Enuresis is associated with attentional and emotional comorbidities in 20 to 30 % of cases. The Short Screening Instrument for Psychological Problems in Enuresis (SSIPPE) is a questionnaire that allows the initial screening of these comorbidities. This study aimed to translate, culturally adapt, and validate the SSIPPE for Brazilian children and adolescents (SSIPPE-Br).
MethodsSix steps were performed for translation and cross-cultural adaptation: translation, synthesis of translations, back-translation, preparation of the pre-final version of the translated instrument, test of comprehensibility of the pre-final version of the tool, and elaboration of the instrument cross-culturally adapted for Brazil, named 13-itens version SSIPPE-Br. To validate the SSIPPE-Br, a cross-sectional study was carried out, in which the validated Brazilian version of the Child and Adolescent Behavior Inventory (CABI) was used.
ResultsValidation was performed on 127 children and adolescents with a mean age of 9.7 ± 2.8 years, 48 % male. The reliability was estimated using Cronbach's alpha, ranging from 0.86 to 0.89, indicating good internal consistency. The factorial analysis had a good agreement adjustment (KMO 0.755, Bartlett's test < 0.001) and explained 70.5 % of the data variability. In the reproducibility analysis, the Kappa coefficient ranged from 0.94 to 1, which can be considered almost perfect. A highly significant (p-value < 0.001) and direct correlation existed between the three SSIPPE-Br domains and all evaluated CABI domains.
ConclusionThe SSIPPE-Br is a valid and reliable tool for emotional problems screening and ADHD symptoms in children and adolescents with enuresis whose first language is Brazilian Portuguese.
According to the International Children's Continence Society (ICCS) criteria, enuresis is a condition characterized by urinary incontinence during sleep in individuals aged at least five years, which occurs at least 1x/month for three consecutive months after the exclusion of all organic causes.1-3 It is a common condition, and its prevalence in the general population occurs in approximately 10.6 % of children at six years of age and in 5.7 % at 11 years of age, being more common in males.4
