to determine the frequency of different phenotypes for congenital anomalies of the kidney and urinary tract (CAKUT) in a Brazilian sample, and to evaluate the association between the CAKUT phenotypes and the BMP4 gene.
Methodsin this study, 457 Brazilian individuals were analyzed in an attempt to establish the association between the BMP4 gene and the CAKUT diagnosis. A case-control sample was genotyped for three BMP4 gene polymorphisms.
Resultsassociation data was established with CAKUT sample as a whole and with the three most important CAKUT phenotypes: multicystic dysplastic kidney disease (MDK), ureteropelvic junction obstruction (UPJO) and vesicoureteral reflux (VUR). When the sample was segregated in these three phenotypes, associations between the BMP4 gene were observed with UPJO and with MDK. Conversely, VUR was not associated to the polymorphisms of the BMP4 gene.
Conclusionsthe present data suggest that Brazilian individuals with polymorphisms of the BMP4 gene have a higher risk to develop CAKUT, especially the malformations related to nephrogenesis and initial branching such as MDK and UPJO. Conversely, VUR appeared not to be related to BMP4 gene.
determinar a frequência de diferentes fenótipos de anomalias congênitas do rim e trato urinário (CAKUT) em uma amostra brasileira e avaliar a associação entre os CAKUT e o gene BMP-4.
Métodosneste estudo, analisamos 457 indivíduos brasileiros em uma tentativa de estabelecer a associação entre o gene BMP-4 e o diagnóstico de CAKUT. As amostras de caso e de controle foram genotipadas em busca de três polimorfismos do gene BMP-4.
Resultadosos dados de associação foram estabelecidos com a amostra de CAKUT como um todo e com os três fenótipos de CAKUT mais importantes: rim displásico multicístico (RDM), obstrução da junção ureteropélvica (UPJO) e refluxo vesico-ureteral (VUR). Quando a amostra foi separada nesses três fenótipos, encontramos associações entre o gene BMP-4 com UPJO e com RDM. Por outro lado, o VUR não foi associado aos polimorfismos do gene BMP-4.
Conclusõesesses dados sugerem que os indivíduos brasileiros com polimorfismos do gene BMP-4 apresentam maior risco de desenvolver CAKUT, principalmente as malformações relacionadas a nefrogênese e ramificação inicial, como RDM e UPJO. Por outro lado, o VUR parece não estar relacionado ao gene BMP-4.
Congenital anomalies of the kidney and urinary tract (CAKUT) occur in 0.5% to 6% of all pregnancies,1,2 and are common causes of end stage renal disease in children.3 CAKUT are polygenic traits and might be the result of multifactorial conditions such as de novo mutations, teratogenic substances, and maternal diet.3 Several candidate genes, including some that are expressed during nephrogenesis, have been associated with CAKUT.
Bone morphogenetic proteins (BMPs) are involved in the organogenesis of almost all vertebrates, regulating many aspects of development, including those of the urinary tract. The BMP4 gene, located in chromosome 14q22.2, is a member of the transforming growth factor-beta (TGF-β) superfamily.4 During urogenital development, BMP4 controls nephrogenesis and ureter branching and outgrowth,5,6 as well as the activity of the metanephric mesenchyma, ensuring that the ureteric bud is formed adjacent to the metanephron mesenchyme.7 Recent data from Chi et al. demonstrated that BMP4 also reduces the expression of important genes related to nephrogenesis process, such as glial cell line-derived neurotrophic factor gene (GDNF), paired box 2 gene (PAX2) and wingless-type MMTV integration site family, member 11 gene (WNT11).8,9
Functional studies showed that the BMP4 mutated gene generates an alternative protein complex with functional impairment.10 Miyazaki et al. demonstrated that mice with reduced expression of BMP4 (BMP4+/−). The authors demonstrated three different patterns of malformations: hydronephrosis with hypo/dysplastic kidneys, hydronephrosis due to ureterovesical junction obstruction, and duplex kidney with bifid ureter.9 In mice BMP4+/-, 60% coursed with hypo/dysplastic kidneys, 32% with ureterovesical junction obstruction, and 8% with bifid ureter.9 In 2008, Weber et al. identified three missense mutations in five CAKUT patients, presenting kidney aplasia or hypoplasia and dysplasia.11 From a mice model, it is known that only some BMP4+/− mice present CAKUT, which leads to the assumption that BMP4 is a fine-tuning protein that modulates the amount of functional nephrons and the ureteric branching.9,11,12 Based on these previous findings, the authors hypothesized that the BMP4 gene would be associated with CAKUT in a Brazilian sample. In this study, the association between three SNPs (rs17563, rs2071047, and rs762642) and CAKUT in general were evaluated, as well as the association to specific phenotypes in a Brazilian CAKUT sample. Since the Brazilian population presents a diverse genetic background,13 this study aimed to evaluate the role of the BMP4 gene in a case/control Brazilian sample.
