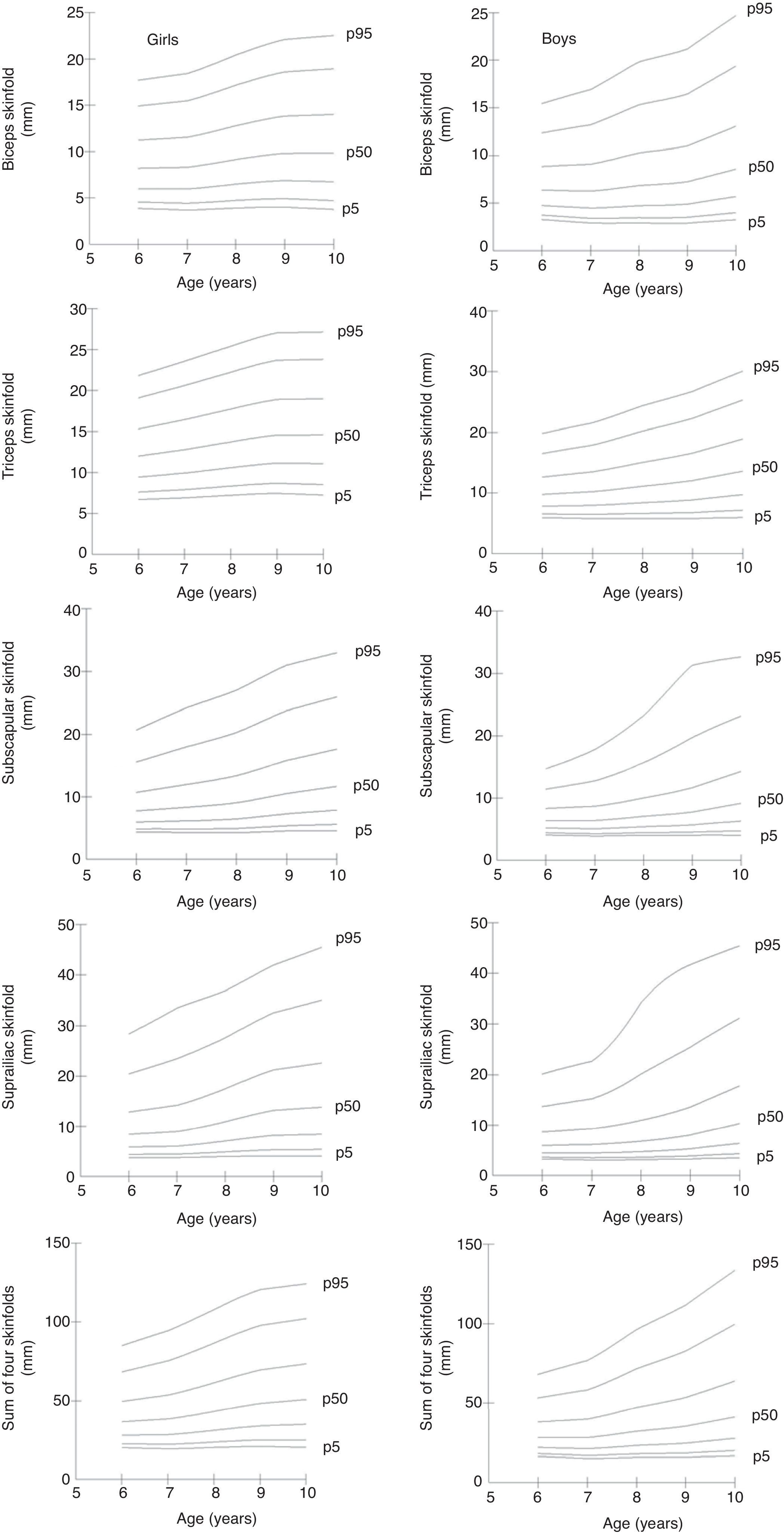
To draw skinfold (SF) reference curves (subscapular, suprailiac, biceps, triceps) and to determine SF cutoff points for predicting the risk of metabolic syndrome (MetS) in children aged 6–10 years old.