Enuresis is associated with psychiatric/psychological comorbidities4 in 20 to 30 % of cases.5 There is an increased risk of conduct problems, oppositional behavior, and Attention Deficit Hyperactivity Disorder (ADHD) in this population,6 the latter being the most common.7,8 Individuals with enuresis are 2.88 times more likely to have ADHD compared to those without enuresis,7 with a prevalence ranging from 28.37 to 74 %.8 These patients are at greater risk of persistent, treatment-resistant enuresis.9,10 The ADHD inattentive presentation is the most prevalent in enuresis, described in approximately 60 %7 to 73.3 %.11 of the patients. Our recent meta-analysis that aimed to answer the central question, "How frequent is the comorbidity of ADHD and enuresis?", demonstrated a significant and reciprocal association between enuresis and ADHD, with the inattentive presentation having a higher relationship with enuresis.10
The condition impacts children's functionality.1,2 It can compromise the quality of life with low self-esteem, changes in school performance, and decreased socialization with peers.12-14 The psychological comorbidities screening is recommended in patients with enuresis.1,2,5 Standardized and validated questionnaires are essential for adequate screening associated with a thorough clinical history and physical examination.5,15
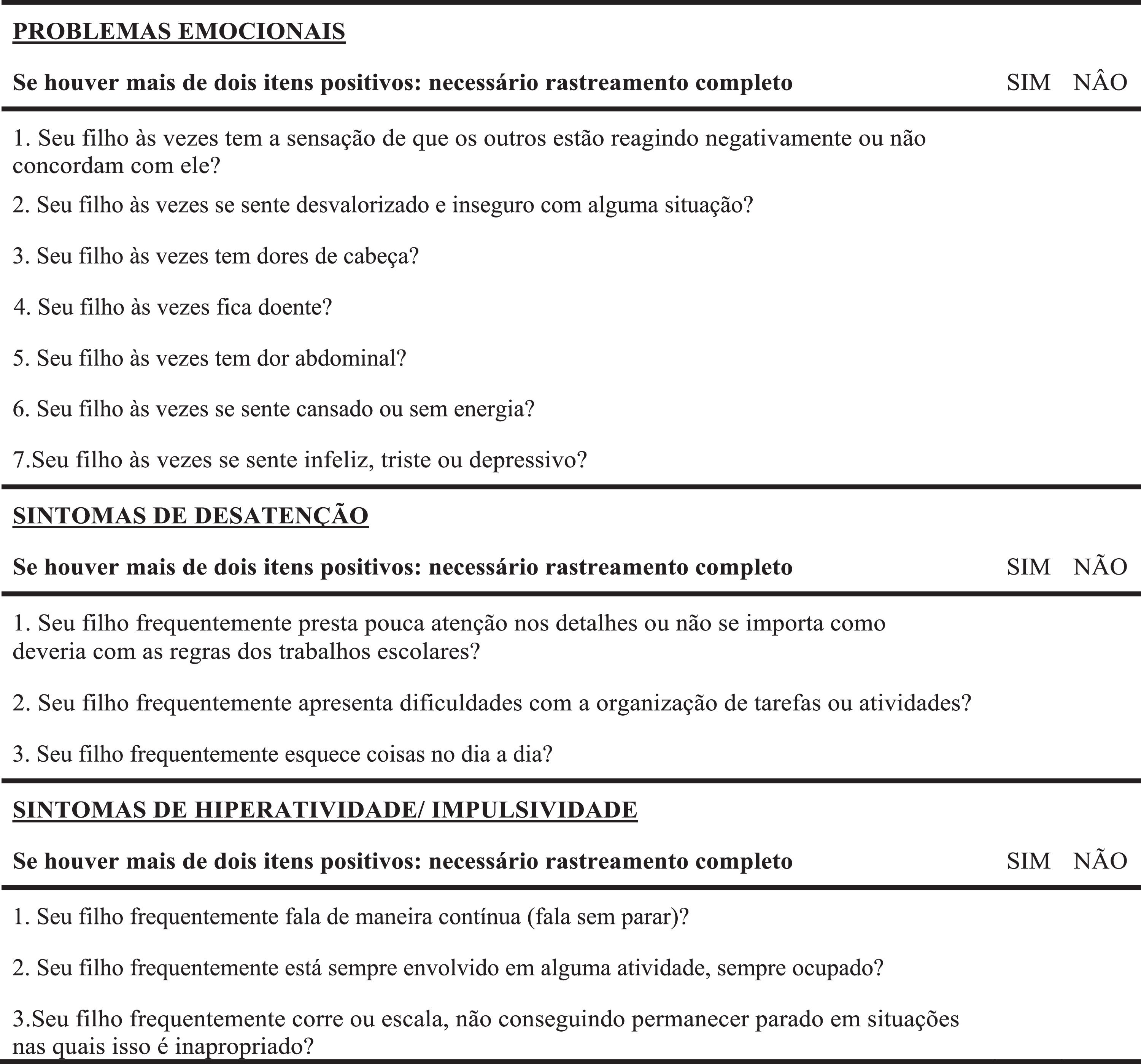
To enable initial screening of emotional, behavioral, and attentional comorbidities in children and adolescents with enuresis, Hoecke et al.16 developed in Belgium and validated in English in 2007, the Short Screening Instrument for Psychological Problems in Enuresis (SSIPPE) based on Child Behavior Checklist (CBCL)17 and Disruptive Behavior Disorders Rating Scale (DBDRS).18 The instrument composed of 13 dichotomous items that assess three main domains: one for emotional problems and two for ADHD symptoms is recommended by the ICCS.1,2 If two or more items score positively in a domain, a full psychiatric/psychological screening is required.16
To use a screening tool, it is important to have a cross-cultural validation process for the population to be applied that follows internationally accepted standards, that is, the items must not only be translated appropriately from a linguistic point of view but also culturally adapted, maintaining the validity of the original19-22 In this context, the objective of this study was to carry out the process of translation, cross-cultural adaptation and validation of the SSIPPE16 for Brazilian children and adolescents with enuresis.19-22 The Child and Adolescent Behavior Inventory (CABI)23,24 validated for Brazilian Portuguese25 was used for this validation process.
MethodsEthical approvalThe project was approved by the ethics board of the Federal University of Minas Gerais, Brazil (registry: 86,171,118.0.0000.5149) and by the principal author of the original SSIPPE study.
