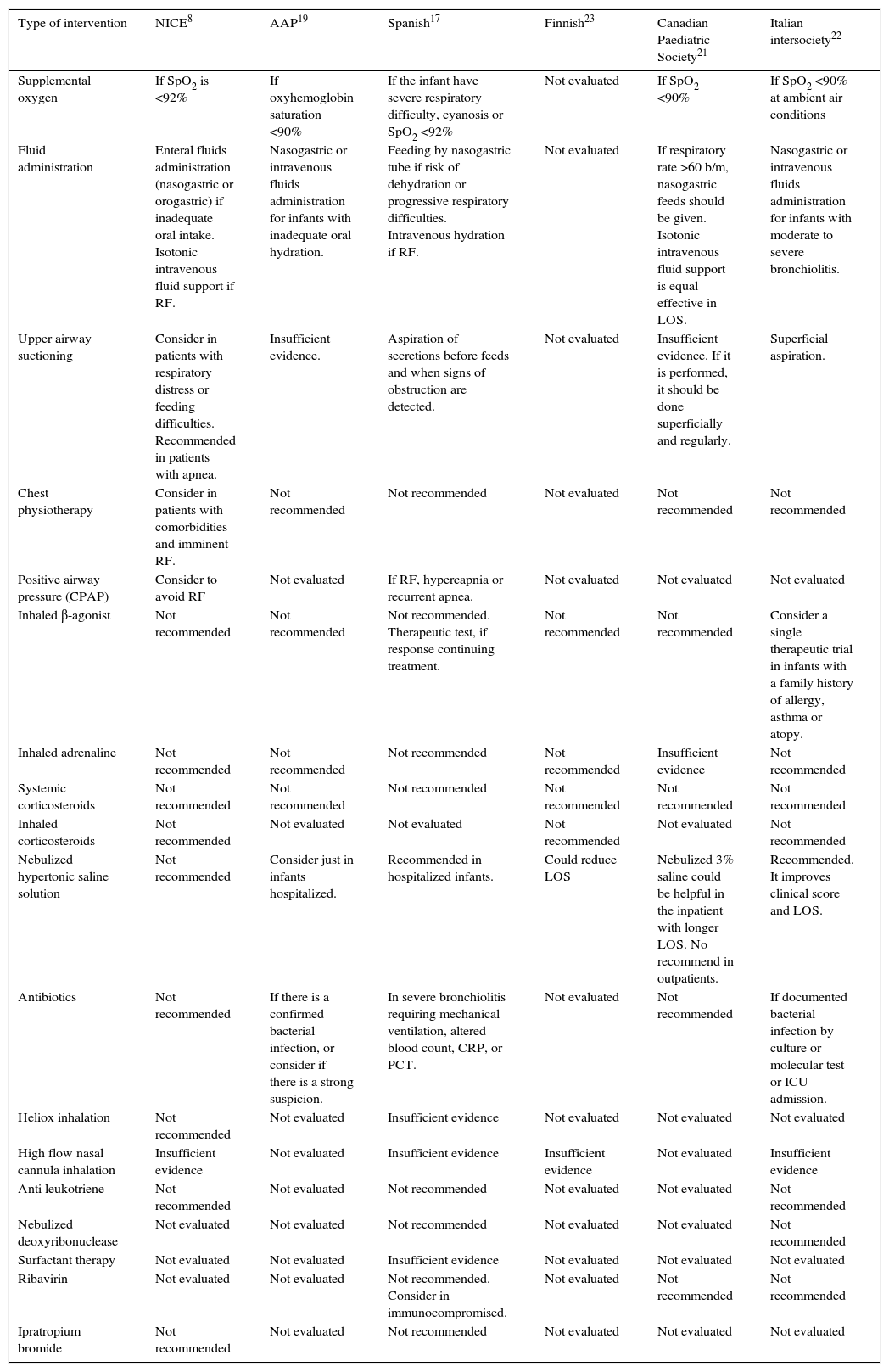
Suggested managementRespiratory supportive careSix guidelines and ten Cochrane database systematic reviews were analyzed to summarize the recommended management in acute viral bronchiolitis (Table 1).8,19,21–34 Overall, few treatment interventions are suggested for bronchiolitis, and the main goal during acute illness is to achieve an adequate fluid balance and normal oxygen saturation levels (Table 1). Infants with viral bronchiolitis present increased mucus production, epithelial debris invading the bronchiolar lumen, peribronchiolar edema, and leukocyte infiltration. In addition, small airways and alveolar sacs in development are more prone to collapse, generating a ventilation/perfusion imbalance that often leads to hypoxemia and, in advanced stages, to hypercapnia.18 Therefore, when SpO2 is below 90–92%, supplemental oxygen should be administrated to increase oxyhemoglobin levels.8,17 Several oxygen supplementation devices are available, including nasal cannulas, facial masks, and endotracheal tubes for severe cases. High-flow nasal cannula (HFNC) allows higher humidified oxygen flows, and can also provide some positive airway pressure, improving the ventilation/perfusion ratio. Despite these potential benefits, HFNC was not superior to standard oxygen supplementation when the main outcome was time on/off supplemental oxygen, time to discharge, and length of stay.31 Continuous positive airway pressure (CPAP) is a non-invasive mechanical ventilation that improves airways resistance, reducing the impact of atelectasis by distending bronchial/bronchiolar lumen diameter. Patients with worsening and severe acute bronchiolitis despite oxygen supplementation may benefit from CPAP.17
Bronchiolitis management recommendations based on guidelines.