MethodsThis was a cross-sectional study with a random sample of 1480 children aged 6–10 years old, 52.2% females, from public and private schools located in the urban and rural areas of the municipality of Uberaba (MG). Anthropometry, blood pressure, and fasting blood samples were taken at school, following specific protocols. The LMS method was used to draw the reference curves and ROC curve analysis to determine the accuracy and cutoff points for the evaluated skinfolds.
ResultsThe four SF evaluated (subscapular, suprailiac, biceps, and triceps) and their sum (∑4SF) were accurate in predicting MetS for both girls and boys. Additionally, cutoffs have been proposed and percentile curves (p5, p10, p25, p50, p75, p90, and p95) were outlined for the four SF and ∑4SF, for both genders.
ConclusionSF measurements were accurate in predicting metabolic syndrome in children aged 6–10 years old. Age- and gender-specific smoothed percentiles curves of SF provide a reference for the detection of risk for MetS in children.
Desenhar curvas de referência de quatro dobras cutâneas (subescapular, suprailíaca, bíceps, tríceps) e determinar pontos de corte para predizer o risco de SM em crianças de seis a 10 anos de idade.
MétodosEstudo epidemiológico de base populacional, corte transversal, com amostra probabilística, estratificada por segmento de ensino, com 1.480 crianças de 6 a 10 anos de idade, 52,2% do sexo feminino, oriundas de escolas públicas e privadas, localizadas na zona urbana e rural do município de Uberaba (MG). Antropometria (dobras cutâneas), pressão arterial e as coletas de sangue em jejum foram realizadas em espaço reservado na escola, seguindo protocolos específicos. O métodolo LMS foi utilizado para desenhar as curvas de referência e análise de curva ROC para determinar a acurácia e pontos de corte para as dobras cutâneas avaliadas.
ResultadosAs quatro DC avaliadas (subescapular, suprailíaca, bíceps e tríceps) e o seu somatório (∑4DC) foram acurados na predição da SM para meninas e meninos. Adicionalmente, pontos de corte foram propostos e curvas percentílicas (p5, p10, p25, p50, p75, p90 e p95) foram delineadas para as quatro DC e o ∑4DC, para ambos os sexos.
ConclusãoMedidas de DC foram acuradas em predizer SM em escolares de seis a 10 anos de idade. As curvas percentílicas de DC desenhadas por idade e sexo fornecem referência na detecção do risco de SM em crianças.
Cardiovascular diseases are the main cause of death in Brazil1 and worldwide.2 Cardiovascular risk factors, such as total and visceral obesity, dyslipidemias, arterial hypertension, hyperglycemia, and hyperinsulinemia have been considered determinants for the development of cardiovascular diseases. Metabolic syndrome (MetS), characterized by the presence of three or more of these risk factors,3 has gained importance due to its consistent association with cardiovascular morbimortality.4
The presence of cardiovascular risk factors and MetS has been observed in adults5 as well as in children and adolescents.6 In recent years, concerns about the diagnosis of and early intervention on these metabolic disorders has increased due to evidence that risk factors observed in childhood and adolescence tend to persist and worsen in adulthood.7
In addition to blood pressure (BP) and waist circumference measurements, biochemical analyses of blood fractions of HDL-c, triglycerides, and glucose should be performed for the diagnosis of MetS. Blood collection is an invasive, expensive and difficult to perform technique, especially in children and adolescents. In turn, the use of practical and low-cost methods may be an important alternative for the screening of MetS at the population level. In this sense, skinfolds (SFs) were shown to be a promising tool for MetS screening in the pediatric population, due to its strong correlation with subcutaneous adiposity.8
According to Ali et al.,9 the accumulation of subcutaneous adiposity is a strong predictor of insulin resistance and hypertriglyceridemia in children and adolescents, and was a stronger predictor of cardiometabolic risk factors than visceral fat. Furthermore, the influence of subcutaneous adiposity on MetS risk is present in children and adolescents, but not in adults.9
Percentile curves for SFs have been developed with samples of young North-American,10 Polish,11 and Indian populations.12 It is noteworthy that the World Health Organization13 presented an important publication in 2007, in which reference curves for anthropometric measurements, including triceps and subscapular SFs were created as an international reference for multi-country populations (Brazil, United States, Ghana, India, Norway, and Oman). However, to the best of the authors’ knowledge, SF percentile curves have not been proposed for Brazilian children, nor are there any SF percentile curves that can be used to predict MetS in the pediatric population. Reference curves from other populations may not be applicable to Brazilian children due to ethnic, cultural, and socioeconomic differences.