MethodsCase and control groupsThe study followed the ethics guidelines of the Declaration of Helsinki, and was approved by the local ethics committee. An informed consent was obtained from all subjects.
CasesAt the Division of Fetal Medicine, all fetuses underwent a detailed ultrasound (US) scan aimed at detecting renal abnormalities and other malformations as previously detailed.14–16 Postnatally, infants who presented fetal renal pelvic dilatation or other renal alterations underwent systematic investigation for urinary tract anomalies, and were prospectively followed up at the Pediatric Nephrology Unit according to a systematic protocol, as previously described.14,16 Renal pelvic dilatation in fetal US was considered to be present if the maximum anteroposterior diameter of the renal pelvis was ≥ 5mm on prenatal US after 28 weeks’ gestation.16 Associated hydronephrosis was defined as dilatation of other segments of the urinary tract, in addition to the renal pelvis. Multicystic dysplastic kidney was defined as present when disconnected cysts of various sizes were located within the parenchyma of a structurally abnormal kidney in which no renal pelvis could be demonstrated.16 The systematic approach to and follow-up of infants with prenatally detected CAKUT at this unit comprised an US scan performed after the first week of postnatal life (7 to 15 days) and a voiding cystourethrogram (VCUG) in a selected subgroup of patients.15 US scans, clinical examination (including growth and blood pressure measurements), and laboratory reviews (including urine culture and serum creatinine) were scheduled at six-month intervals. When VCUG was normal but postnatal ultrasound scans demonstrated renal pelvis dilatation (RPD) ≥10mm, renal scintilography was performed after the first month.14
ControlsA careful screening was performed to provide detailed kinship information of the control group, in order to rule out participants with CAKUT or family relationship to patients with CAKUT. This study recruited a total of 211 isolated CAKUT patients from various regions of Brazil referred to the Hospital das Clínicas da Universidade Federal de Minas Gerais between 2010 and 2011; 246 healthy individuals (control group) from several areas of Brazil were also recruited, and none associated CAKUT were included at the sample. Peripheral blood was collected from all subjects, and DNA was extracted according to the method described by Lahiri and Nurnberger.17
Genotyping and allelic discriminationFor allelic discrimination, the made-to-order TaqMan® (Applied Biosystems) probes for rs17563, rs207147, and rs762642 SNPs, using 50 ng of DNA per sample, were used. rs762642 is located in an intronic region (14:54423053) of chromosome 14. It delimits a genomic region where a promoter and an enhancer of BMP4 are located.18 rs2071047 is located at 14:54418411 in an intronic region, close to the end of the first exon of BMP4.19 rs17563 is located in a coding region (14:54417522), and promotes an amino acid change (Val/Ala).
Allelic discrimination analysis was performed in 96-well plates in a real-time polymerase chain reaction (PCR; Mx3005PTM Stratagene, GE Healthcare Life Sciences) device. Each plate was subjected to the following steps: 10minutes at 95°C, and 50 cycles at 95°C for 15s and at 60°C for 1min. Case and control samples were randomly arranged in well plates; at least 20% of genotypes were retyped as quality control. Based on Penna et al., Brazilian genomic proportions were considered as relatively equal and were not stratified by ethnicity or skin color.13 The three SNPs used were chosen based on the HapMap database, with a selection criterion of r2 > 0.8 and minor allele frequency (MAF) > 0.2.
