To study the effect of two intravenous maintenance fluids on plasma sodium (Na), and acid–base balance in pediatric intensive care patients during the first 24h of hospitalization.
MethodsA prospective randomized controlled study was performed, which allocated 233 patients to groups: (A) NaCl 0.9% or (B) NaCl 0.45%. Patients were aged 1 day to 18 years, had normal electrolyte concentrations, and suffered an acute insult (medical/surgical). Main outcome measured: change in plasma sodium. Parametric tests: t-tests, ANOVA, X2 statistical significance level was set at α=0.05.
ResultsGroup A (n=130): serum Na increased by 2.91 (±3.9)mmol/L at 24h (p<0.01); 2% patients had Na higher than 150mmol/L. Mean urinary Na: 106.6 (±56.8)mmol/L. No change in pH at 0 and 24h. Group B (n=103): serum Na did not display statistically significant changes. Fifteen percent of the patients had Na<135mmol/L at 24h. The two fluids had different effects on respiratory and post-operative situations.
ConclusionsThe use of saline 0.9% was associated with a lower incidence of electrolyte disturbances.
Estudar o efeito de dois fluidos de manutenção intravenosos sobre o sódio (Na) plasmático e o equilíbrio ácido-base em pacientes de terapia intensiva pediátrica durante as primeiras 24horas de internação.
MétodosFoi realizado um estudo controlado randomizado prospectivo. Alocamos aleatoriamente 233 pacientes para os grupos: (A) NaCl a 0,9% ou (B) NaCl a 0,45%. Os pacientes com 1 dia a 18 anos de idade apresentavam concentrações normais de eletrólitos e sofriam de insulto agudo (médico/cirúrgico). Principal resultado: variação no sódio plasmático. Testes paramétricos: teste t, ANOVA, qui-quadrado. O nível de relevância estatística foi estabelecido em α=0,05.
ResultadosGrupo A (n=130): o Na sérico aumentou 2,91 (±3,9) mmolL−1 em 24h (p<0,01); 2% dos pacientes apresentaram Na acima de 150mmolL−1. Concentração média de Na na urina: 106,6 (±56,8)mmolL−1. Sem alteração no pH em 0 e 24h. Grupo B (n=103): o Na sérico não apresentou alterações estatisticamente significativas. 15% dos pacientes apresentaram Na<135mmolL−1 em 24h. Os dois fluidos tiveram efeitos diferentes sobre as situações respiratória e pós-operatória.
ConclusãoO uso de solução fisiológica a 0,9% foi associado à menor incidência de distúrbios eletrolíticos.
The choice of maintenance fluids in hospitalized patients is an issue that physicians frequently have to consider. In the last decade, the recognition of severe complications associated with excess volume administration and iatrogenic hyponatremia has raised increased interest in the prescription of fluids and on its consequences, especially in critical care conditions.1–4
Hyponatremia is defined as a concentration of plasma Na lower than 135mmol/L.5 Nearly all hospitalized patients have the risk of developing hyponatremia as a result of the potential presence of known stimuli for producing anti-diuretic-hormone (ADH).6–9 In children admitted to a PICU,10–12 many such stimuli may be present: (1) those related to hemodynamic status – hypovolemia or hypervolemia and (2) those related to inappropriate antidiuretic hormone release syndrome (IADHRS), present in central nervous system disturbances, pulmonary diseases, tumors, and postoperative conditions.2,3,6,10–12 Until recently, hypotonic fluids (0.2–0.3% saline) were used as maintenance fluids for hospitalized children, based on the Holliday–Segar equation,13,14 but recent studies showed that hyponatremia may be exacerbated by hypotonic intravenous fluids.15–19
Hypernatremia, which may be defined as serum Na higher than 145mmol/L (although values under 150mmol/L are not considered deleterious), has also been extensively described in patients in intensive care units.10,11 At admission, 2–6% of patients are hypernatremic, and during the course of treatment in the ICU, 4–26% of patients become hypernatremic. Many factors may contribute to hypernatremia in these patients, such as patient inability to control free water intake (sedated, intubated, mental status altered), fluid restriction, excessive fluid losses, and treatment with Na containing fluids. As PICU patients are at increased risk of developing electrolyte imbalance, it would be relevant to identify a maintenance fluid not associated with electrolytic disturbances, suitable to administer to a wide range of patients.