Therefore, the present study aimed to design reference curves of four SFs (subscapular, suprailiac, biceps, and triceps) and to determine cutoffs to predict the risk of MetS in children aged 6–10 years.
MethodsA cross-sectional, population-based epidemiological study was carried out with a probabilistic sample of children aged 6–10 years from public and private schools located in the urban and rural areas of the city of Uberaba, MG, Brazil.
The sample calculation considered the number of children enrolled in Elementary School (1st to 9th year of schooling), a prevalence of MetS of 50% (unknown prevalence in the municipality), tolerable error of 3.5% and confidence level of 95%. The minimum sample size was 768 children; after adding 10% to compensate for losses and refusals and 20% to minimize confounding factors, the sample totaled 1014 children.
For sample selection, the schools were stratified according to the type of school as municipal, state, or private.
The World Health Organization14 recommends that 10–15 sample collection points (schools) be used for epidemiological surveys, and that the number of subjects in each age group should vary between 25 and 50 for each site. Therefore, 15 of the 90 eligible schools of the municipality of Uberaba were randomly selected using the random number table. For the adequacy and representativeness of the local population, the number of children in each stratum was determined proportionally to the number of enrollments, according to data provided by the State Education Secretariat. Municipal schools accounted for 43.6% of enrollments, 41.9% of students were enrolled from state schools and 14.5% from private schools.
After approval by the Ethics Committee in Research with Human Beings of Universidade Federal do Triângulo Mineiro (Protocol CEP/UFTM: 1710), the school principals were contacted to obtain authorization and to schedule the collections. Students who met the inclusion criteria and were interested in participating in the research received the informed consent form for the information and signature of their parents and/or guardians. Anthropometry and blood collections were performed at the school itself, following specific protocols.
The biceps (BSF), triceps (TSF), subscapular (SSSF), and suprailiac (SISF) SFs were obtained using an adipometer (Lange Skinfold Caliper, England, UK) exerting constant pressure of 10g/mm2. Measurements were performed on the right side of the body, with three non-consecutive repetitions for each measurement. The final measure constituted the mean of the three values. All measurements were performed by two qualified evaluators submitted to previous training and calibration. The results were interpreted alone, as well as by the sum of the four SFs assessed, and were expressed in millimeters (mm).
BP was measured using a mercury column sphygmomanometer (Unitec, São Paulo, Brazil), with cuffs of appropriate sizes for the circumference of the children's arms, in accordance with the VI Brazilian Guidelines for Hypertension.15 Three BP measurements were performed at the first visit, after which the first was discarded and the mean of the last two were considered. When the child had an alteration in systolic or diastolic BP above the 90th percentile, two further measurements were performed on two different days following the same procedure adopted on the first day.15
The volunteers were invited to go to school, following a 12-h fast, on certain days and at certain times, accompanied by their parents. Nursing professionals collected blood samples (8mL) in BD Vacutainer™ tubes (Becton, Dickinson and Company, New Jersey, USA). Serum samples were analyzed for the measurement of HDL-c and triglycerides and plasma for glycemia. The semi-automated Bio 200F analyzer (Bioplus, São Paulo, Brazil) was used. Standardized methods quantitatively determined blood variables, following the standards and technical specifications of the reagents used.