Data analysisStatistical analysis was performed using UNPHASED version 3.1.4© (2008 Frank Dudbridge MRC Biostatistics Unit Cambridge CB2 0SR - United Kingdom) with 1,000 permutations. Differences between genotype distribution and allele frequency were tested by the chi-squared analysis. A p-value < 0.05 was considered to be statistically significant after correction for multiple testing with 1,000 permutations. To analyze and visualize linkage disequilibrium (LD) and haplotype maps, HAPLOVIEW 4.2© was used in accordance with Barrett et al.20 The markers rs17563 and rs2071047 were at Hardy-Weinberg equilibrium. rs762642 was not at Hardy-Weinberg equilibrium and, for this reason, data were re-analyzed without this marker, and only frequencies for each diagnosis of CAKUT were shown. Therefore, even after the exclusion of marker rs762642, the associations between the other two markers were still observed with CAKUT in general, and the diagnosis of UPJO and MKD. Even considering that the Brazilian population is a result of an admixture of population Amerindians, Asians, Europeans, and Africans, Penna et al. stated that Brazilian genomic proportions are relatively equal and it is not possible to stratify by their ethnicity or skin color.13 Besides this information, 40 indels were genotyped, which confirmed that case and control samples were not stratified for ethnicity (data not show).
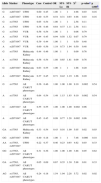
ResultsThe sample consisted of 457 individuals, 211 cases and 246 controls, 36.6% males and 63.4% females. The number of males was higher than the number of females among cases (1.89 male to female ratio). Eleven diverse urinary tract anomalies were observed in the patient's group: vesicoureteral reflux (VUR; n = 49, 23%), ureteropelvic junction obstruction (UPJO; n = 38, 18%), multicystic kidney disease (MKD; n = 32, 15%), idiopathic hydronephrosis (n = 50, 24%), and others (ureterocele, Prune-Belly syndrome, horseshoe kidney, megaureter, urinary tract duplication, isolated unilateral hypoplasia, and posterior urethral valve), none of which represented over 5% of the total the case sample. The presence of allele A at rs17563 was a risk factor for anomalies of CAKUT. When AA genotype was present, the risk was 2.49 higher (chi-squared = 6.64, p = 0.01 after 1,000 permutations = 0.08) than when GG was present. Haplotype composed by allele A from rs17563 and allele G from rs2071047 increased the risk when compared to haplotype composed by GG from the same markers. Since the main diagnosis at CAKUT samples were VUR, UPJO, and (MKD, the association of these phenotypes with the BMP4 gene polymorphisms were analyzed. The allelic and genotype frequencies were significantly different in comparison between patients with UPJO and the control group for the polymorphisms rs2071047 and rs17563 at the BMP4 gene (Table 1). Statistical significance was also obtained when the same polymorphisms between MKD cases and controls were analyzed (Table 1). Conversely, no associations were established between cases of VUR group and the BMP4 gene polymorphisms (Table 1). The presence of allele G at rs2071047 was related to increased risk to present UPJO in comparison with allele A (Table 1). At genotype analysis, the diagnosis of UPJO was associated with the GG homozygosis at rs2071047. At haplotypic analysis, allele A from rs17563 in combination with allele A from rs2071047 also appeared to be associated with UPJO. Detailed results are reported in Tables 1 and 2.
