Fecal calprotectin is an inflammatory marker used for monitoring intestinal diseases. It has been studied as a marker of intestinal inflammation in cystic fibrosis (CF), a multi-systemic genetic disease caused by alterations to the CFTR gene. Manifestations of the disease favor a systemic inflammation not limited to the respiratory tract, therefore, calprotectin is a non-invasive and effective diagnostic method. The aim of the study was to perform a systematic review of the literature with a qualitative synthesis of studies.
SourcesThe articles were selected from PubMed, Web of Science, Scielo and Lilacs.
Summary of the findingsNine studies were selected for that qualitative synthesis, one was a randomized clinical trial, and eight were case-control or cohort designs. Most studies have indicated that calprotectin is a marker of systemic inflammation in CF and not just intestinal inflammation. Calprotectin is an aid in monitoring inflammatory bowel conditions in patients with cystic fibrosis.
ConclusionFurther studies should be conducted to investigate the role of this marker in the systemic inflammation of CF.
Fecal calprotectin is an inflammatory marker used to monitor patients with intestinal diseases.1,2 A calcium and zinc-binding protein, a heterodimer composed of the proteins S100A8 and S100A9, has also been referred to as calgranulin A/B, 14/MRP8 (myeloid-related protein), or cystic fibrosis (CF) antigen, or L1 protein3,4 and found in the cytoplasm of cells such as granulocytes and macrophages when activated.3-5 Calprotectin has antibacterial activity, induces apoptosis and chemotaxis, and is secreted by epithelial cells after the detection of cytokines or bacterial products.4,5 It is an important protein in the acute phase of inflammation.3,4
Calprotectin is present in feces due to the migration of neutrophils to the intestinal cells when there is a pathogen present, causing inflammation. This molecule is resistant to intestinal proteases, as well as to pancreatic secretions that turn it stable in feces for up to one week, facilitating the transport of samples to the laboratory for analysis.3-6 It is widely used as a biomarker in inflammatory bowel diseases, such as Crohn's disease and irritable bowel syndrome. The main method of fecal calprotectin detection is by enzyme-linked immunosorbent assay (ELISA).3 It has been studied as a diagnostic method of intestinal inflammation in CF.2,4
CF is a multi-systemic disease caused by variants in the gene that encodes the cystic fibrosis transmembrane conductance regulator (CFTR) protein, with clinical presentations that include increased secretion viscosity and subsequent epithelial obstruction of ducts.7,8 The pulmonary and gastrointestinal systems are usually affected, and intestinal inflammation requires further study and attention.7-9
The mechanisms of intestinal inflammation in CF are not fully understood. It is hypothesized that inflammation occurs as a primary consequence of a CFTR protein defect, which increases mucus viscosity, in addition to the acidic environment, a consequence of the insufficiency of pancreatic enzymes and mucin glycolysis.8,9 Changes in the intestinal microbiota, the chronic use of antibiotics, and bacterial growth in the intestine are also probable causal factors.9,10 Effective and non-invasive diagnostic methods improve assessment and to monitor intestinal inflammation in CF patients.
ObjectivesThis systematic review aimed to analyze the scientific evidence on fecal calprotectin in CF and its relevance to the disease.
MethodA systematic literature review is consisting of a search method divided into the steps of defining the question that (1) underpins the study, (2) defining a search strategy and databases used, (3) reviewing and selecting studies based on inclusion and exclusion criteria, and (4), evaluating the methodological quality of the studies.
After defining the guiding question, the search strategy consisted of using the following terms in English and Portuguese: "Leukocyte L1 Antigen Complex" OR Calprotectin OR "L1 Antigen" OR "L1 Protein, Leukocyte" OR Calgranulin AND "Cystic Fibrosis" OR Mucoviscidosis involving the platforms PubMed and Web of Science; and "Leukocyte L1 Antigen Complex" OR Calprotectin OR "L1 Antigen" OR "L1 Protein, Leukocyte" OR "Leukocyte L1 Protein" OR "Complexo Antígeno L1 Leucocitário" OR Calgranulin AND "Cystic Fibrosis" OR "Fibrose Cística" OR Mucoviscidose OR Mucoviscidosis OR "Fibrosis, Cystic" involving the platforms Scielo and Lilacs. The strategy was structured from the population, intervention, comparison, and outcome (PICO) method. A manual search of the bibliographic references contained in the articles was also performed to retrieve all existing information.
The inclusion criteria were:
- •
Search in the period of the last ten years;
- •
CF patients of different ages;
- •
Use of fecal calprotectin as a marker of intestinal inflammation;
- •
Texts in English and Portuguese;
- •
Randomized clinical trials, cohort, and case-control studies.