The diagnosis of MetS16 was determined by the presence of at least three of the following alterations: triglycerides ≥100mg/dL; HDL-c <50mg/dL; glycemia ≥110mg/dL; waist circumference ≥75th percentile for age and gender; and alteration in BP (diastolic or systolic) >90th percentile adjusted for age, height and gender.
Variables were tested for their normality using the Kolmogorov–Smirnov test. Outliers were identified and withdrawn using the interval between quartiles. The Mann–Whitney U-test was used to compare independent groups with non-parametric distribution.
For comparison with other studies, the 5th, 10th, 25th, 50th, 75th, 90th, and 95th percentiles were chosen as reference values. Reference curves were created using the Cole LMS method.17 The LMS method assumes that, for independent data with positive values, the age-specific Box–Cox transformation can be employed to make them have a normal distribution; the L, M, and S values are natural cubic splines with knot sequence without each age range. The sample, in each gender, was separated into age groups with 100 or more individuals, the lowest number considered to be adequate for the LMS method.17
The receiver operating characteristic (ROC) curve was used to evaluate the predictive capacity of diagnostic tests. The areas below the ROC curves (AUC) were calculated to assess the discriminatory power of the SFs in the indication of metabolic alterations constituting the MetS. The sensitivity and specificity values of the anthropometric indicators were calculated for each cutoff present in the sample. The cutoff that showed the best equilibrium between sensitivity and specificity was chosen to optimize the association between these two parameters, showing higher accuracy (lower number of false negative and false positive values). The statistical significance of each analysis was verified by the AUC and by the lower limit of the 95% confidence interval >0.5.18
ResultsA total of 1480 elementary school students from the urban and rural areas of Uberaba, MG, Brazil, with a mean age of 8.55 years (SD=1.53 years), of whom 52.2% were females, participated in the study.
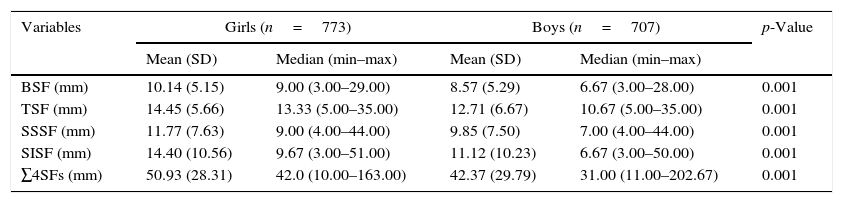
Girls had a higher prevalence of MetS (12.6% vs. 8.5%, p<0.05) and higher SF values in all assessed anatomical points (p<0.05) when compared with boys (Table 1).
Descriptive skinfold characteristics of schoolchildren aged 6–10 years of age, from the municipality of Uberaba, MG, Brazil, by gender.
| Variables | Girls (n=773) | Boys (n=707) | p-Value | ||
|---|---|---|---|---|---|
| Mean (SD) | Median (min–max) | Mean (SD) | Median (min–max) | ||
| BSF (mm) | 10.14 (5.15) | 9.00 (3.00–29.00) | 8.57 (5.29) | 6.67 (3.00–28.00) | 0.001 |
| TSF (mm) | 14.45 (5.66) | 13.33 (5.00–35.00) | 12.71 (6.67) | 10.67 (5.00–35.00) | 0.001 |
| SSSF (mm) | 11.77 (7.63) | 9.00 (4.00–44.00) | 9.85 (7.50) | 7.00 (4.00–44.00) | 0.001 |
| SISF (mm) | 14.40 (10.56) | 9.67 (3.00–51.00) | 11.12 (10.23) | 6.67 (3.00–50.00) | 0.001 |
| ∑4SFs (mm) | 50.93 (28.31) | 42.0 (10.00–163.00) | 42.37 (29.79) | 31.00 (11.00–202.67) | 0.001 |
n, sample number; SD, standard deviation; Min, minimum value; Max, maximum value; TSF, triceps skinfold; BSF, biceps skinfold; SSSF, subscapular skinfold; SISF, suprailiac skinfold; ∑4SFs, sum of the four skinfolds evaluated.
Note: Significant difference between genders p≤0.05, Mann–Whitney test.