Allelic, genotypic, and haplotypic frequencies and association between CAKUT patients and healthy controls.
| Allele | Marker | Phenotype | Case | Control | OR | 95% Lo | 95% Hi | X2 | p-valuea | p 1.000b |
|---|---|---|---|---|---|---|---|---|---|---|
| G | rs2071047 | UPJO | 0.60 | 0.45 | 1.00 | 1 | 1 | 4.98 | 0.03 | 0.16 |
| A | rs2071047 | UPJO | 0.40 | 0.55 | 0.54 | 0.31 | 0.93 | 4.98 | 0.03 | 0.16 |
| G | rs17563 | UPJO | 0.65 | 0.54 | 1.00 | 1 | 1 | 2.58 | 0.11 | |
| A | rs17563 | UPJO | 0.36 | 0.46 | 0.64 | 0.37 | 1.11 | 2.58 | 0.11 | |
| G | rs17563 | VUR | 0.56 | 0.54 | 1.00 | 1 | 1 | 0.06 | 0.79 | |
| A | rs17563 | VUR | 0.44 | 0.45 | 0.94 | 0.58 | 1.52 | 0.07 | 0.79 | |
| G | rs2071047 | VUR | 0.39 | 0.44 | 1.00 | 1 | 1 | 0.50 | 0.48 | |
| A | rs2071047 | VUR | 0.60 | 0.56 | 1.19 | 0.73 | 1.94 | 0.50 | 0.48 | |
| G | rs17563 | Multicystic Kidney | 0.44 | 0.46 | 1.00 | 1 | 1 | 0.09 | 0.76 | |
| A | rs17563 | Multicystic Kidney | 0.56 | 0.54 | 1.08 | 0.65 | 1.81 | 0.09 | 0.76 | |
| G | rs2071047 | Multicystic Kidney | 0.63 | 0.55 | 1.00 | 1 | 1 | 1.66 | 0.20 | |
| A | rs2071047 | Multicystic Kidney | 0.37 | 0.45 | 0.71 | 0.42 | 1.19 | 1.66 | 0.20 | |
| G | rs17563 | All CAKUT phenotypes | 0.36 | 0.46 | 1.00 | 1.00 | 1.00 | 9.10 | 0.002 | 0.54 |
| A | rs17563 | All CAKUT phenotypes | 0.64 | 0.54 | 1.49 | 1.15 | 1.93 | 9.10 | 0.002 | 0.54 |
| G | rs2071047 | All CAKUT phenotypes | 0.55 | 0.55 | 1.00 | 1.00 | 1.00 | 0.002 | 0.96 | |
| A | rs2071047 | All CAKUT phenotypes | 0.45 | 0.45 | 0.99 | 0.77 | 1.28 | 0.002 | 0.96 | |
| GA | rs17563 | Multicystic Kidney | 0.33 | 0.54 | 0.43 | 0.16 | 1.09 | 5.05 | 0.02 | 0.03 |
| GG | rs2071047 | UPJO | 0.40 | 0.18 | 1.00 | 1 | 1 | 7.46 | 0.006 | 0.18 |
| AA | rs17563, rs2071047 | UPJO | 0.22 | 0.37 | 0.46 | 0.23 | 0.93 | 4.62 | 0.03 | 0.33 |
| GG | rs17563, rs2071047 | All CAKUT phenotypes | 0.31 | 0.36 | 1.00 | 1.00 | 1.00 | 3.88 | 0.05 | 0.82 |
| GA | rs17563, rs2071047 | All CAKUT phenotypes | 0.05 | 0.09 | 0.67 | 0.35 | 1.30 | 5.98 | 0.01 | 0.33 |
| AG | rs17563, rs2071047 | All CAKUT phenotypes | 0.24 | 0.18 | 1.54 | 1.04 | 2.28 | 5.72 | 0.02 | 0.02 |
OR, odds-ratio; Lo, lower limit; Hi, hight limit.
The results were obtained by χ2 = chi-squared tests.
