The aim of this study was to evaluate the serum Syndecan-1 (SDC-1) levels in patients with immunoglobulin-A vasculitis (IgAV) in children and its relation with gastrointestinal involvements.
MethodsSixty-eight children with IgAV and 48 healthy children were enrolled in this cross-sectional study. Clinical and related laboratory data were collected from a computerized hospital database. Serum SDC-1 was collected on admission prior to treatment.
ResultsForty-eight patients fully met the IgAV diagnostic criteria at admission (IgAV group), 20 patients with rash only and diagnosed IgAV during hospitalization (Purpura group). In IgAV group, 30 patients with gastrointestinal involvements (IgAV-GI group) and 18 patients without gastrointestinal involvements (IgAV-NGI group). SDC-1 serum levels were significantly higher in the IgAV group (86.37 ng/mL (IQR 59.16–117.14 ng/mL)) than in the controls (20.37 ng/mL (IQR 15.52–26.45 ng/mL)) and the Purpura group (32.66 ng/mL (IQR 14.87–49.89 ng/mL)). Additionally, SDC-1 (OR = 1.08) was independently associated with IgAV with a cut-off value (sensitivity and specificity) of 66.55 ng/mL (68.8%, 95.0%), and the area under the curve was 0.908. The serum SDC-1 levels of the IgAV-GI group (106.92 ± 50.12 ng/mL) were significantly higher than those in the IgAV-NGI group (67.52 ± 17.59 ng/mL). Logistic regression analysis showed that SDC-1 (OR = 1.03) was independently associated with IgAV-GI with a cut-off value of 89.39 ng/mL.
ConclusionsSDC-1 serum levels may mirror vascular endothelium injury and mucosal damage in IgAV. Its applicability as a surrogate biomarker in IgAV remains to be determined.
Immunoglobulin-A vasculitis (IgAV)—also referred to as Henoch-Schönlein purpura (HSP)—is one of the most common vasculitis in children,1 with an incidence of 18.6 per 100,000.2 IgAV is a systemic vasculitis accompanied by IgA deposits, complementary factors, and large neutrophil infiltrates3 that affect small vessels of the skin, the gastrointestinal tract, and the kidneys.4 IgAV diagnosis is criteria-based and made with patients who have purpura or petechiae with lower limb predominance and at least one of the following criteria: abdominal pain, histological features, proven IgA vasculitis, arthritis or arthralgia, and renal involvements, according to the Ankara 2008 criteria.5 Gastrointestinal involvements are relatively frequent and may cause severe complications. Abdominal symptoms occur in 75% of patients with IgAV,6 and 11% IgAV patients may be presented with intermittent abdominal pain in the first 30 days of disease.7 Moreover, complications can mimic acute abdomen conditions, including perforations, intussusception, and bowel infarctions that can be fatal without timely surgical procedures.6 Thus, timely diagnosis and intervention therapy are essential to improve the prognosis of patients with IgAV.
IgAV could produce IgA antibodies against endothelial cells.8 The interaction between leukocytes and endothelial cells could contribute to IgAV pathogenesis, and endothelial damage could be associated with IgAV.9 The endothelial glycocalyx serves as a protective barrier that covers the luminal surface of the vascular endothelium and the intestinal epithelial.10 Syndecan-1(SDC-1), the most prominent of four syndecan subtypes, is structurally connected to the endothelial cell membrane and binds with glycosaminoglycans.11 Meanwhile, SDC-1 is one of the major components of the glycocalyx and plays a central role in maintaining normal intestinal barrier function.12 When inflammatory responses, trauma, and other pathological conditions occur, the ectodomain of SDC-1 is shed from the cell surface and accumulates in the serum of patients.13 Recent data suggest that the serum SDC-1 level is a biomarker for endothelial glycocalyx damage.14,15 As systemic vasculitis, IgAV theoretically damages vascular endothelial cells and releases SDC-1 into the blood. Additionally, significant increases in serum SDC-1 in patients with inflammatory bowel disease (IBD) and celiac disease suggest SDC-1 predicts intestinal mucosal barrier damage.16,17
Early and accurate diagnosis of IgAV is crucial. Although the Ankara criteria5 have high sensitivity and specificity, which is a post-symptom diagnosis and disease-specific laboratory diagnostic indicators are lacking. Beside, for patients with only rashes present, they usually are refused a skin biopsy, which makes diagnosis more difficult. Thus, simple and effective predictive biomarkers for IgAV are required. In the light of the above, as one category of small vessel vasculitis, IgAV is associated with vascular endothelial glycocalyx damage in theory. In addition, the integrity of intestinal epithelium and mucosa is damaged by the abdominal effects of IgAV, which may release more SDC-1. Therefore, this study aimed to evaluate the serum SDC-1 levels in IgAV and its relation with gastrointestinal involvement.