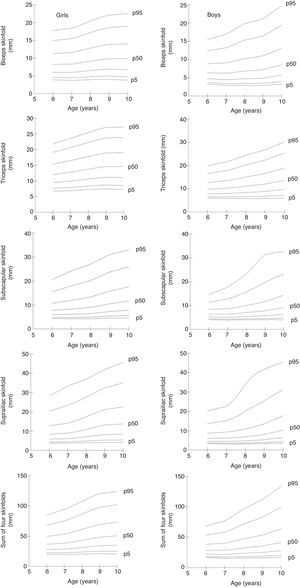
Percentile curves (p5, p10, p25, p50, p75, p90, and p95) were created for the four assessed SFs, as well as for their sum, for both genders (Fig. 1). Overall, all SFs showed a linear increase according to age and gender, with higher values being observed in girls.
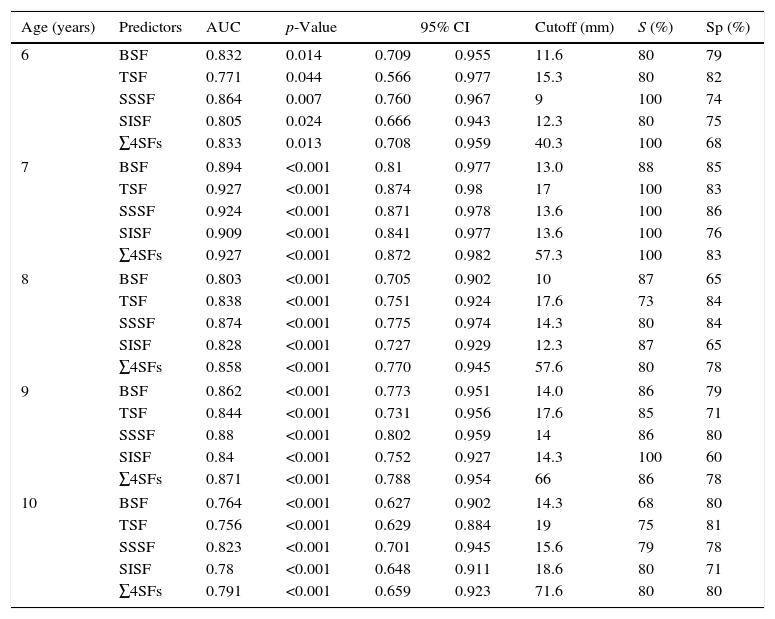
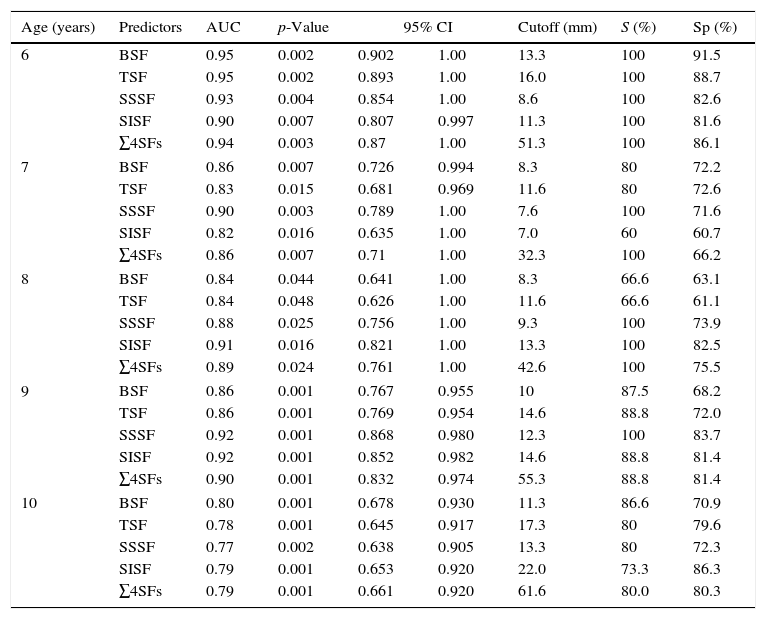
The four assessed SFs and their sum were accurate in predicting MetS for girls (Table 2) and boys (Table 3). Most of the suggested values are above the 75th percentile for age and gender.