The exclusion criteria were:
- •
Use of calprotectin under other conditions;
- •
CF citations in other contexts;
- •
Study designs different from those of interest.
Next, the articles were sent to the Mendeley bibliographic reference manager, and duplicates were removed. Then titles and abstracts were analyzed to verify if they met the inclusion and exclusion criteria. Subsequently, the full article was read.
A form verified the population, type of intervention, if any, comparison group, and outcomes in the selected articles.
The quality of evidence represents the reliability of the effects presented in the studies. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) tool was used to check quality and risk of bias using 22 criteria. The method by Mendes et al.10 was used to score the articles, with each criterion being scored 0 and 1 for each article.
After evaluating all criteria, the scores were added. The articles studied received grades from 0 to 22 by each of two reviewers (EL, +), and the final grade was obtained through the mean. This score was analyzed as a percentage, and studies were considered good if the score was > 50%.
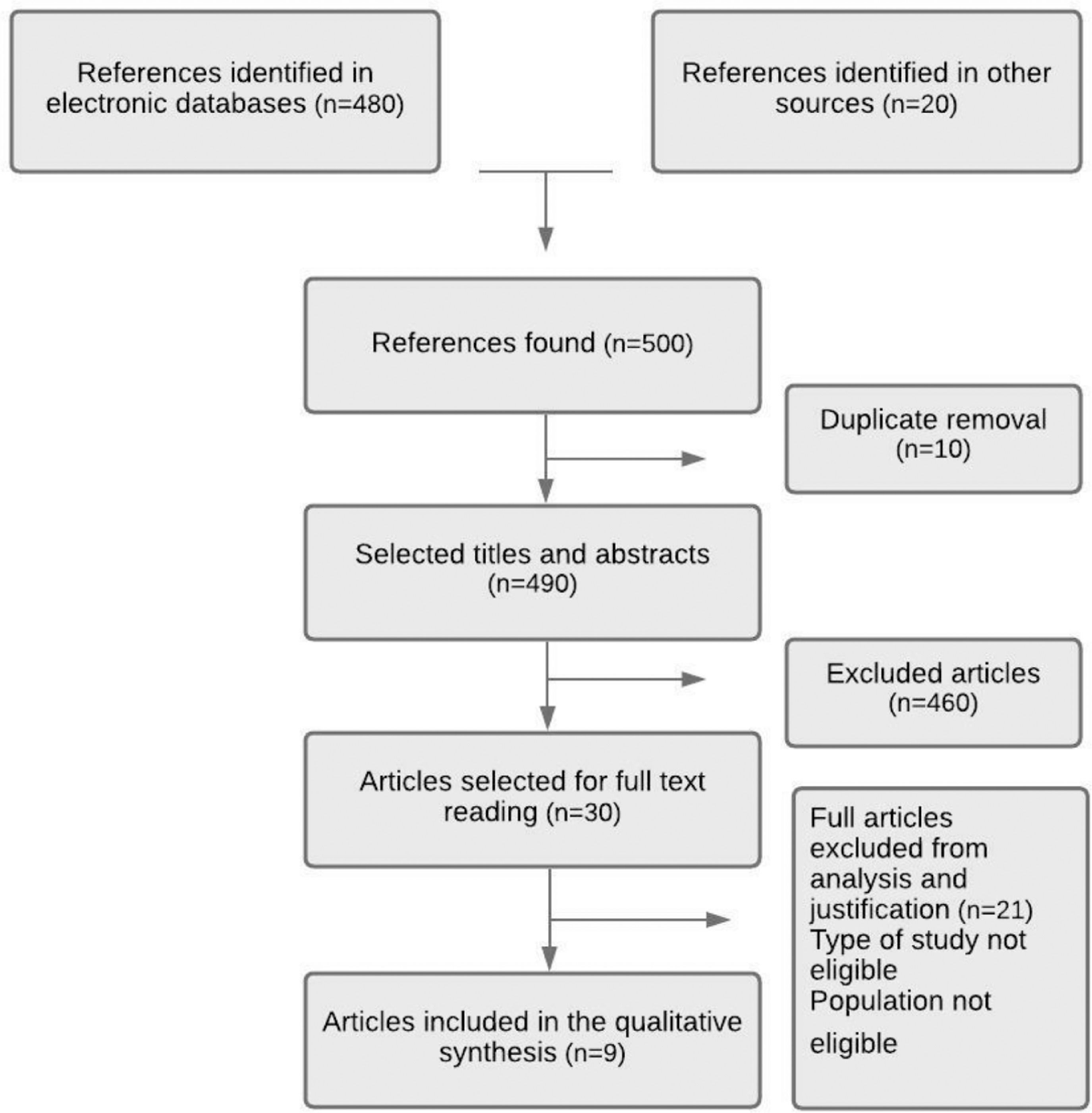
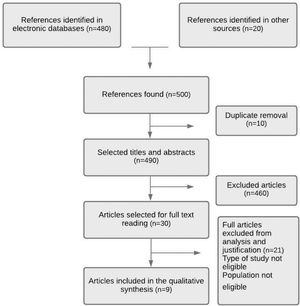
ResultsAccording to the flowchart presented in Figure 1, the search in databases and other sources resulted in approximately 500 articles, of which 50 addressed the subject defined in the guiding question. After eliminating duplicates and reading the titles and abstracts, 30 articles were selected for a complete reading. Of the articles read, nine were selected for qualitative synthesis (Table 1).