Data regarding all genotype and haplotype analysis of all markers and associations with CAKUT patients and controls.
| Genotype | Marker | Phenotype | Case | Control | O-R | 95% Lo | 95% Hi | X2 | p-valuea | p 1.000b |
|---|---|---|---|---|---|---|---|---|---|---|
| GG | rs1753 | Multicystic Kidney | 0.27 | 0.18 | 1.00 | 1 | 1 | 1.31 | 0.25 | |
| GA | rs17563 | Multicystic Kidney | 0.33 | 0.54 | 0.43 | 0.16 | 1.09 | 5.05 | 0.02 | 0.03 |
| AA | rs17563 | Multicystic Kidney | 0.39 | 0.27 | 1.01 | 0.39 | 2.53 | 2.18 | 0.14 | |
| GG | rs2071047 | Multicystic Kidney | 0.38 | 0.29 | 1.00 | 1.00 | 1.00 | 1.19 | 0.27 | |
| GA | rs2071047 | Multicystic Kidney | 0.50 | 0.52 | 0.73 | 0.34 | 1.59 | 0.04 | 0.85 | |
| AA | rs2071047 | Multicystic Kidney | 0.12 | 0.19 | 0.47 | 0.14 | 1.52 | 1.09 | 0.30 | |
| GG | rs17563 | VUR | 0.23 | 0.27 | 1.00 | 1 | 1 | 0.37 | 0.54 | |
| GA | rs17563 | VUR | 0.67 | 0.54 | 1.48 | 0.65 | 3.34 | 2.11 | 0.15 | |
| AA | rs17563 | VUR | 0.10 | 0.18 | 0.68 | 0.19 | 2.35 | 1.45 | 0.23 | |
| GG | rs2071047 | VUR | 0.13 | 0.18 | 1.00 | 1 | 1 | 0.71 | 0.39 | |
| GA | rs2071047 | VUR | 0.54 | 0.51 | 1.51 | 0.53 | 4.24 | 0.08 | 0.77 | |
| AA | rs2071047 | VUR | 0.33 | 0.30 | 1.57 | 0.52 | 4.72 | 0.14 | 0.71 | |
| GG | rs17563 | UPJO | 0.42 | 0.26 | 1.00 | 1 | 1 | 3.26 | 0.07 | |
| GA | rs17563 | UPJO | 0.45 | 0.54 | 0.52 | 0.23 | 1.176 | 0.97 | 0.32 | |
| AA | rs17563 | UPJO | 0.13 | 0.19 | 0.43 | 0.13 | 1.39 | 0.68 | 0.41 | |
| GG | rs2071047 | UPJO | 0.40 | 0.18 | 1.00 | 1 | 1 | 7.46 | 0.006 | 0.18 |
| GA | rs2071047 | UPJO | 0.40 | 0.52 | 0.35 | 0.15 | 0.84 | 1.67 | 0.19 | |
| AA | rs2071047 | UPJO | 0.20 | 0.29 | 0.32 | 0.11 | 0.91 | 1.07 | 0.30 | |
| GG | rs17563, rs2071047 | VUR | 0.3665 | 0.3521 | 1 | 1 | 1 | 0.002543 | 0.9598 | |
| GA | rs17563, rs2071047 | VUR | 0.2232 | 0.1906 | 1.125 | 0.57 | 2.21 | 1.193 | 0.2747 | |
| AG | rs17563, rs2071047 | VUR | 0.01807 | 0.09021 | 0.1924 | 0.02 | 1.53 | 3.615 | 0.05727 | |
| AA | rs17563, rs2071047 | VUR | 0.3922 | 0.3671 | 1.026 | 0.59 | 1.78 | 0.01922 | 0.8897 | |
| GG | rs17563, rs2071047 | UPJO | 0.45 | 0.35 | 1.00 | 1 | 1 | 3.33 | 0.06 | |
| GA | rs17563, rs2071047 | UPJO | 0.18 | 0.19 | 0.76 | 0.32 | 1.83 | 0.13 | 0.72 | |
| AG | rs17563, rs2071047 | UPJO | 0.15 | 0.09 | 1.18 | 0.44 | 3.19 | 0.98 | 0.32 | |
| AA | rs17563, rs2071047 | UPJO | 0.22 | 0.37 | 0.46 | 0.23 | 0.93 | 4.62 | 0.03 | 0.33 |
| GG | rs17563, rs2071047 | Multicystic Kidney | 0.42 | 0.37 | 1.00 | 1.00 | 1.00 | 0.46 | 0.50 | |
| GA | rs17563, rs2071047 | Multicystic Kidney | 0.01 | 0.09 | 0.17 | 0.02 | 1.36 | 3.55 | 0.06 | |
| AG | rs17563, rs2071047 | Multicystic Kidney | 0.22 | 0.18 | 1.02 | 0.50 | 2.07 | 0.85 | 0.36 | |
| AA | rs17563, rs2071047 | Multicystic Kidney | 0.35 | 0.36 | 0.84 | 0.47 | 1.52 | 0.15 | 0.69 | |
| GG | rs17563, rs2071047 | All CAKUT phenotypes | 0.31 | 0.36 | 1.00 | 1.00 | 1.00 | 3.88 | 0.05 | 0.82 |
| GA | rs17563, rs2071047 | All CAKUT phenotypes | 0.05 | 0.09 | 0.67 | 0.35 | 1.30 | 5.98 | 0.01 | 0.33 |
| AG | rs17563, rs2071047 | All CAKUT phenotypes | 0.24 | 0.18 | 1.54 | 1.04 | 2.28 | 5.72 | 0.02 | 0.02 |
| AA | rs17563, rs2071047 | All CAKUT phenotypes | 0.39 | 0.36 | 13.0 | 0.96 | 1.76 | 1.31 | 0.25 |