Skinfold cutoffs for predicting metabolic syndrome in girls, by age, municipality of Uberaba, MG, Brazil.
| Age (years) | Predictors | AUC | p-Value | 95% CI | Cutoff (mm) | S (%) | Sp (%) | |
|---|---|---|---|---|---|---|---|---|
| 6 | BSF | 0.832 | 0.014 | 0.709 | 0.955 | 11.6 | 80 | 79 |
| TSF | 0.771 | 0.044 | 0.566 | 0.977 | 15.3 | 80 | 82 | |
| SSSF | 0.864 | 0.007 | 0.760 | 0.967 | 9 | 100 | 74 | |
| SISF | 0.805 | 0.024 | 0.666 | 0.943 | 12.3 | 80 | 75 | |
| ∑4SFs | 0.833 | 0.013 | 0.708 | 0.959 | 40.3 | 100 | 68 | |
| 7 | BSF | 0.894 | <0.001 | 0.81 | 0.977 | 13.0 | 88 | 85 |
| TSF | 0.927 | <0.001 | 0.874 | 0.98 | 17 | 100 | 83 | |
| SSSF | 0.924 | <0.001 | 0.871 | 0.978 | 13.6 | 100 | 86 | |
| SISF | 0.909 | <0.001 | 0.841 | 0.977 | 13.6 | 100 | 76 | |
| ∑4SFs | 0.927 | <0.001 | 0.872 | 0.982 | 57.3 | 100 | 83 | |
| 8 | BSF | 0.803 | <0.001 | 0.705 | 0.902 | 10 | 87 | 65 |
| TSF | 0.838 | <0.001 | 0.751 | 0.924 | 17.6 | 73 | 84 | |
| SSSF | 0.874 | <0.001 | 0.775 | 0.974 | 14.3 | 80 | 84 | |
| SISF | 0.828 | <0.001 | 0.727 | 0.929 | 12.3 | 87 | 65 | |
| ∑4SFs | 0.858 | <0.001 | 0.770 | 0.945 | 57.6 | 80 | 78 | |
| 9 | BSF | 0.862 | <0.001 | 0.773 | 0.951 | 14.0 | 86 | 79 |
| TSF | 0.844 | <0.001 | 0.731 | 0.956 | 17.6 | 85 | 71 | |
| SSSF | 0.88 | <0.001 | 0.802 | 0.959 | 14 | 86 | 80 | |
| SISF | 0.84 | <0.001 | 0.752 | 0.927 | 14.3 | 100 | 60 | |
| ∑4SFs | 0.871 | <0.001 | 0.788 | 0.954 | 66 | 86 | 78 | |
| 10 | BSF | 0.764 | <0.001 | 0.627 | 0.902 | 14.3 | 68 | 80 |
| TSF | 0.756 | <0.001 | 0.629 | 0.884 | 19 | 75 | 81 | |
| SSSF | 0.823 | <0.001 | 0.701 | 0.945 | 15.6 | 79 | 78 | |
| SISF | 0.78 | <0.001 | 0.648 | 0.911 | 18.6 | 80 | 71 | |
| ∑4SFs | 0.791 | <0.001 | 0.659 | 0.923 | 71.6 | 80 | 80 | |
AUC, area under the receiver operating characteristic curve; 95% CI, 95% confidence interval; S, sensitivity; Sp, specificity; TSF, triceps skinfold; BSF, biceps skinfold; SSSF, subscapular skinfold; SISF, suprailiac skinfold; ∑4SFs, sum of the four assessed skinfolds.