Scoring and quality analysis of articles selected for synthesis.
| Author/year of publication | Quality of articles. Score: mean (%) |
|---|---|
| Lisowska et al. (2010)13 | 19.6 (89) |
| Fallahi et al. (2013)14 | 20.4 (93) |
| Rumman et al. (2014)15 | 15.8 (72) |
| Adriaanse et al. (2015)16 | 19.7 (90) |
| Więcek et al. (2017)17 | 14.3 (65) |
| Ellemunter et al. (2017)11 | 16.3 (74) |
| Garg et al. (2017)18 | 17.8 (81) |
| Parisi et al. (2017)19 | 20.0 (91) |
| Schnapp et al. (2019)12 | 18.8 (85) |
One of the studies included was a randomized, controlled, and double-blind clinical trial. The other articles were case-control and cohort studies (eight articles).
The largest number of patients evaluated was 171 in a study by Ellemunter et al.,11 and the lowest was 14 patients in a study by Schnapp et al.12 Predominantly, the studies presented sample collection and calprotectin measurement, except for a longitudinal study, in which 2,434 samples were collected and analyzed.11
As for the location of the studies, a large proportion was derived from Europe (n = 5), mainly from countries such as Poland, Italy, and France. The second most frequent location was the Middle East (n = 4), with three studies from Israel and one from Iran, in addition to countries such as Australia (one study). Some studies included pancreatic insufficiency as a factor in the likelihood of patients having higher levels of calprotectin and inflammation.
Data such as the use of enzyme replacement therapy (ERT), weight and height, body mass index (BMI), pulmonary symptoms, liver insufficiency, and history of meconium ileus were also included. Patients with different genetic variants were analyzed in some of the studies in order to examine fecal calprotectin levels with disease phenotypes.
Most of the articles analyzed the possibility of fecal calprotectin testing functioning as an effective marker of intestinal inflammation.
The objectives of the studies and their results were summarized and are shown in Table 2.13-19
Results of the analysis of selected articles.
| Authors | Population | Objective | Result |
|---|---|---|---|
| Lisowska et al. (2010)Poland13 | Children and teenagersn = 55Cross-sectional study | To correlate small intestinal bacterial overgrowth with intestinal inflammation. | Fecal calprotectin levels were higher in CF patients compared to the control group. There was no difference in calprotectin levels between the group with and without SIBO assessed by BT. Indirect measures of intestinal inflammation may give false positive results. |
| Fallahi et al. (2013) Iran14 | Children over 4 yearsoldn = 47Doubleblind randomizedcontrolled trial | To verify intestinal inflammation after administration of probiotics by measuring fecal calprotectin. | The administration of probiotic (FOS) significantly reduced fecal calprotectin levels (p = 0.031) and, consequently, intestinal inflammation. |
| Rumman et al. (2014)Israel15 | Childrenn = 62. Prospective study | To relate high levels to clinical signs of disease. | No significant value in the clinical presentations, including the use of probiotics, pancreatic enzymes, and PS or PI. There was a higher percentage of sufficient pancreatic function with abnormal levels (81%) than insufficient (65%). The use of inhaled antibiotics reduced the marker levels. |
| Adriaanse et al. (2015)Netherlands16 | Children and adultsn = 175 prospective study | To assess enterocyte lesion with (I-FAPB) and correlation with intestinalinflammation(calprotectin). | They observed a correlation between CF and enteropathy (enterocyte injury) and intestinal inflammation. I-FAPB levels were increased in CF patients. Increased fecal calprotectin levels were observed in the CF group directly correlated with age and inversely with pulmonary function values in children (< 18 years) and adults. I-FAPB levels were not related to comorbidities, but there was an association of calprotectin with CFRD, PI, and use of proton pump inhibitors. |