OR, odds-ratio; Lo, lower limit; Hi, hight limit.
The results were obtained by χ2: chi-squared tests.
Association studies of candidate genes have helped to decipher the genetic basis of many complex diseases, and are meaningful to establish a genotype-phenotype relationship. Mackie and Stephens21 postulated that the primary event for CAKUT occurs during the start of the budding process. These changes during the budding process also impact many developmental disturbances, even at renal parenchyma, by producing hypo- and/or dysplastic kidneys.21 Since then, much attention has been directed to the beginning of the budding. After the hypothesis that all kidney malformations might be derived from a single mutation and a consequent inadequately punctual signaling event, several genes have been indicated as candidates for nephrogenesis abnormalities.22,23 It is well known that the BMP family takes part in the ureteric bud development, and that the BMP4 gene inhibits this process.9,11 High concentration of BMP4 in the kidneys of mice resulted in lower numbers of bud tips when compared with non-treated embryonic mice kidneys.24,25 In addition, BMP4 appeared to be a regulator of the ureteric growth rate,9,11 since the growth of the ureteric stalk was affected by exogenous BMP4 and its expression at the peri-ureteric region might regulate ureteric elongation.9 In this context, the present study evaluated the potential role of BMP4 gene in a large CAKUT sample. Furthermore, associations were searched for between the BMP4 gene and specific CAKUT phenotypes, such as UPJO, MKD, and VRU.
BMP4 is one of the molecules responsible for the assembly of muscle coating of the urothelium, the insertion of the most caudal part of the ureter into the bladder, and the following aggregation of cells around the ureter that will differentiate into smooth muscles.9,11 Therefore, the proper functioning of the ureter depends on the positioning of the ureter emerging from Wolffian duct. Consequently, it has already been established, from a mice model, that the BMP4 gene regulates in a dose-dependent rate the loss of ureteral smooth muscles and determines the UPJO phenotype.25,26 In that way, the association with UPJO, as observed in the present CAKUT sample, is at least expected.
Major changes in kidney structure might result from disturbances of the GDNF–RET signaling.3,6 In fact, the GDNF is downstream to BMP4.22,26 In this regard, the association found between the BMP4 gene and MKD in the present sample might be related to alterations in GDNF-RET pathway. Moreover, when the CAKUT patients were segregated according to the three main endophenotypes, a different pattern of association for MKD/UPJO in comparison to VUR was identified. The polymorphisms evaluated for the BMP4 gene were not associated with VUR. This finding at least suggests that different pathways might regulate the genesis of VUR, and this microenvironment does not include the BMP4 gene. Despite the role of BMP4 in ureteric elongation, the gene does not appear to regulate the tuning and the insertion of the ureter into the bladder, which are considered critical for the pathogenesis of VUR. Further studies with other genetic markers are necessary to elucidate the pathogenesis of VUR.