This study compares the effects on Na homeostasis and acid–base balance, in patients admitted to the PICU, of two intravenous maintenance fluids: NaCl 0.9%, with 154mEq Na and Cl/L (Fluid A), and NaCl 0.45%, with 75mEq Na and Cl/L (Fluid B), both in 5% dextrose. Fluid A is hypernatremic and hyperosmotic, while Fluid B is hyponatremic and close to plasma osmolarity. These are the two maintenance fluids most used in this department, where lower Na concentration intravenous fluids (including saline 0.18%) have not been used since 2000.
The primary objective of the study was to determine and compare the effects at 24h on plasma Na of the two fluids studied. Other outcomes considered were the incidence of hypernatremia and hyponatremia, urinary excretion of electrolytes, and acid–base balance (pH, bicarbonate, base excess, total CO2, chloride) after 24h under treatment with the two maintenance fluids.
In contrast with the postoperative pediatric patient, there is still a paucity of clinical trials comparing isotonic and hypotonic fluids in other pediatric settings, namely critical care children. This study is useful as it addresses the PICU population and includes aspects not considered in previous works.
MethodsStudy designA prospective, randomized, open, label-controlled study was used, with a simple randomization list. Patients were distributed between two groups, A and B. In Group A patients were treated with saline 0.9% in 5% dextrose as maintenance fluid, while in Group B patients received saline 0.45% in 5% dextrose as maintenance fluid. Data were collected at 24h. The study was terminated when enteral fluids were administered at a rate higher than mL/kg/h in children less than 10kg or 10mL/h in older children.
ParticipantsThis study was conducted in a secondary PICU with 11 beds that admits 450 patients annually. Patients aged 1 day to 18 years old were included, with an acute insult (medical or surgical), who needed exclusive administration of intravenous fluids for more than 24h. Patients with severe electrolyte and acid–base disturbances, metabolic disease, plasma Na lower than 135mEq/L or higher than 150mEq/L, renal insufficiency, or who refused to sign an informed consent were excluded from the study. During the study, patients in whom the maintenance fluid was switched or interrupted before 24h were excluded.
Interventions and therapeutic protocolIn both arms of the study, fluid volume protocols were the same. In patients with respiratory disease, fluid restriction with administration of 50–90% of the volume proposed by the Holliday–Segar equation was used. In abdominal surgery, 100–120% of the daily proposed intake was administered. During this study, every fluid ressuscitation was done with NaCl 0.9%, 20mL/kg for 20–30min, when shock or pre-shock were detected. A daily water balance of zero was aimed in this set of patients, and diuretic (furosemide), when needed, was administered to achieve this goal.
Data collection, variables, and outcomesThis study used a questionnaire designed to collect epidemiologic, clinical, and laboratory data. Variables included were: date, gender, age, weight, neonate (y/n), diagnosis, diagnostic group, previous existence of a chronic disease, PIM2 (Pediatric Index of Mortality), need for fluid expansion, diuretics, volume of fluids administered, urinary output, water balance, Na, Cl, potassium, pH and bicarbonate at T0h (prior to fluid administration) and 24h, and urinary ionogram at T24h.
Primary outcome was the difference between Na at T0 and T 24h in mmol/L (ΔNa 24h/Na 0h) with maintenance Fluids A and B. Secondary outcomes were: incidence of hyper or hyponatremia, ΔpH, bicarbonate, base excess, tCO2 and chloride at T24h, and urinary excretion of Na and Cl.