| Type of intervention | NICE8 | AAP19 | Spanish17 | Finnish23 | Canadian Paediatric Society21 | Italian intersociety22 |
|---|---|---|---|---|---|---|
| Supplemental oxygen | If SpO2 is <92% | If oxyhemoglobin saturation <90% | If the infant have severe respiratory difficulty, cyanosis or SpO2 <92% | Not evaluated | If SpO2 <90% | If SpO2 <90% at ambient air conditions |
| Fluid administration | Enteral fluids administration (nasogastric or orogastric) if inadequate oral intake. Isotonic intravenous fluid support if RF. | Nasogastric or intravenous fluids administration for infants with inadequate oral hydration. | Feeding by nasogastric tube if risk of dehydration or progressive respiratory difficulties. Intravenous hydration if RF. | Not evaluated | If respiratory rate >60 b/m, nasogastric feeds should be given. Isotonic intravenous fluid support is equal effective in LOS. | Nasogastric or intravenous fluids administration for infants with moderate to severe bronchiolitis. |
| Upper airway suctioning | Consider in patients with respiratory distress or feeding difficulties. Recommended in patients with apnea. | Insufficient evidence. | Aspiration of secretions before feeds and when signs of obstruction are detected. | Not evaluated | Insufficient evidence. If it is performed, it should be done superficially and regularly. | Superficial aspiration. |
| Chest physiotherapy | Consider in patients with comorbidities and imminent RF. | Not recommended | Not recommended | Not evaluated | Not recommended | Not recommended |
| Positive airway pressure (CPAP) | Consider to avoid RF | Not evaluated | If RF, hypercapnia or recurrent apnea. | Not evaluated | Not evaluated | Not evaluated |
| Inhaled β-agonist | Not recommended | Not recommended | Not recommended. Therapeutic test, if response continuing treatment. | Not recommended | Not recommended | Consider a single therapeutic trial in infants with a family history of allergy, asthma or atopy. |
| Inhaled adrenaline | Not recommended | Not recommended | Not recommended | Not recommended | Insufficient evidence | Not recommended |
| Systemic corticosteroids | Not recommended | Not recommended | Not recommended | Not recommended | Not recommended | Not recommended |
| Inhaled corticosteroids | Not recommended | Not evaluated | Not evaluated | Not recommended | Not evaluated | Not recommended |
| Nebulized hypertonic saline solution | Not recommended | Consider just in infants hospitalized. | Recommended in hospitalized infants. | Could reduce LOS | Nebulized 3% saline could be helpful in the inpatient with longer LOS. No recommend in outpatients. | Recommended. It improves clinical score and LOS. |
| Antibiotics | Not recommended | If there is a confirmed bacterial infection, or consider if there is a strong suspicion. | In severe bronchiolitis requiring mechanical ventilation, altered blood count, CRP, or PCT. | Not evaluated | Not recommended | If documented bacterial infection by culture or molecular test or ICU admission. |
| Heliox inhalation | Not recommended | Not evaluated | Insufficient evidence | Not evaluated | Not evaluated | Not evaluated |
| High flow nasal cannula inhalation | Insufficient evidence | Not evaluated | Insufficient evidence | Insufficient evidence | Not evaluated | Insufficient evidence |
| Anti leukotriene | Not recommended | Not evaluated | Not recommended | Not evaluated | Not evaluated | Not recommended |
| Nebulized deoxyribonuclease | Not evaluated | Not evaluated | Not recommended | Not evaluated | Not evaluated | Not recommended |
| Surfactant therapy | Not evaluated | Not evaluated | Insufficient evidence | Not evaluated | Not evaluated | Not evaluated |
| Ribavirin | Not evaluated | Not evaluated | Not recommended. Consider in immunocompromised. | Not evaluated | Not recommended | Not recommended |
| Ipratropium bromide | Not recommended | Not evaluated | Not recommended | Not evaluated | Not evaluated | Not evaluated |
NICE, National Institute for Health and Care Excellence; AAP, American Academy of Pediatrics; SpO2, oxygen saturation; CPAP, continuous positive airway pressure; RF, risk factor; CRP, C-reactive protein; PCT, procalcitonin; ICU, intensive care unit.