MethodsPatients and healthy control subjectsSixty-eight pediatric patients hospitalized for IgAV in the Jinhua Municipal Central Hospital were enrolled. IgAV diagnosis was made in accordance with the Ankara 2008 criteria.5 Blood specimens were obtained before the treatment of patients. All the patients hospitalized with IgAV were discharged after their clinical symptoms and signs were under control. A retrospective analysis was performed on all the cases, and the entire course of the disease was evaluated to exclude misdiagnosed cases. Meanwhile, forty-eight healthy children in the physical examination center were selected as the control group. This study was approved by the medical ethics committee of the Jinhua Municipal Central Hospital (Permission number: 2019-014-001); written informed consent was obtained from parents.
Sample size calculationThe authors used G-Power software (www.gpower.hhu.de) to estimate the sample size, which is a free power analysis program. The authors also considered a type I error of 5% (α = 0.05) and a type II error of 20% (β = 0.2; power = 80%), the minimum total sample size was calculated to be 66 subjects, and the minimum sample size for each group calculated is 19, so the sample size of the present study is within a reasonable range.
Patients were grouped according to IgAV diagnostic criteriaThe selected patients were divided into a definite diagnosed IgAV group (IgAV group) and a purpura-only group (Purpura group) according to the IgAV diagnostic criteria at admission. All patients had purpura or petechiae with lower limb predominance at the time of admission. However, the IgAV group had a rash plus one of four criteria before admission:(1) abdominal pain, (2) histopathology (IgA), (3) arthritis or arthralgia, or (4) renal involvement. The only purpuric rash was observed for the Purpura group at the time of enrollment; the group met the aforementioned criteria during the hospitalization.
Patients were grouped according to gastrointestinal symptomsNext, patients in the IgAV group were divided into an IgAV with gastrointestinal involvements group (IgAV-GI group) and an IgAV without gastrointestinal involvements group (IgAV-NGI group) based on the presence or absence of developed gastrointestinal symptoms or signs. Patients in the Purpura group were divided into an IgAV with delayed gastrointestinal involvements group (Delayed- IgAV-GI group) and an IgAV without delayed gastrointestinal involvements group (No-Delayed- IgAV-GI group) according to whether they had gastrointestinal symptoms during the hospitalization.
Date collectionClinical data, signs, symptoms of the disease and laboratory data were collected from the computerized hospital database and included white blood cell count (WBC), neutrophil count (NE), red cell volume distribution width (RDW), platelet count (PLT), platelet hematocrit (PCT), platelet distribution width (PDW), mean platelet volume (MPV), C-reactive protein (CRP), IgA, immunoglobulin G (IgG), and immunoglobulin M (IgM). The hematologic parameters of healthy children were reviewed from the same computerized database.
SDC-1 serum level measurementsUpon admission, the blood samples were collected from the IgAV, Purpura, and control groups prior to treatment. Samples were centrifuged at 3000 × g for 3 min, and the serum was collected and stored at −80 °C. Serum concentrations of SDC-1 were all determined by enzyme-linked immunosorbent assay (ELISA) kits (Abcam, UK) according to manufacturer instructions. Each of 100 μl of standards and samples was pipetted into the wells. The intensity of the color was measured spectrophotometrically at a wavelength of 450 nm.
Statistical analysisStatistical analyses were performed using SPSS version 20.0 (IBM, Hangzhou, China) and Prism version 6.0 software (GraphPad Software, La Jolla, CA, USA). The assumption of normality was assessed using the Kolmogorov–Smirnov test. Continuous variables are presented as means with standard deviation or median (Interquartile range) and categorical variables as numbers (percentage). Comparisons of frequencies between groups were analyzed using χ2 tests. Two sample t-tests and ANOVA were performed to compare the normally distributed continuous variables between the two groups or among the three groups, respectively. Mann-Whitney U tests and Kruskal-Wallis tests were performed for abnormally distributed continuous variables to compare the two groups or the three groups, respectively. Binary logistic regression was used to identify independent predictors of the presence of IgAV and IgAV with gastrointestinal involvements. Receiver–operating characteristic (ROC) curves were plotted, and areas under the curves (AUC) were calculated to determine a cut-off value for predicting IgAV and IgAV with gastrointestinal involvements. All p values were 2-sided, and a p value < 0.05 was regarded as significant.