Sample sizeIn a prior pilot study,20 the present authors detected a difference in serum Na of 2mmol/L after 24h using normal saline or 0.45% saline in PICU patients. For α=0.05, a power of 0.9, and an expected difference of 2mmol/L in serum Na at 24h between the two groups, it was concluded that 66 patients were needed in each arm of the study. For a difference between the two values of 1.5mmol/L, each group would need 117 patients.
RandomizationPatients were randomized using a single sequence of random assignments, with a simple randomization table between the two arms of the study (Fluids A and B). The physician in charge of the patient consulted this randomization table and assigned the child to Group A or B.
Statistical methodsDescriptive statistics were calculated for the whole sample and for Groups A and B. The data were tested for normality, as required by subsequent parametric statistical tests. To analyze differences in Na between T0 and T24h (main outcome) and differences in Cl and acid–base balance between T0 and T24 and between electrolytes in Groups A and B, independent and paired t-tests and ANOVA were used. The univariate analysis also utilized X2. Statistical significance level was set at 0.05. Data were analyzed using SPSS version 19 (SPSS, Inc., SPSS for Windows, Version 19.0, Chicago, USA).
EthicsInformed consent was obtained from the parents/guardians of patients under 16 years old, and from adolescents aged 16 or older. The study was approved by the Ethics Committee of Hospital Fernando Fonseca.
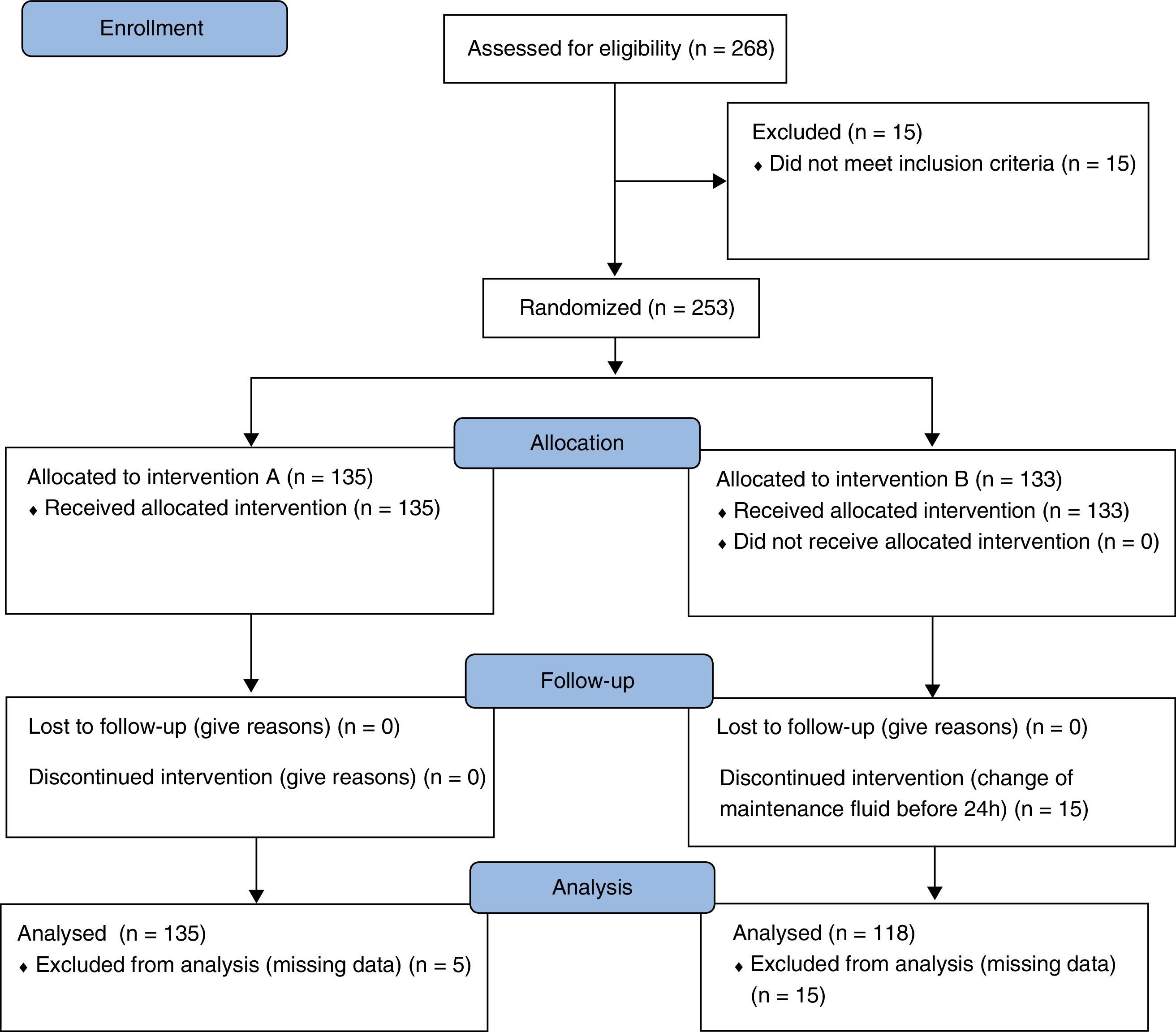
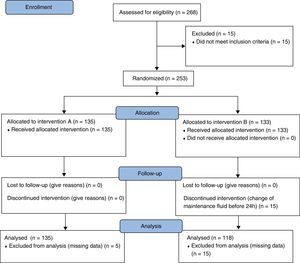
ResultsFlowchartDuring 2011, 268 episodes were included and randomized to arm A – Fluid A (n=135) or B – Fluid B (n=133) of the study. In Groups A and B, 5 and 30 episodes, respectively, were excluded because of missing data considered relevant for the study. At the end, there were 130 patients in Group A and 103 in Group B, for a total of 233 patients analyzed (Fig. 1).
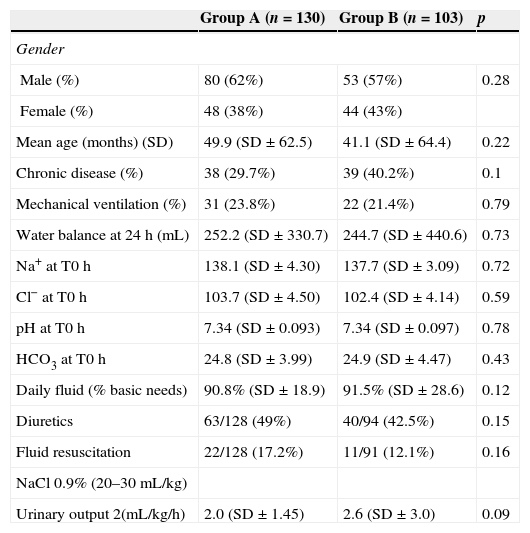
ResultsFrom the 233 patients included in the study, 137 were male and 42 were neonates. Their median age was 12 months (minimum age 0.37 months, maximum 204 months). Seventy-five (33.6%) had a previous chronic disease (Table 1). Median PIM2 was 1.6. One hundred twenty one were admitted for a respiratory problem, 65 after major surgery, 24 for shock, and 23 had miscellaneous conditions. Fifty-three patients were mechanically ventilated.
Emographic and clinical characterization of Groups A and B.