| Więcek et al. (2017) Poland17 | Children and teenagers,n = 41Crosssectional study | Calprotectin in CF patients diagnosed by neonatal screening (mean age at diagnosis = 11 m) and without screening (mean = 7 years). | Increased fecal calprotectin levels were observed in 4/41 patients. There was no relationship between calprotectin and clinical variables. Patients presenting changes belonged to the group with homozygous delta F508 mutation. |
| Ellemunter et al. (2017)Austria11 | Children and adults,n = 171Retrospective study | Calprotectin as a marker of intestinal inflammation in CF. | Patients with PI unrelated to abdominal symptoms, forced expiratory volume (FEV1), and BMI had higher levels compared to patients with PS. Median fecal calprotectin levels in delta F508 homozygotes were not significantly higher than in heterozygotes. The levels of fecal calprotectin reduced over the years, which was attributed to the use of probiotics. There was a significant association between CRP and fecal elastase levels. |
| Garg et al. (2017) Australia18 | Children aged up to10 years,n = 165Prospective study | To compare calprotectin dosage in fibrocystic and healthy participants. | Healthy participants had higher fecal calprotectin levels at birth and decreased levels up to four years, and CF patients presented the opposite, with lower levels at birth, which increased up to four years and continued high. |
| Parisi et al. (2017)Italy19 | Children and adults,n = 104cohort study | To relate CF signs and symptoms to calprotectin levels. | CF patients had higher levels than healthy subjects. In severe CF phenotype (FEV1 < 50%, PI, BMI < 18.5, chronic P. aeruginosa colonization, > 18 ys), had higher levels of fecal elastase, as well as patients with pulmonary exacerbation. |
| Schnapp et al. (2019)Israel12 | Childrenn = 14 Prospective study | To verify the effects of pulmonary exacerbation treatment on fecal calprotectin. | Fecal calprotectin levels decreased after systemic use of antimicrobials to treat pulmonary exacerbation. Fecal calprotectin levels were high even in the absence of gastrointestinal symptoms. This study indicated fecal calprotectin as an intestinal as well as a systemic marker of inflammation. |
CF was an inclusion criterion. CRP: C-reactive protein; CF: cystic fibrosis; DBPC: double-blind placebo-controlled; FEV1: Forced expiratory volume in the first second; FOS: fructooligosaccharides; I-FAPB: intestinal fatty acid-binding protein; CFRD: cystic fibrosis-related diabetes; PI: pancreatic insufficiency; PS: pancreatic sufficiency; SIBO: small intestinal bacterial overgrowth; BT: breath testing with methane and hydrogen.; BMI: body mass index.
DiscussionThis review evaluated the use of fecal calprotectin dosage as a marker of inflammation in CF, especially in intestinal inflammation. It evaluated the effectiveness of the marker and possible limitations of its clinical usefulness.
The fecal calprotectin test as a marker of intestinal inflammation is recognized in patients with gastrointestinal diseases in general and has become increasingly evident in CF subjects.20,21
The determination of fecal calprotectin assists in differentiating between active and inactive inflammatory bowel diseases and between inflammatory bowel diseases and irritable bowel syndrome.22 However, the number of studies that demonstrated effectiveness for CF is low, and there are questions regarding their clinical relevance. Quantity and quality information on this topic is restricted. Some studies had a low number of patients, while others demonstrated analysis time as a limitation. The main issue observed in this review was that further studies are needed to specifically assess the use of this marker in CF.