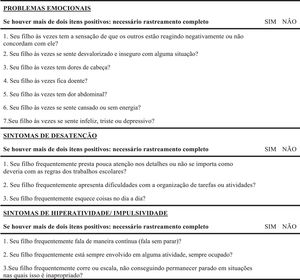
InstrumentsShort Screening Instrument for Psychological Problems in Enuresis (SSIPPE)This instrument was developed and validated by Hoecke et al.16 as a screening tool for early detection of emotional problems, inattention, and hyperactivity/impulsivity in children and adolescents with enuresis. The SSIPPE was based on the items with the highest load on the CBCL17/DBDRS18 subscales in a sample of enuretic individuals with psychological and psychiatrics comorbidities symptoms. The classification accuracy (absent/present) for each subscale is around 88 %. The three SSIPPE subscales had an excellent specificity (0.91 to 0.99), which indicates that a negative prediction (less than two positive items) on the SSIPPE is reliable and leads to few false-negative results. The questionnaire has 13 questions divided into three parts. In the first part, the person responsible for the participant must answer seven questions related to emotional problems. The second and third parts are composed of three questions each, assessing symptoms of inattention, hyperactivity and impulsivity, respectively. The questions’ answer format is yes, if signs or symptoms are present, and no, for their absence. If more than two items are marked as yes in any SSIPPE domain, the participant should be referred for a mental health evaluation16 (Supplement 1).
Child and Adolescent Behavior Inventory (CABI)The CABI is a parental questionnaire that assesses different domains of child behavior and psychopathology based on the Diagnostic and Statistical Manual of Mental Disorders (DSM-5)26 referring to the last six months. The inventory includes 75 items grouped by psychopathological areas, which facilitate application and interpretation with the following distribution: five items for anxiety, four for somatic symptoms, ten for depression, five for Oppositional Defiant Disorder, five for Conduct Disorder and nine for ADHD.23,24 The CABI has validity and reliability similar to the CBCL.17 For our study, the authors used the Brazilian version of the CABI because it is a valid and reliable inventory that assesses individuals’ behavior aged between six and 18 years25 (Supplement 2).
Study designThe study design was carried out in two stages, following rigorous guidelines.19-22 First, the instrument was translated into Brazilian Portuguese, and culturally adapted for the Brazilian population by a committee of experts (composed of six physicians and a physiotherapist, with experience in urology and pediatric psychiatry). Second, the validation process was carried out (Supplement 3).
Stage 1: the translation and the cross-cultural adaptationThe original SSIPPE was translated independently by two translators from the expert committee whose mother language is Brazilian Portuguese and who are fluent in English. Two translated versions were generated (T1 and T2). A third translator, whose native language is Brazilian Portuguese, fluent in English and who did not participate in the initial translation, prepared the translations’ synthesis (T3). The experts’ committee resolved the ambiguities and discrepancies during the translations’ synthesis and preparation.
The translations’ synthesis was then back-translated, independently into English, by a bilingual translator whose mother language is English. This translator did not participate in the previous stages, was not a health professional, and was not informed about the instrument's concepts explored. The back-translation into English, carried out without knowledge of the questionnaire's original version, resulted in an instrument version called R1.
The experts’ committee analyzed the versions generated in the previous stages (T1, T2, T3 and R1) and compared them with the original questionnaire. The committee evaluated, reviewed, and consolidated the instructions, items, and response format of the translated and R1 versions about the conceptual, semantic, and content equivalence of the instrument in the original language. The committee also prepared the pre-final version of the instrument in Brazilian Portuguese (T4) for the transcultural adaptation.
A pre-test was carried out to evaluate the operational equivalence, verbal understanding, and clarity of the items in the instrument's pre-final version (T4). This version was then applied to 40 randomly recruited people from different age groups and education levels. The expert committee analyzed the pre-test results. The necessary changes to the questionnaire were made according to the difficulties encountered by participants in the pre-test phase. The guiding question for evaluating the T4 version was: “Did you understand what was asked?” with an answer in the format “YES” for when they understood or “NO” for when they did not understand. After the final consensus, the SSIPPE's final version was created, translated, and cross-culturally adapted for the Brazilian population, called SSIPPE-Br (T5) and shown in Figure 1. Subsequently, this version of the SSIPPE-Br was sent to the principal author of the original instrument, for approval.
Brazilian version of the Short Screening Instrument for Psychological Problems in Enuresis (SSIPPE-Br) authorized by Van Hoecke et al.16
In the second stage, a cross-sectional observational study was carried out in a sample of Brazilian children and adolescents to assess the equivalence of the measure through psychometric analysis of the reliability and validity of the culturally adapted instrument.19-22 The CABI's Brazilian version25 was used to validate the SSIPPE-Br. A trained researcher read the cross-culturally adapted version of the SSIPPE with the parents during the application. The face-to-face application of the questionnaires was repeated seven days after, to assess the test-retest reliability. The researchers used a stopwatch and recorded the time in minutes taken to complete the questionnaires.