| Group A (n=130) | Group B (n=103) | p | |
|---|---|---|---|
| Gender | |||
| Male (%) | 80 (62%) | 53 (57%) | 0.28 |
| Female (%) | 48 (38%) | 44 (43%) | |
| Mean age (months) (SD) | 49.9 (SD±62.5) | 41.1 (SD±64.4) | 0.22 |
| Chronic disease (%) | 38 (29.7%) | 39 (40.2%) | 0.1 |
| Mechanical ventilation (%) | 31 (23.8%) | 22 (21.4%) | 0.79 |
| Water balance at 24h (mL) | 252.2 (SD±330.7) | 244.7 (SD±440.6) | 0.73 |
| Na+ at T0h | 138.1 (SD±4.30) | 137.7 (SD±3.09) | 0.72 |
| Cl− at T0h | 103.7 (SD±4.50) | 102.4 (SD±4.14) | 0.59 |
| pH at T0h | 7.34 (SD±0.093) | 7.34 (SD±0.097) | 0.78 |
| HCO3 at T0h | 24.8 (SD±3.99) | 24.9 (SD±4.47) | 0.43 |
| Daily fluid (% basic needs) | 90.8% (SD±18.9) | 91.5% (SD±28.6) | 0.12 |
| Diuretics | 63/128 (49%) | 40/94 (42.5%) | 0.15 |
| Fluid resuscitation | 22/128 (17.2%) | 11/91 (12.1%) | 0.16 |
| NaCl 0.9% (20–30mL/kg) | |||
| Urinary output 2(mL/kg/h) | 2.0 (SD±1.45) | 2.6 (SD±3.0) | 0.09 |
Fluid volume aimed for a daily balance of 0 and varied between 24% (respiratory patient) and 193% (surgical patient) of basal fluid requirements, with a mean of 91.0%. Daily water balance had a mean of +2.4mL, with a standard deviation of 388mL. The median was +125mL, with minimum of −688 and maximum of 2066mL. Mean and median urine output were, respectively, 2.15mL/kg/h (±1.6) and 1.7mL/kg/h (min 0.25, max 10.5). Fifty-one had hemodynamic instability. Volume expansion was needed in 43 patients, diuretics in 105, blood products in 35, and inotropics in 30. Plasma Na, Cl, pH, and urinary Na and Cl at T0 and T24 are shown in Table 1.
The 35 patients excluded from the study had a median age of 13 months, 19 were male, and their mean sodium was 137.9mmol/L.
Comparison of effects of Fluids A and BDescriptive statistics show that both groups were similar regarding age, gender, chronic disease, PIM2, diagnostic groups, mechanical ventilation, Na, Cl, pH at the beginning of the study, water balance, volume administered, and urine output. Use of diuretics and volume expansion were statistically similar in both groups (Table 1). Based on this, the effects of both fluids (A and B) on Na and pH balance were compared (Table 2).
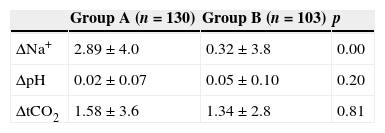
Effects on NaGroup A – NaCl 0.9% (n=130)Using Fluid A, serum Na increased from 138.1mmol/L (±4.3) at time T0 to 140.9mmol/L (±4.9) at T24h, with a mean increase of 2.9mmol/L (±3.9) (p<0.01). Ten patients (5%) had Na higher than 145mmol/L and three of them had sodium higher than 150mmol/L (151.4; 152.6; 155.0). This corresponds to an attributable risk of 2%. Seven patients in this group had hyponatremia, with Na higher than 130mmol/L. Urinary Na in this group had a mean of 106.6mmol/L (±56.8). Clinical symptomatology due to hypernatremia or hyponatremia was not registered.
Group B – NaCl 0.45% (n=103)With Fluid B, at T0, serum Na was 137.86mmol/L (±3.1), and at T24, 137.81mmol/L (±3.0), with a mean difference of −0.2mmol/L, which was not statistically significant. Fourteen patients had Na values under 135mmol/L (130–134). In these 14 patients, the differences in Na between T0 and T24 varied from 0.5 to −10mmol/L and corresponded to an attributable risk of 15%. None of these patients had clinical signs of hyponatremia. Two patients had hypernatremia (Na of 146.5 and 147mmol/L). Urinary Na in this group had a mean of 81.8mmol/L (±5.0), and this value was statistically significantly different from the Na urinary excretion with Fluid A (p=0.04).
Effect of both fluids on acid–base balanceHCO3 pH, total content in CO2 and base excess increased in both groups from T0 to T24. There were no statistically significant differences using these maintenance fluids. Saline 0.9% and saline 0.45% increased the total CO2 at 24h (Table 2).