More than one study indicated fecal calprotectin as a marker of systemic inflammation, and not just of intestinal inflammation since they showed variations in the presence of signs such as pulmonary exacerbation and use of antibiotics.12 Another point to be considered is the association of intestinal inflammation with pancreatic function in CF.23
Several studies report an association of pancreatic insufficiency with intestinal inflammation using the pancreatic elastase marker as a parameter. However, others dispute this observation, as the fecal calprotectin dosage was similar in the two groups in some studies. According to the Australian guidelines for the use of pancreatic enzyme replacement therapy in CF, intestinal inflammation exists because pancreatic insufficiency can increase intestinal permeability. The difficulty in the secretion of enzymes into the duodenum influences the extent of inflammation detected by pancreatic elastase.23
A possible explanation for the similar results involving sufficient and insufficient pancreatic groups in the studies would be the use of Pancreatic Enzyme Replacement Therapy (PERT) in insufficient patients, impacting the analysis of fecal calprotectin. The enzymes used in therapy improve symptoms related to the pancreas and may consequently affect the intestinal system.23
It is speculated whether intestinal inflammation could be linked to the respiratory system or if it is a local disorder, still poorly understood, as an association between fecal calprotectin levels and lung disease occurs in some cases. Serum and sputum calprotectin are elevated in pulmonary exacerbation.24,25 Gray et al. have demonstrated that treatment of an exacerbation with antibiotic therapy in CF results in decreasing levels of sputum and serum calprotectin. Thus, the change in sputum calprotectin following antibiotic therapy implies a direct association of calprotectin with a changing state of airways inflammation. The assessment of the possible role of calprotectin as a pro-inflammatory molecule in the lung requires further work.25
The onset of respiratory infections in patients and colonization by microorganisms can also induce increased fecal calprotectin since it interacts with inflammation levels, and, therefore, marker levels are likely to be increased in CF-related to lung disease. High levels of fecal calprotectin were found in some other gastrointestinal tract disorders, infectious gastroenteritis diverticular disease, microscopic colitis, cystic fibrosis, and patients treated with non-steroidal anti-inflammatory drugs or proton pump inhibitors.6,26
Essentially, this information only guarantees that there is an intestinal presentation of the disease but not a relationship with the pancreatic condition. Some authors suggest that the two factors are independent. Until recently, the diagnosis of inflammatory bowel diseases was mainly based on both clinical and endoscopic arguments. Fecal calprotectin is the most documented method in this situation because it is an easy, fast, reliable, non-invasive, and inexpensive biological assay not only in diagnosis but also in progress and therapeutic monitoring. This marker allows the discrimination between functional and organic bowel processes with good performance.26
The use of antibiotics was addressed in a few studies. Schnapp et al., demonstrated that fecal calprotectin levels decreased with antibiotic treatment of acute pulmonary exacerbations. The treatment of one system can affect the other, acting on both lung and intestinal microbiota, thus reducing intestinal inflammation.12
CF pulmonary exacerbation may represent part of a systemic inflammatory exacerbation that includes the GI tract, even with no GI complaints, possibly representing "silent" GI inflammation. Antibiotic treatment may alter the microbiome in both organs reducing the inflammatory burden.
The use of antibiotics impacts the microbial population present in the body, causing changes due to their frequent use.25,27
Changing the intestinal microbiota positively impacts some patients, modifying the inflammation in response to the presence of opportunistic microorganisms.28,29 The microbiota of CF patients has been shown to change from birth. Therefore, this aspect must be considered since the microbiome composition can lead to intestinal dysbiosis and imbalance in the microbiota responsible for reducing nutrient absorption capacity.29,30
The structure of intestinal microbiota of CF patients is probably shaped by the microecological effects resulting from these alterations in gut functions and also by the increased local concentrations of antimicrobials, either secreted by bile or accessing the intestinal tract after inhalation.31
A double-blind prospective study reported the positive impact of probiotics on intestinal microbiota since there may also be changes in the organism microbiota, helping the growth of beneficial microorganisms and balancing intestinal microbiota after treatment with probiotics.33
The studies report the possibility of interference in cases in which patients swallow sputum, which contains a certain amount of calprotectin. However, the amount swallowed does not seem to affect fecal calprotectin, as it is partially degraded in the intestinal transit.13 Lisowska et al. compared marker levels in sputum and feces samples, reporting that, despite the high levels, there was little interference by sputum in feces samples.13
The inflammatory condition in CF becomes an impacting factor in defining the functionality of the fecal calprotectin marker since it appears, in many cases, to be changed in inflammatory conditions resulting from the disease.32
Despite the outcomes found and studies showing the need for larger numbers of patients and longer analysis time, fecal calprotectin seems to be a useful marker of intestinal inflammation in CF, although some studies reported it as a marker of systemic inflammation.33
ConclusionIn summary, this systematic review showed that serial fecal calprotectin measurements could aid in following-up on inflammatory bowel conditions in CF patients.
In-depth studies with appropriate methods should be conducted aiming at understanding the role of this marker in CF and to determine its potential as a marker of systemic inflammation.
The authors are grateful to the Postgraduate Program in Internal Medicine and Health Sciences for providing the facilities for the conduction of the experiments and data analysis and Editage for the translation and editing services.
Study linked to the Universidade Federal do Paraná, Hospital de Clínicas, Unidade de Alergia, Imunologia e Pneumologia, Curitiba, PR, Brazil.