ResultsPatient demographicsOf 68 potentially eligible patients, 48 subjects fully met IgAV diagnostic criteria at admission and were enrolled in the IgAV group with a median age of 6.0 years. Twenty subjects met the criteria during the hospitalization and were assigned to Purpura group with a median age of 5.0 years. After retrospective analysis of all cases, there were no misdiagnosed cases. Additionally, the control group contained 48 patients, with a median age of 10.0 years.
Clinical characteristics and serum SDC-1 levels in subjectsDemographic and laboratory information were compared to evaluate significant differences in the characteristics among the IgAV, Purpura, and control groups (Table 1). There was no difference in frequency of arthritis, gastrointestinal or renal complication between the groups of IgAV and Purpura. In addition, no significant differences among the three groups in PCT, PDW, or IgG serum levels were found. Whereas WBC, NE, RDW, PLT, CRP, IgA, and IgM were significantly higher in the IgAV and Purpura groups (p < 0.05) than in the control group. Serum SDC-1 levels were significantly higher in the IgAV group (86.37 ng/mL (IQR 59.16–117.14 ng/mL)) than in the control group (20.37 ng/mL (IQR 15.52–26.45 ng/mL)) and the Purpura group (32.66 ng/mL (IQR 14.87–49.89 ng/mL)) (p = 0.000). Beside, children in IgAV group had significantly higher serum IgA, IgM, and SDC-1 levels compared with patients in the Purpura group (p < 0.05).
Clinical characteristics and serum SDC-1 levels among the IgAV, Purpura, and control groups.
| IgAV group (n = 48) | Purpura group (n = 20) | Controls (n = 48) | p | |
|---|---|---|---|---|
| Demographics, n (%) | ||||
| Gender, (male/female) | 25/23 | 13/7 | 10/38 | 0.060 |
| Age, years | 6.0 (4.0, 8.0) | 5.0 (4.0, 6.8)a | 10 (9, 11) | 0.000 |
| Purpura/petechiae | 48 (100%) | 20 (100%) | / | |
| Arthritis/arthralgia | 28 (58%) | 11 (55%) | 0.800 | |
| Abdominal pain | 30 (62%) | 12 (60%) | 0.847 | |
| Gastrointestinal bleeding | 2 (4%) | 0 (0%) | 1.000 | |
| Renal involvement | 8 (16%) | 0 (0%) | 0.126 | |
| Initial laboratory finding | ||||
| WBC, ⅹ109/L | 10.35 (8.68, 12.1)b | 10.70 (7.43, 12.35)a | 6.90 (5.62, 7.70) | 0.000 |
| Neutrophil, ⅹ109/L | 5.17 (3.85, 6.52)b | 4.91 (2.74, 7.86)a | 3.25 (2.45, 4.15) | 0.000 |
| RDW, % | 13.05 (12.73, 13.58)b | 13.00 (12.60, 13.75)a | 12.5 (12.1, 12.9) | 0.000 |
| PLT, ⅹ109/L | 369 (315, 425)b | 346 (276, 449)a | 304 (241, 353) | 0.000 |
| MPV, fL | 9.83 ± 0.83b | 9.95 ± 1.08 | 10.42 ± 1.03 | 0.010 |
| PCT, % | 0.31 (0.26, 0.34) | 0.30 (0.22, 0.34) | 0.28 (0.25, 0.33) | 0.284 |
| PDW, % | 11.24 ± 2.22 | 10.85 ± 1.34 | 11.44 ± 1.27 | 0.453 |
| CRP, mg/L | 3.70 (0.60, 7.80)b,c | 6.75 (4.18, 10.75)a | 0.65 (0.5, 1.86) | 0.000 |
| IgA, g/L | 3.36 (2.52, 3.89)b,c | 2.41 (2.17, 2.79)a | 1.16 (0.97, 1.44) | 0.000 |
| IgG, g/L | 9.78 (8.46, 10.90) | 9.48 (8.13, 11.86) | 9.76 (8.54, 10.80) | 0.987 |
| IgM, g/L | 1.25 (0.87, 1.43)b | 0.83 (0.73, 1.18) | 0.80 (0.65, 1.01) | 0.000 |
| SDC-1, ng/mL | 86.37 (59.16, 117.14)b,c | 32.66 (14.87, 49.89)a | 20.37 (15.52, 26,45) | 0.000 |
IgAV group, definite diagnosed IgAV group; Purpura group, pure purpura group; WBC, white blood cells counts; Neutrophil, neutrophil cell counts; RDW, red cell volume distribution width; PLT, platelet; MPV, mean platelet volume; PCT, platelet hematocrit; PDW, platelet distribution width; CRP, C-reactive protein; IgA, immunoglobulin A; IgG, immunoglobulin G; IgM, immunoglobulin M; SDC-1, Syndecan-1.