Fluid A increased serum Cl levels from 104.0mmol/L (±4.7) to 106.7mmol/L (±6.7) (p<0.01). Fluid B changed the initial Cl from 102.7mmol/L (±4.2) to 102.3mmol/L (±6.1); this was not a significant difference (Table 2).
Neonates (n=42)Based on different physiologic knowledge, neonates are expected to behave differently from older children in relation to electrolyte balance. This study observed those aspects and concluded that neonates in Group A had greater increases in Na serum concentration (5.0±4.6mmol/L versus 2.5±3.8mmol/L in older children) at 24h, and one neonate had a plasma Na level of 152.6mmol/L. In Group B, no differences were detected in these parameters between neonates and older children. No differences were seen between neonates and children in relation to urinary electrolyte excretion and urinary output.
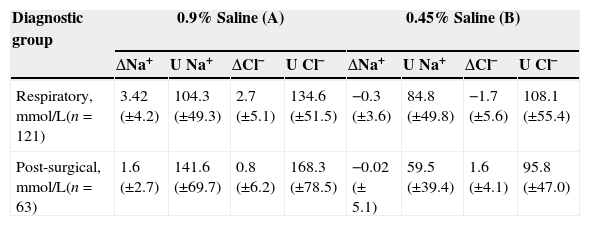
Respiratory and post-operatory patientsDifferences were detected between major diagnostic groups, as subsequently explained (Table 3).
Differences in electrolytes variation between diagnostic groups: respiratory and post-surgical.
| Diagnostic group | 0.9% Saline (A) | 0.45% Saline (B) | ||||||
|---|---|---|---|---|---|---|---|---|
| ΔNa+ | U Na+ | ΔCl− | U Cl− | ΔNa+ | U Na+ | ΔCl− | U Cl− | |
| Respiratory, mmol/L(n=121) | 3.42 (±4.2) | 104.3 (±49.3) | 2.7 (±5.1) | 134.6 (±51.5) | −0.3 (±3.6) | 84.8 (±49.8) | −1.7 (±5.6) | 108.1 (±55.4) |
| Post-surgical, mmol/L(n=63) | 1.6 (±2.7) | 141.6 (±69.7) | 0.8 (±6.2) | 168.3 (±78.5) | −0.02 (± 5.1) | 59.5 (±39.4) | 1.6 (±4.1) | 95.8 (±47.0) |
When using Fluid A (n=63), the Na plasma concentration increased by 3.42mmol/L (±4.2), and decreased by 0.3mmol/L (±3.6) when using Fluid B (n=58) (p<0.001). Urinary excretion with Fluid A was 104.3mmol/L (±49.3) and 84.8mmol/L (±49.8) with Fluid B, with no statistically difference between these values.
Post-operative group (n=65)In post-operative patients, urinary excretion of Na was 141.6mmol/L (±69.7) with Fluid A (n=33) and 59.5mmol/L (±39.4) with Fluid B (n=20), p<0.001. The differences in serum Na when using Fluids A or B were not statistically significant. In this group of patients, electrolyte urinary excretion was significantly different between the two groups, and the difference in serum electrolytes was not statistically significant.
DiscussionSaline 0.9% was compared to saline 0.45%. The risk of hypernatremia using saline 0.9% was significantly lower (2%) than the risk of hyponatremia using saline 0.45% (15%), and hyponatremia is a relevant issue in intensive care units.21 Both fluids induced marked urinary excretion of Na.
Saba et al.,22 in their clinical trial with 36 children, compared the use of normal saline to saline 0.45% and found that the difference between the change in Na concentration was not significant. They did not analyze changes in pH or chloride concentrations, nor the urinary excretion of electrolytes.