Study populationThe study population consisted of 150 children and adolescents aged between six and 17 years, randomly recruited from local public and private schools and the Enuresis Ambulatory from March to October 2022. The socioeconomic level was measured using the Brazil Socioeconomic Classification Criteria, 2022. Patients diagnosed with ADHD undergoing drug treatment were excluded.
Sample calculationThere has yet to be a consensus on the ideal sample size for validation studies.21 Kline27 suggested an interval rule of four to 10 subjects per variable, with a minimum number of 100 participants that can be used for the sample calculation of these studies, which was used as a guideline for the definition of our sample.
Statistical analysisStatistical analysis was performed using the IBM® SPSS® Statistics Version 21 statistical package (Microsoft Co., New York, NY, USA). For all statistical procedures, a confidence interval (CI) of 95 % was applied, and the level of significance was set as p < 0.05.
Sample characterizationSample characterization included frequency distribution tables to express categorical variables and measures of central tendency, position, and variability to show numeric variables.
ReliabilityInternal consistency analysisThe internal consistency of each dimension proposed for the SSIPPE-Br was assessed using Cronbach's alpha coefficient. Values between 0.70 and 0.95 indicated a measure of good internal consistency.21
Reproducibility analysisThe reproducibility of the SSIPPE-Br was evaluated using the Kappa coefficient, comparing the responses obtained for each item and domain in the test and retest (seven-day interval). The interpretation of the Kappa coefficient values was as follows: < 0 no agreement; 0–0.19 poor agreement; 0.20–0.39 fair agreement; 040–0.59 moderate agreement; 0.60 −0.79 substantial agreement and 0.80–1.00 almost perfect agreement.21
Validity assessmentContent validity index (CVI)It measured the proportion or percentage of an experts ‘panel who agree with specific aspects and items of the instrument. The number of experts for content validation must be at least six, and acceptable CVI values must be at least 0.83.
