to prospectively validate a previously constructed transcutaneous bilirubin (TcB) nomogram for identifying severe hyperbilirubinemia in healthy Chinese term and late‐preterm infants.
Methodsthis was a multicenter study that included 9,174 healthy term and late‐preterm infants in eight hospitals of China. TcB measurements were performed using a JM‐103 bilirubinometer. TcB values were plotted on a previously developed TcB nomogram, to identify the predictive ability for subsequent significant hyperbilirubinemia.
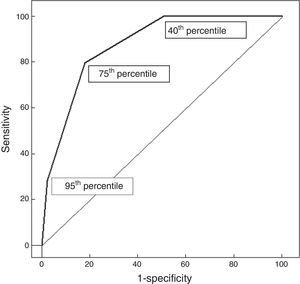
Resultsin the present study, 972 neonates (10.6%) developed significant hyperbilirubinemia. The 40th percentile of the nomogram could identify all neonates who were at risk of significant hyperbilirubinemia, but with a low positive predictive value (PPV) (18.9%). Of the 453 neonates above the 95th percentile, 275 subsequently developed significant hyperbilirubinemia, with a high PPV (60.7%), but with low sensitivity (28.3%). The 75th percentile was highly specific (81.9%) and moderately sensitive (79.8%). The area under the curve (AUC) for the TcB nomogram was 0.875.
Conclusionsthis study validated the previously developed TcB nomogram, which could be used to predict subsequent significant hyperbilirubinemia in healthy Chinese term and late‐preterm infants. However, combining TcB nomogram and clinical risk factors could improve the predictive accuracy for severe hyperbilirubinemia, which was not assessed in the study. Further studies are necessary to confirm this combination.
validar de forma prospectiva um nomograma de bilirrubina transcutânea (BTc) para identificar hiperbilirrubinemia grave em neonatos a termo e pré‐termo tardios saudáveis na China.
Métodosfoi realizado um estudo multicêntrico que incluiu 9174 neonatos a termo e pré‐termo tardios saudáveis em oito unidades da China. Foram realizadas dosagens de BTc utilizando um bilirrubinômetro. Os valores de BTc foram traçados em um nomograma de BTc para identificar a capacidade de predição de hiperbilirrubinemia significativa.
Resultados972 recém‐nascidos (10,6%) desenvolveram hiperbilirrubinemia significativa. O percentil 40 de nosso nomograma pode identificar todos os recém‐nascidos com risco de hiperbilirrubinemia significativa, porém com baixo valor preditivo positivo (VPP) (18,9%). De 453 recém‐nascidos acima do percentil 95, 275 recém‐nascidos desenvolveram posteriormente hiperbilirrubinemia significativa, com VPP elevado (60,7%), porém com baixa sensibilidade (28,3%). O percentil de 75 foi altamente específico (81,9%) e moderadamente sensível (79,8%). A área sob a curva (ASC) de nosso nomograma de BTc foi de 0,875.
Conclusõeseste estudo validou o nomograma de BTc, que pode ser utilizado para prever hiperbilirrubinemia significativa em neonatos a termo e pré‐termo tardios saudáveis na China. Contudo, combinar o nomograma de BTc e fatores de risco clínicos pode melhorar a precisão de predição da hiperbilirrubinemia grave, o que não foi avaliado neste estudo. São necessários estudos adicionais para confirmar essa combinação.
Hyperbilirubinemia causes severe damage in term and late‐preterm infants; the American Academy of Pediatrics (AAP) has formulated methods of surveillance, prediction, and therapy.1 In China, bilirubin encephalopathy continues to occur, and 348 cases were reported from 28 hospitals from January to December of 2009.2 Therefore, the identification of neonates at risk of developing significant hyperbilirubinemia and prevention of bilirubin encephalopathy remain a high priority among public health institutions.