To identify the potential IgAV independent predictors based on univariate analyses among the IgAV and Purpura groups, p < 0.05 was considered a condition for inclusion in logistic regression analysis. Multivariate analysis with logistic regression confirmed that SDC-1 (odds ratio (OR) = 1.08, p = 0.001) was independently associated with IgAV. Additionally, IgA, and IgM were not as useful biomarkers (Table S1).
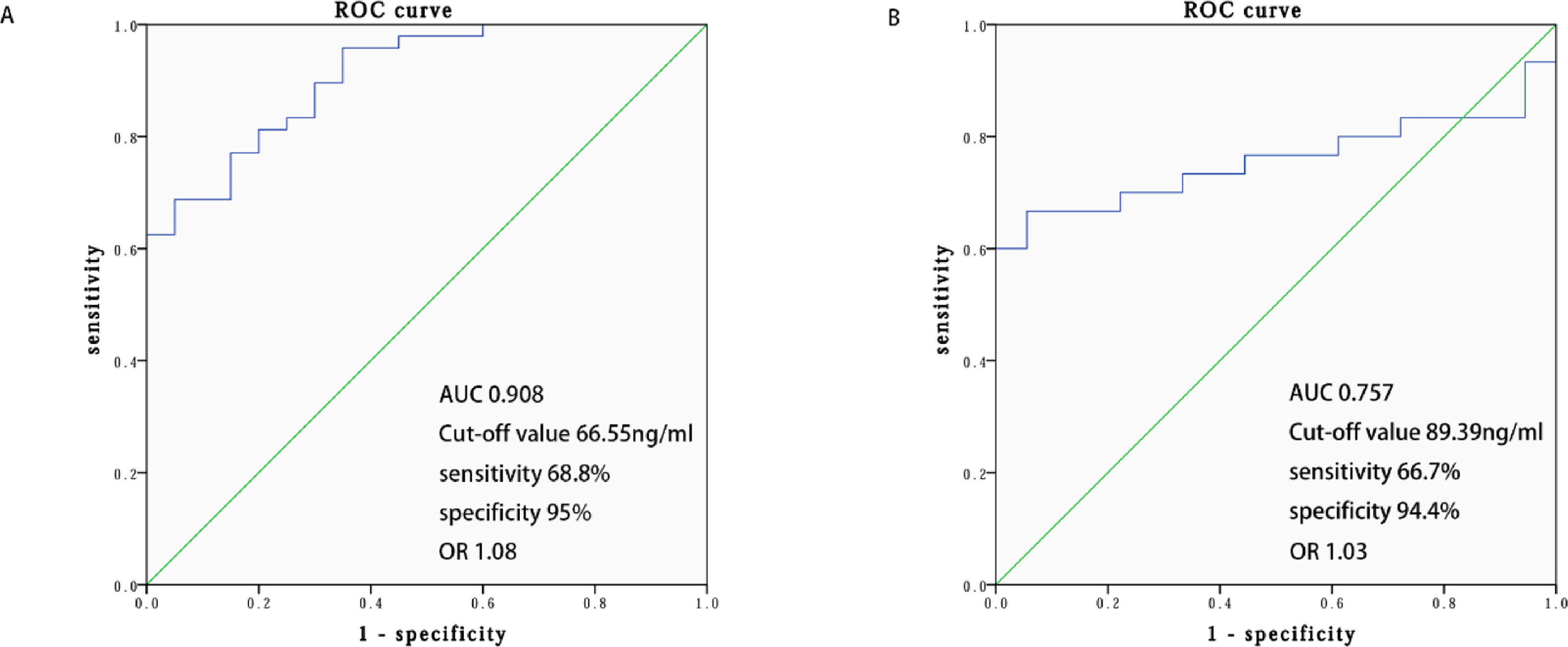
The clinical utility of SDC-1 was evaluated using AUC based on ROC analysis, and the sensitivity, specificity, Jordan index, and cut-off point were calculated. The optimal cut-off value (sensitivity and specificity) was 66.55 ng/mL (68.8%, 95.0%) for SDC-1, and the area under the curve was 0.908 (Figure 1A).