Rey et al.,12 in a prospective randomized study, compared the utilization of fluids with 30–50mmol/L Na to fluids with 136mmol/L Na in a group of 134 ICU patients. They also concluded that hypotonic fluids increased the risk of hyponatremia. They described only one case of hypernatremia with normal saline. In the present study hypernatremia was detected in three patients, which corresponds to an attributable risk of 2%. This discrepancy may be due to different clinical and therapeutic situations between studies (different hemodynamic status), although they were both conducted in PICUs.
Yung and Keeley23 compared normal saline with 0.18% saline in a PICU population, concluding that both maintenance fluids were related with hyponatremia. Their sample was smaller and in some aspects different from the population studied in this study: most of the patients were surgical, mildly ill, and not ventilated. They also studied the Na urinary output, which was similar in both groups and lower than that of the present sample. In the present study, the hypotonic fluid studied also induced hyponatremia.
There are studies including only post-operative patients24–26: Choong et al.24 conducted a randomized controlled trial including 258 patients (some of them admitted to the PICU) comparing 0.45% saline to 0.9% saline. The 0.9% fluid proved to be less deleterious. Coulthard et al.26 compared the effects on plasma sodium at 16–18h of Hartmann's solution and 5% dextrose or 0.45% saline and 5% dextrose in 82 patients following major surgery; they concluded that the postoperative fall in Na was smaller in children who received Hartmann's solution and 5% dextrose. The present results agree with these conclusions.
Effects on acid–base balance were also regarded. Saline 0.9%, when used in large volumes, is known to cause hyperchloremic metabolic acidosis27; based on these results it is possible to conclude that when used as maintenance fluid, saline 0.9% or saline 0.45% did not have that effect, although both fluids increased the total CO2.
In this sample of 233 acutely ill medical and surgical patients, 0.45% normal saline induced hyponatremia in 15% of the patients, and none of the fluids induced significant changes in acid–base balance. These results strengthen the choice of an isonatremic maintenance fluid in this group of patients.
This study has several strengths beyond the size of the sample. To the authors’ knowledge, no other studies focused on the effect of maintenance fluids on serum Cl concentrations or acid–base balance. In this study, no effects on acid–base balance were seen with the use of the two different fluids. Individualizing two diagnostic groups (respiratory and postoperative) showed different effects on Na and Cl concentrations. This raises the possibility of interference of different hormonal or multifactorial mechanisms. Postoperative patients excreted higher concentrations of Na and Cl, which may be related to ADH secretion. McCluskey et al.28 showed that in adult noncardiac surgery, hyperchloremia is associated with mortality and longer hospitalization.
Certain limitations inherent to this study must be recognized: 35 patients were excluded (5 from Group A and 30 from Group B) due to missing data or interruption of the study; demonstrating that the two groups studied were similar at the beginning of the study, and that the 35 patients excluded had the same epidemiologic characteristics as the 233 patients included attenuates this limitation.
With this prospective controlled randomized study, it has been observed that although both saline 0.9% and saline 0.45% may be used in a PICU during 24h as maintenance fluids, normal saline should be preferred, as it was able to increase plasma Na, in contrast to 0.45% saline, which was associated with a relevant risk of hyponatremia. No clinical signs of hypernatremia or hyponatremia were detected. None of the fluids induced hyperchloremic metabolic acidosis. The study shows that the prescription of maintenance fluids must take into account age, diagnosis, fluid balance, and the use of diuretics or expansion fluids. Studies addressing these different variables need to be performed. The use of balanced fluids like Ringer's lactate or other polyelectrolyte solutions needs to be studied, as they may have substantial clinical advantages in relation to NaCl fluids.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Almeida HI, Mascarenhas MI, Loureiro HC, Abadesso CS, Nunes PS, Moniz MS, et al. The effect of NaCl 0.9% and NaCl 0.45% on sodium, chloride, and acid–base balance in a PICU population. J Pediatr (Rio J). 2015;91:499–505.
Study conducted at Pediatric Intensive Care Unit, Department of Pediatrics, Hospital Prof. Doutor Fernando Fonseca EPE, Amadora, Portugal.