The authors use three parameters: I-CVI (item-level content validity index), which measures the proportion of content experts who give the item a relevance rating of 3 or 4; S-CVI/Ave (scale-level content validity index using the mean), which shows the average I-CVI scores for all scale items; S-CVI/UA (scale-level content validity index based on universal agreement method): the proportion of scale items that achieve a relevance scale of 3 or 4 by all experts. The universal agreement (UA) score is 1 when the item has reached 100 % expert agreement; otherwise, the UA score is zero.28
Factor analysisFactor analysis was performed using the principal components method with the variables that constitute the SSIPPE. In this analysis, the adjustment's quality of the factor analysis model was estimated using the indices "Kaiser Meyer Olkin test (KMO)" and "Bartlett's sphericity test" (Bartlett's test).
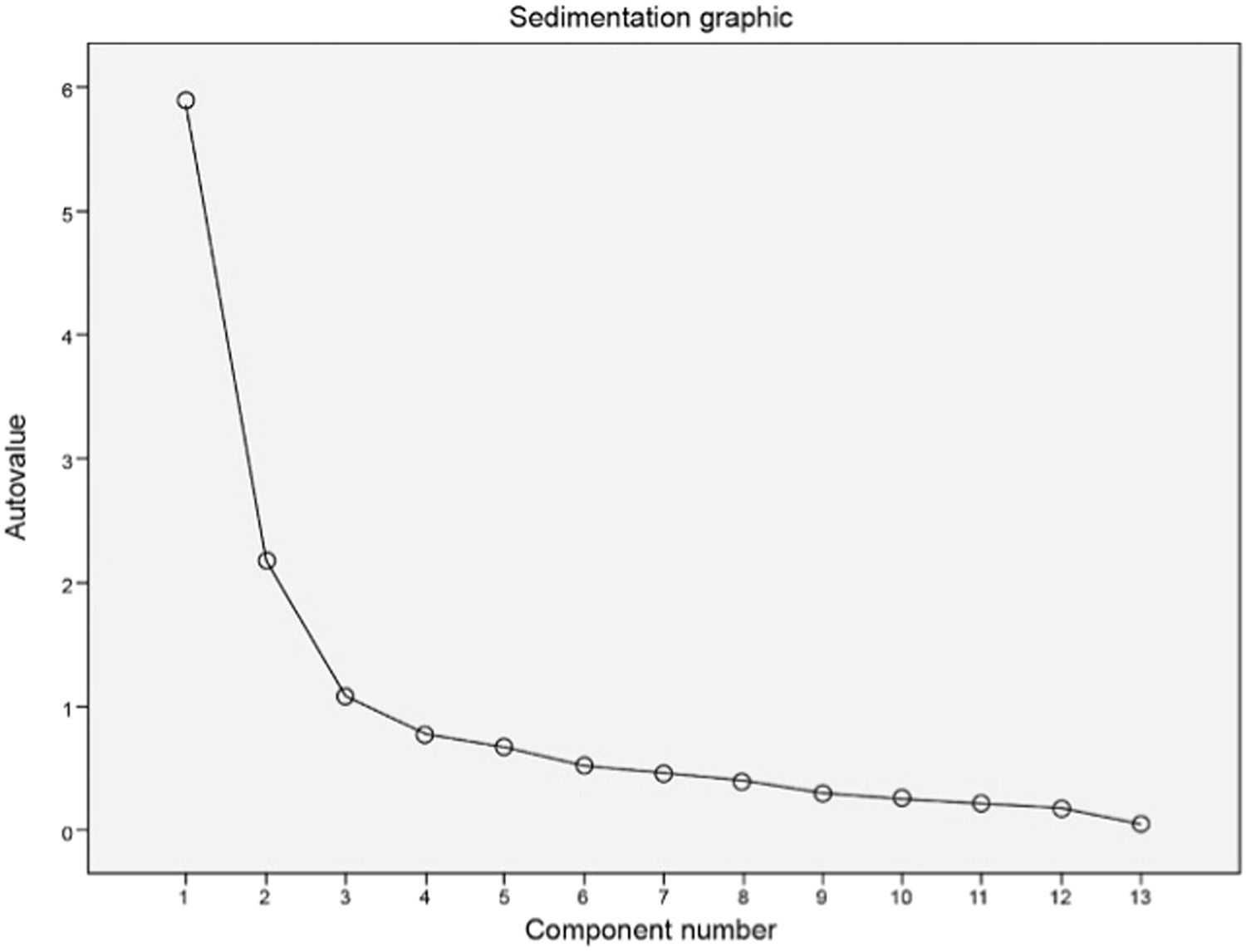
The total percentage of variance explained by the model, eigenvalues and scree plot were evaluated to define the number of factors to be considered. The factors’ maximum number was considered in the stability point of the scree plot, representing the factors’ number to be considered in the analysis. The factor matrix was created using varimax rotation, and items with a factor loading less than 0.40 were excluded.
Comparison between SSIPPE and CABI scoresThe scores of the following CABI domains were considered: somatic symptoms (items 1 to 4), depression (items 19 to 28), impulsivity (items 43 to 45), hyperactivity (items 46 to 48), and inattention (items 49 to 51). These domains were selected considering the similarity with the constructs measured by the SSIPPE-Br and were also calculated by the sum of their respective items. Both instruments’ scores were compared using the Spearman Correlation Coefficient (rsp).
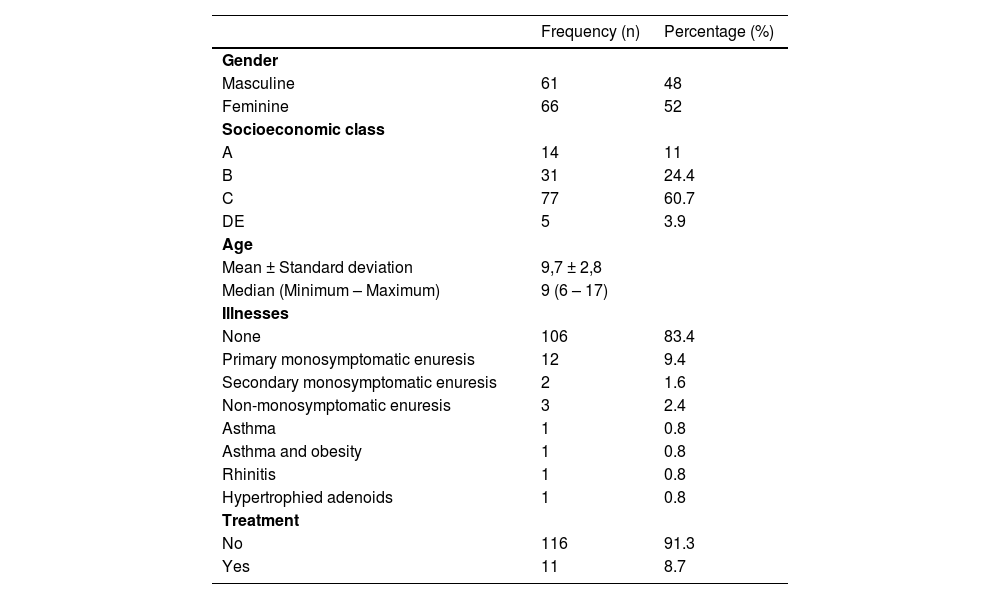
ResultsSample characterizationOf the 150 participants recruited for this study, 127 were eligible with a mean age of 9.7 ± 2.8 years (48 % male). Eight patients diagnosed with ADHD undergoing drug treatment and 15 participants who did not respond to the questionnaires were excluded. Table 1 displays a description of the sample. The SSIPPE-Br items with positive responses are shown in Supplement 4.