The total serum bilirubin (TSB) level after birth was plotted on an hour‐specific nomogram by Bhutani et al., and is a valuable method for assessing the risk of subsequent severe hyperbilirubinemia.3 The AAP has recommended the measurement of TSB in a predischarge newborn population for identification of severe hyperbilirubinemia, based on the Bhutani's nomogram.1 However, measurements of TSB levels remain an invasive, stressful, and time‐consuming procedure. Transcutaneous bilirubin (TcB) is less time‐consuming, and can be used to screen for the need for blood sampling for serum bilirubin level, and thus reduce the measurements of TSB.4 The values of TcB after birth have also been plotted on an hour‐specific TcB nomogram to predict severe hyperbilirubinemia in term and late‐preterm infants.5 These hour‐specific TcB nomograms assessed pre‐test predictive ability using retrospective data from the same developed TcB nomogram.6 Theoretically, a predictive nomogram should be developed in one sample and validated in another, and some studies prospectively assessed the post‐test predictivity of TcB nomograms in different samples.7,8 The after‐effect evaluation of the constructed TcB nomogram is very important to explore the possibility for future clinical application.
In 2010, the authors developed an hour‐specific TcB nomogram based on TcB levels for the first 168h after birth in 6,035 healthy term and late‐preterm infants.9 Subsequently, they have conducted a multicenter study to verify the predictive value of the constructed TcB nomogram to identify severe hyperbilirubinemia in healthy term and late‐preterm infants.
MethodsSettingEight hospitals, including two general hospitals and six maternity hospitals, participated in the study. They were selected because they are the main tertiary centers in their areas and because they agreed to participate in this study. The Ethics Committee of the Nanjing Maternity and Child Healthcare Hospital of the Nanjing Medical University approved the study and it was adopted by each participating center. The Nanjing Maternity and Child Healthcare Hospital of the Nanjing Medical University performed the statistical analysis of the collaborative data. The Children's Hospital of Fudan University did not participate in this survey, but served as a coordinating center and supervised the study.
Selection of participantsThis multicenter prospective study was conducted between August 1, 2010, and December 31, 2011. Neonates with gestational age (GA) ≥ 35 weeks and birth weight ≥ 2,000g were included, and all sick neonates who were admitted to the intensive care unit and those who required phototherapy before discharge were excluded. The decision to use phototherapy was made by the attending physicians according to AAP guidelines.1 No prophylactic intervention for hyperbilirubinemia was used.
Measurements of TcB and TSBTcB measurements were performed with a transcutaneous jaundice meter model JM‐103 (Minolta ‐ Osaka, Japan). A single device was used for all measurements in each participating unit. All measurements were performed by trained physicians according to the instructions of the manufacturer and using the standard technique.10 The physicians obtained TcB measurements, which were performed at two sites (the forehead and mid‐sternum), and the mean of both measurements was calculated. According to previous studies, the JM‐103 is less accurate at TcB levels > 222μmol/L, which were confirmed with a TSB measurement.10 The blood samples (50μL) were drawn by heel stick, and special care was taken to avoid exposure of the collected samples to light. TSB assessment was performed in the clinical chemistry laboratory of each participating unit. Skilled physicians performed the TSB measurements using a UNISTAT reflectance bilirubinometer (Reichert‐Jung ‐ Buffalo, NY, USA), according to the manufacturer's instructions. Significant hyperbilirubinemia was defined as TSB above the 95th percentile for age (high‐risk zone), according to the hour‐specific percentile nomogram presented by the AAP guidelines.1
Follow‐up of studied neonatesIn each participating unit, the physicians obtained TcB measurements between 7:30 a.m. and 8:00 p.m., and then at time intervals of 12±2h. At least six measurements were obtained for each infant. A follow‐up evaluation within 24h to 96h after discharge was offered to all neonates, depending on TcB levels before discharge, which were described in the authors’ previous study.9 All perinatal and postpartum data of neonates were recorded in a single database for each unit during the study period. Each participating unit adopted the same clinical protocol study, method for sample collection, patient recruitment, and measurements of TcB and TSB. The coordinating center trained the investigators and supervised the implementation, so that the data from each unit could be pooled.