Maintenance of good oral hydration and breastfeeding are crucial measures in the management of bronchiolitis. Nevertheless, if a hospitalized infant cannot receive oral feedings due to a high respiratory rate (>60breaths/min), a nasogastric tube can be placed to restore adequate feeding and hydration.8,17,19,21,22 Although intravenous isotonic fluids administration does not appear to be better than nasogastric hydration, it is used in patients admitted to PICU, those with clinical signs of exhaustion, and those intolerant to nasogastric tube feeding.8,17,19,21,22
Bronchodilators and inhaled steroidsNo evidence supports the administration of systemic corticosteroids and/or inhaled β-agonist and/or epinephrine for the treatment of hospitalized patients with viral bronchiolitis.8,17,19,21–24 Nevertheless, both the Spanish and Italian guidelines consider that inhaled β-agonists could be tried once at the beginning of treatment, especially if a patient has a personal or family history of atopy, asthma, or eczema.17,22 Some studies have suggested a potential benefit when epinephrine was used in children in an emergency room setting, lowering the risk of hospital admission.24 However, the observed clinical impact is very modest, and the patients’ length of hospital stay and days on oxygen supplementation were not significantly affected.
Hypertonic salineNebulized hypertonic saline solution has osmotic properties and proven effectiveness in patients with COPD and cystic fibrosis.35 This intervention improves airway clearance by reducing airway edema, mucus production, and rehydrating the airway surface liquid.36 Recent studies and systematic reviews suggest that nebulized hypertonic saline may be beneficial only to infants already hospitalized, but its impact in preventing admissions is poor.17,19,21–23
AntibioticsThe misuse of antibiotics in patients with viral bronchiolitis is often observed in clinical practice. Although it is sometimes difficult to distinguish between viral and bacterial infections through clinical and radiological criteria, infants with RSV LRTI are only exceptionally co-infected and need antibiotics.8,17,19 Children who progress to severe disease with respiratory failure are admitted to a PICU and will likely receive empiric antibiotic therapy for bacterial co-infections.2,17,19,22,37,38
New treatment perspectivesTo date, no effective and accessible treatments for RSV bronchiolitis are available. Recent experimental trials in adults yielded encouraging results with novel candidate antivirals. In two separate sophisticated studies, the administration of fusion inhibiting and nucleoside analog formulations improved respiratory symptoms when compared with placebo.39,40 However, in these controlled, early studies, the drugs were administrated simultaneously with experimental inoculation. Therefore, they acted against RSV before any observable signs and symptoms. Whether a similar benefit will be observed in infants when treatment is initiated days later, upon presentation to the hospital, remains unclear.
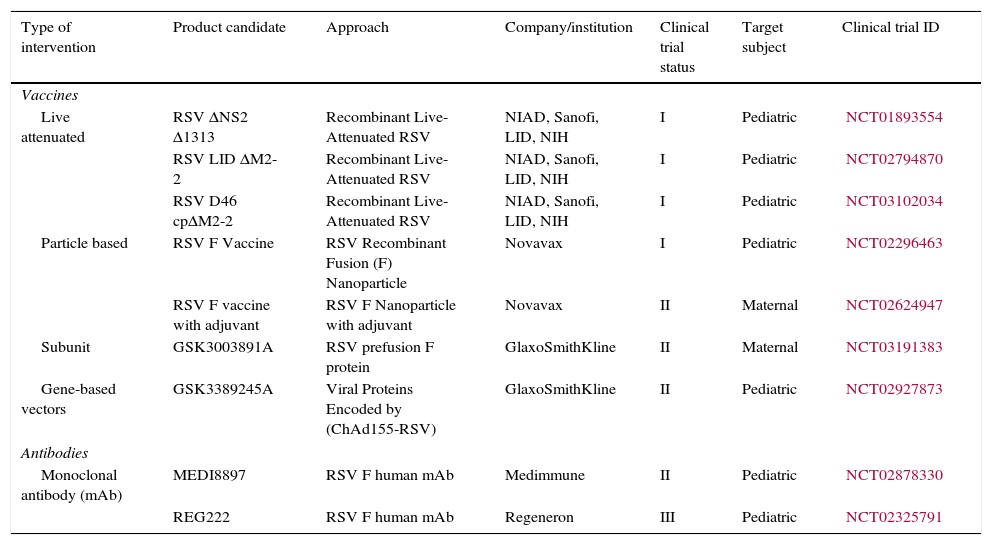
The administration of palivizumab in specific risk groups is limited by its expensive cost in many low to middle income countries.16 Consequently prevention of RSV LRTI is a public health priority, and global initiatives have advanced numerous efforts to expand the field (Table 2).