ROC curve of the serum SDC-1 for predicting IgAV and IgAV with gastrointestinal involvements. A, ROC curve of the serum SDC-1 for predicting IgAV. B, ROC curve of the serum SDC-1 for predicting IgAV with gastrointestinal involvements. ROC receiver operating characteristic curve, AUC area under curve, IgAV Immunoglobulin-A vasculitis, SDC-1 Syndecan-1.
The IgAV group was categorized into IgAV-GI and IgAV-NGI groups based on gastrointestinal symptoms at admission. Table 2 shows baseline information and laboratory indicators between the two groups. All IgAV patients were treated with glucocorticoid after diagnosis. Beside, patients with gastrointestinal symptoms were given additional treatment with omeprazole or cimetidine. Serum SDC-1 was significantly higher in the IgAV-GI group (p = 0.000) than in the IgAV-NGI group (106.92 ± 50.12 and 67.52 ± 17.59, respectively); the other laboratory indicators were not significantly different.
Clinical characteristics and serum SDC-1 levels in the group of IgAV with gastrointestinal involvements and IgAV without gastrointestinal involvements.
IgAV-GI group, IgAV with gastrointestinal involvements group; IgAV-NGI group, IgAV without gastrointestinal involvements group; WBC, white blood cells counts; Neutrophil, neutrophil cell counts; RDW, red cell volume distribution width; PLT, platelet; MPV, mean platelet volume; PCT, platelet hematocrit; PDW, platelet distribution width; CRP, C-reactive protein; IgA, immunoglobulin A; IgG, immunoglobulin G; IgM, immunoglobulin M; SDC-1, Syndecan-1.
Logistic regression analysis showed that SDC-1 (OR: 1.03, p = 0.007) was independently associated with gastrointestinal involvements in IgAV patients (Table S2). When assessing the presence of gastrointestinal involvements with SDC-1 in the patients, a cut-off value of 89.39 ng/mL with a sensitivity of 66.7% and specificity of 94.4% was observed according to ROC curve analysis. The area under the curve was 0.757 (Figure 1B).
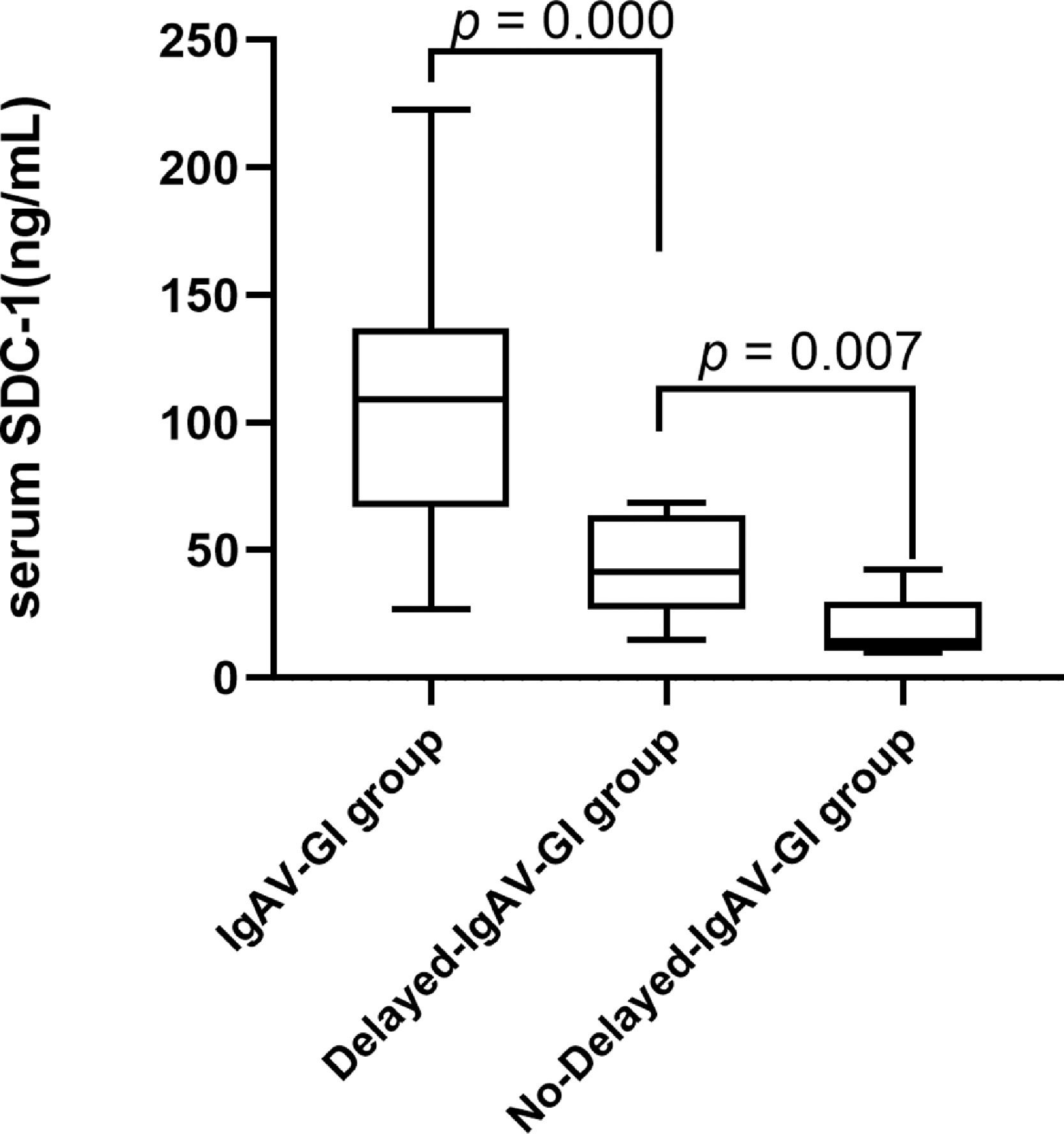
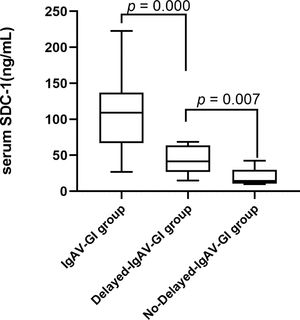