Statistical analysisData collected in the eight participating units were pooled by the Nanjing Maternity and Child Healthcare Hospital of the Nanjing Medical University, which conducted the statistical analysis. These data were entered into a custom‐designed spreadsheet (Microsoft Excel 2003, Microsoft Corporation ‐ Redmond, WA, USA) and checked for completeness, consistency, and accuracy by two researchers (Qing Sun and Xiaoyue Dong). After checking and verifying these data, the TcB values were plotted on the previously constructed TcB nomogram, separately by two researchers (Qing Sun and Xiaofan Sun).9 The sensitivity, specificity, positive predictive values (PPV), negative predictive values (NPV), and positive likelihood ratio (PLR) were calculated for the 40th, 75th, and 95th percentile of the TcB nomogram. Receiver operating characteristic (ROC) curve analysis was performed with the Statistical Package for Social Sciences (SPSS), version 16.0 (SPSS Inc. ‐ Chicago, IL, USA), which was used to assess the predictive ability of the TcB nomogram.
ResultsEight hospitals participated in the multicenter study. The number of neonates from each hospital are listed in Table 1; 9,174 neonates (5,385 males and 3,789 females), of whom 945 (10.3%) were late‐preterm, were enrolled in the study. Mean GA was 38.6±2.9 weeks and mean birth weight was 2,875±412g; 5,275 (57.5%) neonates were born by cesarean section. Regarding feeding, 3,165 (34.5%) neonates were exclusively breast‐fed, and 3,376 (36.8%) were exclusively bottle‐fed. Of the total population studied, 514 (5.6%) neonates were small for gestational age, 661 (7.2%) experienced a weight loss greater than 10%, and 147 (1.6%) were diagnosed as having ABO incompatibility.
Number of neonates from each hospital participating in the study.
| Collaborative units | Participants, n |
|---|---|
| Nanjing Maternity and Child Healthcare Hospital, Nanjing Medical University | 1,796 |
| Inner Mongolia Maternal and Child Healthcare Hospital | 1,703 |
| The First Affiliated Hospital of Xinjiang Medical University | 1,394 |
| Changzhou Maternal and Child Healthcare Hospital | 1,271 |
| Gynecology and Obstetrics Hospital, Fudan University | 1,046 |
| The Fifth People's Hospital of Shenzhen | 927 |
| Guangxi Maternal and Child Health Hospital | 565 |
| Guangdong Maternal and Children's Hospital, Guangzhou Medical College | 472 |
| Total | 9,174 |
Regarding the percentiles, 972/9,174 (10.6%) neonates were above the 95th percentile based on AAP guidelines (significant hyperbilirubinemia). In 453 (4.9%) neonates, predischarge TcB was > 95th percentile (Table 2). Of these, 275 neonates subsequently developed significant hyperbilirubinemia (PPV: 60.7%, sensitivity: 28.3%). In 4,037 (36.8%) neonates, predischarge TcB was < 40th percentile; none of whom developed significant hyperbilirubinemia (NPV: 100.0% and specificity: 49.2%). The 75th percentile curve showed a sensitivity of 79.8% and an NPV of 97.2%; the specificity was 81.9%. The AUC for predischarge TcB percentiles was 0.875 (Figure 1).