Characterization of the analysed sample referring to sociodemographic and clinical data (n = 127).
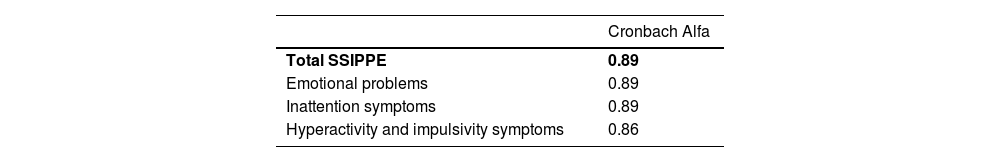
Cronbach's alpha values calculated for the complete SSIPPE-Br and for each proposed domain ranged from 0.86 to 0.89, indicating very good internal consistency (Table 2). In addition, Cronbach's alpha values were calculated if any scale item was excluded (Supplement 6).
Values of Cronbach alfa coefficient considering total SSIPPE-Br and each of the three proposed dimensions (emotional problems, inattention symptoms and hyperactivity and impulsivity symptoms) – (n = 127).
| Cronbach Alfa | |
|---|---|
| Total SSIPPE | 0.89 |
| Emotional problems | 0.89 |
| Inattention symptoms | 0.89 |
| Hyperactivity and impulsivity symptoms | 0.86 |
SSIPPE-Br Brazilian version of the Short Screening Instrument for Psychological Problems in Enuresis.
One hundred and eleven individuals were evaluated in the test and retest analysis (87.4 % of the original sample) (Supplement 6). Among the items in the emotional problems domain, the Kappa coefficient assessment in the total sample varied between 0.96 and 1 (almost perfect).
Content validity indexThe I-CVI was equal to 1 for 12 questions; only question four was not scored by three experts. S-CVI/AVE was 0.96, and S-CVI/UA was 0.92. There was agreement on 12 questions among six experts, except for question number four in the emotional domain, “Your child sometimes feels sick” (Supplement 7).
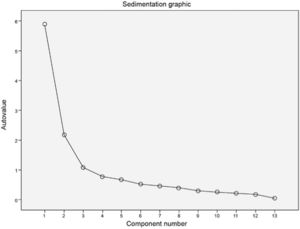
Factor analysisThe 13 SSIPPE variables distributed across three domains (theoretical model) were included in the factor analysis. A “scree plot” was created (Figure 2) that helped define the factors’ number for analysis and build a model with three factors and 13 items (Supplement 8).
No item presented reduced factor loading (< 0.40). The standard factor analysis model showed good adjustment according to the KMO test (0.755) and Bartlett's test (< 0.001) and explained 70.5 % of the data variability with results identical to the theoretical model.
Correlation between SSIPPE-Br and CABI scoresThere was a highly significant (p-value < 0.001) and direct correlation (positive coefficients) between the three SSIPPE-Br domains and all evaluated CABI domains (Supplement 9).
The median time for applying the SSIPPE-Br, total test-retest, was two minutes (two to three). In relation to the CABI's Brazilian version,25 the median time to perform the test and retest was nine (eight to 10) and 10 (nine to 12) minutes, respectively.