Skinfold cutoffs for predicting metabolic syndrome in girls, by age, municipality of Uberaba, MG, Brazil.
| Age (years) | Predictors | AUC | p-Value | 95% CI | Cutoff (mm) | S (%) | Sp (%) | |
|---|---|---|---|---|---|---|---|---|
| 6 | BSF | 0.95 | 0.002 | 0.902 | 1.00 | 13.3 | 100 | 91.5 |
| TSF | 0.95 | 0.002 | 0.893 | 1.00 | 16.0 | 100 | 88.7 | |
| SSSF | 0.93 | 0.004 | 0.854 | 1.00 | 8.6 | 100 | 82.6 | |
| SISF | 0.90 | 0.007 | 0.807 | 0.997 | 11.3 | 100 | 81.6 | |
| ∑4SFs | 0.94 | 0.003 | 0.87 | 1.00 | 51.3 | 100 | 86.1 | |
| 7 | BSF | 0.86 | 0.007 | 0.726 | 0.994 | 8.3 | 80 | 72.2 |
| TSF | 0.83 | 0.015 | 0.681 | 0.969 | 11.6 | 80 | 72.6 | |
| SSSF | 0.90 | 0.003 | 0.789 | 1.00 | 7.6 | 100 | 71.6 | |
| SISF | 0.82 | 0.016 | 0.635 | 1.00 | 7.0 | 60 | 60.7 | |
| ∑4SFs | 0.86 | 0.007 | 0.71 | 1.00 | 32.3 | 100 | 66.2 | |
| 8 | BSF | 0.84 | 0.044 | 0.641 | 1.00 | 8.3 | 66.6 | 63.1 |
| TSF | 0.84 | 0.048 | 0.626 | 1.00 | 11.6 | 66.6 | 61.1 | |
| SSSF | 0.88 | 0.025 | 0.756 | 1.00 | 9.3 | 100 | 73.9 | |
| SISF | 0.91 | 0.016 | 0.821 | 1.00 | 13.3 | 100 | 82.5 | |
| ∑4SFs | 0.89 | 0.024 | 0.761 | 1.00 | 42.6 | 100 | 75.5 | |
| 9 | BSF | 0.86 | 0.001 | 0.767 | 0.955 | 10 | 87.5 | 68.2 |
| TSF | 0.86 | 0.001 | 0.769 | 0.954 | 14.6 | 88.8 | 72.0 | |
| SSSF | 0.92 | 0.001 | 0.868 | 0.980 | 12.3 | 100 | 83.7 | |
| SISF | 0.92 | 0.001 | 0.852 | 0.982 | 14.6 | 88.8 | 81.4 | |
| ∑4SFs | 0.90 | 0.001 | 0.832 | 0.974 | 55.3 | 88.8 | 81.4 | |
| 10 | BSF | 0.80 | 0.001 | 0.678 | 0.930 | 11.3 | 86.6 | 70.9 |
| TSF | 0.78 | 0.001 | 0.645 | 0.917 | 17.3 | 80 | 79.6 | |
| SSSF | 0.77 | 0.002 | 0.638 | 0.905 | 13.3 | 80 | 72.3 | |
| SISF | 0.79 | 0.001 | 0.653 | 0.920 | 22.0 | 73.3 | 86.3 | |
| ∑4SFs | 0.79 | 0.001 | 0.661 | 0.920 | 61.6 | 80.0 | 80.3 | |
AUC, area under the receiver operating characteristic curve; 95% CI, 95% confidence interval; S, sensitivity; Sp, specificity; TSF, triceps skinfold; BSF, biceps skinfold; SSSF, subscapular skinfold; SISF, suprailiac skinfold; ∑4SFs, sum of the four assessed skinfolds.
The present study showed percentile SF curves of the central and peripheral regions of the body, as well as the sum of the four SFs to predict the risk of MetS according to age and gender in Brazilian children. The curves outlined here may be a useful strategy to prevent the risk of MetS in childhood, with the possibility of being used in schools, family health units, clinics, and hospitals. According to the present findings, both the central adiposity, as well as the peripheral adiposity SFs and the sum of four SFs showed cutoffs with high sensitivity and specificity values to predict the risk of MetS in children. Approximately 75% of the children in the present study with SF values above the 75th percentile, regardless of the assessed SF, had MetS.