Another aspect to be considered is the role of the BMP4 gene in tissue fibrosis. Studies on the role of the BMP4 gene in asthma showed a modulation of fibrosis, and consequently, of remodeling and of determination of a worse prognosis;27 this function and pathway might be another interesting point to evaluate the BMP4 function on kidney disorders, since renal fibrosis is a common final pathway for end-stage disease, a potential consequence of CAKUT. In this regard, the study of Tominaga et al.28 tried to elucidate the direct function of BMP4 in kidneys; however, the focus was on the glomeruli function in adult mice. Their findings demonstrated that heterozygous BMP4 knockout mice presented less glomerular injury resulting from diabetes when compared to wild type mice, suggesting that BMP4 controls the deposit of extracellular matrix and, consequently, might modulate the fibrosis process in such disorders.28
The present study has certain limitations. Since CAKUT are multifactorial and polygenic conditions,3 it is necessary to perform an epidemiological study in the Brazilian population, in order to verify whether the primary cause of CAKUT is directly related to genetic mutations, which alter the function of proteins involved in the urinary tract development, such as BMP4, WNT11, PAX2, and GDNF.8,9 This genetic-epidemiological approach may provide information such as environmental factors related to CAKUT, in the absence of changes in CAKUT candidate genes. In recent decades, studies have reported various environmental factors that affect the development of the urinary tract. Drugs such as dexamethasone and antibiotics affect renal development in animal models.29,30 In 2009, Andiman et al. confirmed the increased incidence of changes in the urinary tract of HIV-infected patients on antiretroviral therapy since birth.31
Nevertheless, the data obtained in this study at least partly contribute to the understanding of CAKUT origins in the Brazilian population. The present data suggest that BMP4 has many roles that might determine the phenotype and the major consequences of kidney malformation, such as the end-stage renal disease. These results are in line with the study of Wang et al.,22 who reported that decreased BMP4 signaling results in a gradual decrease in the coat of smooth muscle, which surrounds the ureter. This decrease raises the possibility that abnormalities in BMP4 signaling may have a role in the development of congenital UPJO. It could explain why an association between UPJO and the polymorphisms in the BMP4 gene was observed.
ConclusionData show that the BMP4 gene is associated MKD and UPJO, but not with VUR in this sample of Brazilian patients. This gene might have an essential role in nephrogenesis. Therefore, further studies are necessary in order to identify the molecular pathways of BMP4 in CAKUT, and also to confirm these associations in other populations with different endophenotypes of urinary tract malformations.
FundingThis study was partially supported by CNPq (Brazilian National Research Council, Grant 401949/2010-9), FAPEMIG (Fundação de Amparo à Pesquisa do Estado de Minas Gerais, Grant PPM-00152-09), and the INCT-MM Grant (FAPEMIG: CBB-APQ-00075-09 / CNPq 573646/2008-2). ISF and TRH were the recipients of CNPq fellowships. Dr. AC Simões e Silva, Dr De Marco LA, Dr. EA Oliveira and Dr DM Miranda received a research productivity grant from CNPq.
Conflicts of interestThe authors declare no conflicts of interest.
The authors would like to thank the Universidade Federal de Minas Gerais, the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), the Fundação de Amparo à Pesquisa do estado de Minas Gerais (FAPEMIG), the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), and the Instituto Nacional de Ciência e Tecnologia de Medicina Molecular (INCT MM).
Please cite this article as: Reis GS, Simões e Silva AC, Freitas IS, Heilbuth TR, Marco LA, Oliveira EA, et al. Study of the association between the BMP4 gene and congenital anomalies of the kidney and urinary tract. J Pediatr (Rio J). 2014;90:58–64.