New vaccines and antibodies currently being tested.
| Type of intervention | Product candidate | Approach | Company/institution | Clinical trial status | Target subject | Clinical trial ID |
|---|---|---|---|---|---|---|
| Vaccines | ||||||
| Live attenuated | RSV ΔNS2 Δ1313 | Recombinant Live-Attenuated RSV | NIAD, Sanofi, LID, NIH | I | Pediatric | NCT01893554 |
| RSV LID ΔM2-2 | Recombinant Live-Attenuated RSV | NIAD, Sanofi, LID, NIH | I | Pediatric | NCT02794870 | |
| RSV D46 cpΔM2-2 | Recombinant Live-Attenuated RSV | NIAD, Sanofi, LID, NIH | I | Pediatric | NCT03102034 | |
| Particle based | RSV F Vaccine | RSV Recombinant Fusion (F) Nanoparticle | Novavax | I | Pediatric | NCT02296463 |
| RSV F vaccine with adjuvant | RSV F Nanoparticle with adjuvant | Novavax | II | Maternal | NCT02624947 | |
| Subunit | GSK3003891A | RSV prefusion F protein | GlaxoSmithKline | II | Maternal | NCT03191383 |
| Gene-based vectors | GSK3389245A | Viral Proteins Encoded by (ChAd155-RSV) | GlaxoSmithKline | II | Pediatric | NCT02927873 |
| Antibodies | ||||||
| Monoclonal antibody (mAb) | MEDI8897 | RSV F human mAb | Medimmune | II | Pediatric | NCT02878330 |
| REG222 | RSV F human mAb | Regeneron | III | Pediatric | NCT02325791 | |
RSV, respiratory syncytial virus.
The development of RSV vaccines is challenging. The history of enhanced respiratory syncytial virus disease (ERD), the need to immunize in early life, and the possible interference by natural maternal antibodies complicates immunization strategies.41 A suitable vaccine must ideally generate protective antibodies in infants younger than 2 months of age, who represent the group at greater risk of hospitalizations.1–3 Six different formulations of RSV candidate vaccines are being tested in preclinical and clinical studies: live attenuated or chimeric, whole inactivated, particle based, subunit, nucleic acid, and gene based vectors (Table 2).42 Furthermore, passive protection through administration of monoclonal antibodies of prolonged half-life in early life represents an attractive alternative under evaluation.43
Although palivizumab reduces severe RSV infections by 55%, its administration is cumbersome and the drug is expensive.16,43 Therefore, its use is restricted to populations at high risk for severe disease.16 A new monoclonal antibody against the pre-fusion conformation of RSV F protein (MEDI8897) has an extended half-life and higher potency (allowing a single intramuscular dose), and is an attractive potential alternative for the future.43,44 Other similar formulations are also under evaluation.45
Other perspectivesWhile several of the aforementioned strategies are under evaluation, it is important to modify preventable risk factors to protect young infants. For example, breastfeeding can significantly reduce hospitalizations due to respiratory infections. Indicted for the vast majority of children, its beneficial effect against LRTI is most notable in preterm girls.46,47 Supporting breastfeeding is therefore a critical public health action. Human milk is a bonafide, inexpensive intervention of excellent effectiveness for all infants and should also complement palivizumab in high risk infants.47
Other dietary and habit interventions that have been associated with severe LRTI include a high intake of carbohydrates or alcohol during the last trimester of pregnancy.4,48 Reducing alcohol and exposure to tobacco smoke during and after pregnancy will benefit not only the baby, but also the mother.
Other studies suggest that TLR4 heterozygosity (Asp299Gly, rs4986790) and urban habits may explain a poor response to palivizumab in preterm infants, and promote severe RSV LRTI in a subgroup of term infants in the community.49,50 It remains to be seen whether these infants will respond adequately to new generation mAbs and transplacental immunity.
ConclusionRSV bronchiolitis is the main cause of infant hospitalization worldwide, and an important cause of death in developing countries.1 A number of promising new technologies are advancing in the field. Until new interventions became feasible, early detection and modification of preventable risk factors is essential to improve outcomes. Pediatricians, families, and public health officials should contribute to these efforts through individual actions (e.g., smoking cessation) and by addressing modifiable risk factors for severe disease, while providing the best possible medical care.
Conflicts of interestMTC declares no conflicts of interest, FPP served in Advisory Boards at Pfizer, Janssen, Novavax, Bavarian Nordic and Sanofi, and RTS was a speaker for Abbvie.
Please cite this article as: Caballero MT, Polack FP, Stein RT. Viral bronchiolitis in young infants: new perspectives for management and treatment. J Pediatr (Rio J). 2017;93:75–83.