The prevalence of MetS in Brazilian children and adolescents varied from 0% to 42.4%, according to data from a systematic review.19 In the present study, MetS was prevalent in 12.6% of the girls and 8.5% of the boys, with a significant difference between the genders. The heterogeneity of definitions and cutoffs for the MetS components may explain, at least partly, the different prevalence rates reported in the literature.19 However, it is true that the prevalence of MetS has been increasing among children and adolescents, with a significantly higher proportion among those who are obese.20
The percentile distribution of the triceps and subscapular SFs were performed in 8568 Chinese schoolchildren aged 7–18 years of age21 and 32,783 North-American children and adolescents.10 The percentile values for TSF and SSSF of Chinese and American children were lower than the percentiles of boys and girls in the present study. This result is of concern, due to the association between SFs and central obesity, unfavorable lipid profile, increased levels of insulin and BP, and left ventricular mass.22 The accumulation of subcutaneous fat, translated into high SF values, increase the chances of children having metabolic alterations.
Pre-pubertal Mexican children aged 6–10 years had a three-fold higher chance of having alterations in MetS components when the distribution of SSSF was in the fourth quartile of the sample (low HDL-c levels [OR=3.16; 95% CI: 1.41–7.10; p<0.01]) and high triglyceride levels (OR=3.27; 95% CI: 2.02–5.29; p<0.001).23 However, high values of Σ3SF (TSF+BSF+SSSF; >90th percentile) in German children aged 3–11 years increased the chance of having three or more cardiovascular risk factors by 1.6-fold (95% CI: 1.1–2.2; p<0.05) and the chance of having arterial hypertension by 1.7-fold (95% CI: 1.1–2.7; p<0.05)].24
The results of the present study demonstrated that all isolated SF measures predict the risk of MetS in both genders. In the scientific investigations related to the subject, studies with TSF and SSSF are predominant.10,13,21,25 Many studies have used SF values alone as a predictor of the amount of body fat25–29 and isolated cardiometabolic risk factors.12,30 Conversely, studies investigating the power of SF in predicting MetS in the pediatric population remain scarce.
The use of the sum of SF absolute values may become an interesting predictor of MetS, as it minimizes biases in predictive body composition equations, as well as suggesting values that show equilibrium/disequilibrium of body fat distribution (TSF+BSF+SSSF+SISF). Among the different anthropometric measures tested, ∑4SF was the most accurate predictor of MetS in Brazilian girls and boys, with AUC=0.908 and AUC=0.897, respectively.6
In the present study, the ∑4SF showed an AUC of 0.859 for girls and 0.879 for boys, and the suggested cutoffs showed high sensitivity values (>80%).
To the best of the authors’ knowledge, this is the first study with percentile curves of the central and peripheral region SFs, as well as the sum of four SFs, used to predict MetS in a representative sample of Brazilian schools. However, this study had a cross-sectional design, which did not allow the determination of whether alterations in subcutaneous adiposity reflect changes in the MetS components. Thus, longitudinal studies are necessary to define the causal association between MetS and subcutaneous adiposity. It is worth mentioning that, although the study population was delimited among students from the 1st to the 5th year of Elementary School, the State Education Secretariat provided updated data on all Elementary School years, which may have overestimated the sample calculation.
Reference curves from other countries can either underestimate or overestimate the disease due to ethnic, socioeconomic, and cultural differences. The development of percentile curves with populations of different geographic areas is a relevant challenge to allow a more accurate screening of MetS in young individuals through the evaluation of SFs.
FundingFundação de Amparo à Pesquisa do Estado de Minas Gerais (Fapemig).
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Andaki AC, Quadros TM, Gordia AP, Mota J, Tinôco AL, Mendes EL. Skinfold reference curves and their use in predicting metabolic syndrome risk in children. J Pediatr (Rio J). 2017;93:490–6.
Study carried out at Universidade Federal do Triângulo Mineiro, Uberaba, MG, Brazil.