DiscussionThe SSIPPE is a cost-free screening tool originally developed in English to assess emotional problems and ADHD symptoms in children and adolescents with enuresis.16 In this study, the authors translated and cross-culturally adapted the SSIPPE to a Brazilian version following a strict methodological approach and examined its reliability (internal consistency through Cronbach's Alpha and stability/reproducibility through KAPPA) and validity (content validity through the I-CVI, S-CVI/AVE and S-CVI/UA and factor analysis through the KMO and Bartlett test) in an adequate sample size.19–22 Our results support SSIPPE-Br as a valid, reliable, and easy-to-use tool.
The authors evaluated all the SSIPPE-Br's psychometric properties in 127 participants aged six to 17 years. The reliability estimate through internal consistency was considered good,21 with Cronbach's alpha of the total SSIPPE-Br of 0.89, ranging from 0.86 to 0.89 for one of the three domains. Deleting any items that make up the SSIPPE's three domains would not increase its internal consistency. Our results further supported the reliability, homogeneity, and construct validity of the SSIPPE16 The three original SSIPPE domains exhibit excellent specificity (0.91 to 0.99), which indicates that a negative prediction (less than two positive items) determines a few false-negative results.16 The authors also demonstrated excellent stability/reproducibility of SSIPPE-Br through test-retest in our sample (Kappa 1). The authors opted for a week to repeat the examination, as there seems unlikely to be any change in the reported clinical symptoms in such a short time. In addition, the time must be sufficient to avoid recalling the answers given.21
Regarding content validation, the SSIPPE-Br proved to be valid according to experts' assessment, with CVI values above the critical limit of 0.8328 for all items evaluated. The authors emphasize that some changes were made to the instrument to meet the demands presented in the pre-test in the cross-cultural adaptation phase. At the end of this stage, it was understood that the content of the SSIPPE-Br was clear, applicable, and relevant for the assessment of the proposed symptoms.
In the original SSIPPE study, to assess validity, seven items with factor loadings > 0.65 were used for emotional problems based on the CBCL scale and three items with factor loadings > 0.80 and 0.75, respectively, for Inattention and Hyperactivity/Impulsivity based on DBDRS scale. Our results showed a factor analysis similar to the original study, with factor loading values for the seven items related to emotional problems between 0.64 and 0.79. For the three symptoms of inattention, factor loadings values were between 0.77 and 0.88 and, for the three symptoms of hyperactivity, between 0.82 and 0.86. These factor loading values were considered with good to excellent adequacy, being similar to the original study.16
The present study showed a high positive correlation between the SSIPPE-Br and all the Brazilian version CABI25 domains evaluated. The highest coefficients were observed when comparing the emotional problems domain of the SSIPPE-Br and somatic symptoms of the CABI (rsp = 0.82) and when comparing the inattention constructs of both questionnaires (rsp = 0.66). The psychometric analysis of the validated Brazilian version of the CABI25 showed high reliability for internalizing and externalizing symptoms (hyperactivity and impulsivity) and inattention. Cianchetti et al.29 reported that the CABI has good predictive validity compared to the CBCL,17 with the advantage of being a shorter instrument.
Our study has several limitations. Although the sample size was adequate, a convenience sample may not represent the Brazilian population in terms of gender and socioeconomic level. The SSIPPE-Br was applied to the general population with 13 % (17/127) of children and adolescents with enuresis, which may, at least in part, limit the criterion validity analysis. It is worth noting that the present study has some strengths, as it adopted the steps recommended for translation, cross-cultural adaptation, and validation of the SSIPPE-Br and tested the validity and reliability of the instrument.
The SSIPPE-Br is a valid and reliable tool for screening emotional problems and ADHD symptoms in children and adolescents (six to 17 years) with enuresis whose first language is Brazilian Portuguese. The use of a quick, cost-free, and easy-to-apply screening instrument, such as the SSIPPE-Br, allows an efficient assessment of these comorbidities by general pediatricians and will indicate if a complete psychiatric/psychological evaluation is necessary.
FundingThis study was financed by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) Finance Code 001and by Pró-reitoria de Pesquisa-Universidade Federal de Minas Gerais Grant PRPQ-UFMG 26048*104.
The authors would like to thank Dr Eline Van Hoecke for the permission to use the Short Screening Instrument for Psychological Problems in Enuresis, in this study. The authors also thank the patients and their parents who participated in this study.
Institution where the research was carried out: Universidade Federal de Minas Gerais.