Predictive characteristics of percentile values of predischarge TcB for subsequent significant hyperbilirubinemia.
| Predischarge TcB | Significant hyperbilirubinemia | Predictive characteristics | |||||
|---|---|---|---|---|---|---|---|
| Percentile | Number(9174) | Present(972) | Absent(8202) | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
| Above the 95th percentile | 453 | 275 | 178 | 28.3 | 97.8 | 60.7 | 92.0 |
| Below the 95th percentile | 8,721 | 697 | 8,024 | ||||
| Above the 75th percentile | 2,258 | 776 | 1,482 | 79.8 | 81.9 | 34.4 | 97.2 |
| Below the 75th percentile | 6,916 | 196 | 6,720 | ||||
| Above the 40th percentile | 5,137 | 972 | 4,165 | 100.0 | 49.2 | 18.9 | 100.0 |
| Below the 40th percentile | 4,037 | 0 | 4,037 | ||||
NPV, negative predictive value; PPV, positive predictive value; TcB, transcutaneous bilirubin.
The PLR that determines the risk assessment for subsequent significant hyperbilirubinemia for each risk zone is presented in Table 3. Among 453 neonates with predischarge TcB in the high‐risk zone (> 95th percentile), 275 (60.7%) subsequently developed significant hyperbilirubinemia. Conversely, 178 newborns in the 95th percentile did not develop significant hyperbilirubinemia (PLR = 12.9) (Table 3). Of the 1,805 newborns in the upper‐intermediate risk zone (76th to 95th percentile), 501 (27.8%) jumped to the high‐risk zone (PLR = 4.4). Of the 2,879 neonates in the lower‐intermediate risk zone (40th to 75th percentile), 196 (6.8%) climbed to the high‐risk zone (PLR = 2.0). Of the 4,037 neonates in the low‐risk zone (< 40th percentile), none moved upwards into the high‐risk zone (Table 3).
Predictive ability of percentile values of predischarge TcB for subsequent significant hyperbilirubinemia.
| Predischarge TcB | Significant hyperbilirubinemia | Predictive ability | ||||
|---|---|---|---|---|---|---|
| Risk zone | Percentile | Number(9,174) | Present(972) | Absent(8,202) | Probability of disease | PLR |
| High‐risk | > 95th | 453 | 275 | 178 | 60.7% | 12.9 |
| Upper‐intermediate | 76th‐95th | 1,805 | 501 | 1,304 | 27.8% | 4.4 |
| Lower‐intermediate | 40th‐75th | 2,879 | 196 | 2,683 | 6.8% | 2.0 |
| Low‐risk | < 40th | 4,037 | 0 | 4,037 | 0.0% | 0 |
PLR, positive likelihood ratio; TcB, transcutaneous bilirubin
TSB measurements are an invasive procedure that involves pain, neonatal stress, and risk of infection. A noninvasive determination of bilirubin concentrations (TcB) is advantageous, and is suitable for universal neonatal screening.11 The new generation of noninvasive TcB‐measuring devices (BiliCheck™ and JM‐103) have presented good correlations with TSB measurements (BiliCheck™: 0.8212, JM‐103: 0.8686).12 Recently, some studies developed predictive nomograms based on measurements of TcB or TSB to assess the risk for significant hyperbilirubinemia in healthy term and late‐preterm infants.13–18 The results showed that the TcB nomogram was equivalent to the TSB nomogram, and both could be used to identify subsequent significant hyperbilirubinemia.
A predictive nomogram should be developed in one sample and validated in another. The present study was a multicenter study to verify the predictive value of the TcB nomogram constructed in 2010.9 The result showed that the AUC was 0.875, which was lower than the pre‐test predictive ability in the previous study (AUC = 0.920). The previously constructed TcB nomogram was developed from a single hospital, which could not represent the demographic characteristics of the Chinese neonatal population. The multicenter study included eight units, which showed different genetic and environmental features. Therefore, a population‐based TcB nomogram should be constructed, which should show a better predictive ability.
The rate of TcB increase is affected by smaller gestational age, blood incompatibilities, glucose‐6‐phosphate dehydrogenase deficiency, increased weight loss, and exclusive breastfeeding.19 Therefore, risk factors should been assessed when planning appropriate follow‐up strategies according to the predischarge TcB.20 Six centers assessed the predictive value of predischarge TcB, and the AUC was 0.86; however, combined clinical risk factors (earlier GA, bruising, positive direct antiglobulin test, Asian race, exclusive breastfeeding, blood type incompatibility, and jaundice extent) was better (AUC = 0.95).21 Another study evaluated the predictive performance of predischarge bilirubin risk zone (AUC = 0.88); however, combined clinical risk factors (GA, and percentage of weight loss per day on the first two days) showed better accuracy (AUC = 0.96).22 Thus, the risk factors for developing significant hyperbilirubinemia in the Chinese neonatal population should have been investigated in combination with a TcB nomogram, which could improve the predictive accuracy.
The study has some limitations. Firstly, the previous TcB nomogram was constructed from a single, tertiary‐care center, which does not represent population‐based study data. Secondly, the previous TcB nomogram did not combine the TcB nomogram with other clinical risk factors, such as GA and exclusive breastfeeding, which may improve the prediction of subsequent hyperbilirubinemia. Due to the relative limitations of previous TcB nomogram, the authors are currently conducting a multicenter study (ClinicalTrials.gov Identifier: NCT01763632), in which 17 hospitals in China will participate from January to December, 2013, to develop an hour‐specific TcB nomogram. The constructed TcB nomogram, which will combine predischarge TcB with other clinical risk factors, may better represent the Chinese neonatal population.
ConclusionThe multicenter study validated the TcB nomogram, which is a useful tool for predicting subsequent severe hyperbilirubinemia in Chinese healthy term and late‐preterm infants. However, the study did not combine predischarge TcB with clinical risk factors (such as GA, exclusive breastfeeding, cephalhematoma, significant bruising, or previous sibling with jaundice) to determine the risk for healthy term and late‐preterm infants developing subsequent severe hyperbilirubinemia. Further studies are necessary to confirm this combination.
FundingThis work was supported by grants from the Key Medical Personnel Foundation of Jiangsu Province (Grant No. RC2011021), the Nanjing Medical Science and Technique Development Foundation (QRX11107), and the Nanjing Municipal Medical Science Development Foundation, Nanjing, Jiangsu, China (ZKX12044).
Conflicts of interestThe authors declare no conflicts of interest.
The authors are grateful to the staff of the Affiliation for their support and comments during the preparation of this manuscript. The authors would like to thank the following investigators who participated in the multicenter study: Jin Wang (Department of Neonatology, Children's Hospital of Fudan University, Shanghai, China), Qing Sun (Department of Pediatrics, Nanjing Maternity and Child Health Care Hospital of Nanjing Medical University, Nanjing, China), Xiaoyue Dong (Department of Pediatrics, Nanjing Maternity and Child Health Care Hospital of Nanjing Medical University, Nanjing, China), Xiaofan Sun (Department of Pediatrics, Nanjing Maternity and Child Health Care Hospital of Nanjing Medical University, Nanjing, China), Hongyun Wang (Department of Neonatology, Inner Mongolia Maternal and Child Health Care Hospital, Hohehot, China), Yanping Zhu (Department of Neonatology, The First Affiliated Hospital of Xinjiang Medical University, Urumqi, China), Ying Wang (Department of Neonatology, Changzhou Maternal and Child Health Care Hospital, Changzhou, China), Xiaolei Zhuang (Department of Neonatology, Gynecology and Obstetrics Hospital, Fudan University, Shanghai, China), Jun Long (Department of Pediatrics, The Fifth People's Hospital of Shenzhen, Shenzhen, China), and Qiufen Wei (Department of Neonatology, Guangxi Maternal and Child Health Hospital, Nanning, China).
Please cite this article as: Yu Z, Han S, Wu J, Li M, Wang H, Wang J, et al. Validation of transcutaneous bilirubin nomogram for identifying neonatal hyperbilirubinemia in healthy Chinese term and late‐preterm infants: a multicenter study. J Pediatr (Rio J). 2014;90:273–8.