Clinical characteristics and serum SDC-1 levels in the groups of Delayed-IgAV-GI and No-Delayed-IgAV-GIPatients in the Purpura group were divided into Delayed-IgAV-GI and No-Delayed-IgAV-GI groups according to their gastrointestinal symptoms after admission. The Delayed-IgAV-GI group had significantly higher serum SDC-1 than the No-Delayed-IgAV-GI group (43.28 ± 18.93 and 20.19 ± 11.94, respectively), indicating that the gastrointestinal epithelium may have been damaged in the early IgAV stages (p = 0.007). Additionally, the serum SDC-1 level was significantly higher in the IgAV-GI group (p = 0.000) than in the Delay-IgAV-GI group (106.92 ± 50.12 and 43.28 ± 18.93, respectively), further indicating that the serum SDC-1 level increased when IgAV patients presented gastrointestinal symptoms (Figure 2).
The value of SDC-1 in predicting of IgAV with gastrointestinal involvements. IgAV-GI group IgAV with gastrointestinal involvements group, Delay- IgAV-GI group IgAV with delayed gastrointestinal involvements group, No-delayed- IgAV-GI group IgAV without delayed gastrointestinal involvements group, SDC-1 Syndecan-1.
Despite the theoretical appeal, there is no evidence of the correlation between SDC-1 and IgAV. This study focused on the association between SDC-1 and IgAV. The authors found that SDC-1 was significantly higher in patients with IgAV compared to the Purpura group and controls. The ROC curve indicated that the SDC-1 cut-off value of 66.55 ng/mL can predict IgAV. SDC-1 values, meanwhile, were higher in patients with gastrointestinal involvements than in those without gastrointestinal involvements, and SDC-1 was independently associated with the presence of gastrointestinal involvements in IgAV patients. To our knowledge, this is the first study to investigate the levels of serum SDC-1 in children with IgAV and to evaluate any potential association with vascular endothelium injury and mucosal damage in this condition.
IgAV is characterized by IgA1-autoantibodies; however, the antigen to which IgA1 binds remains unknown. Anti-endothelial cell antibodies (AECAs) are a heterogeneous group of antibodies directed against poorly characterized antigens on human endothelial cells. AECAs have also been detected in other vascular disorders, including systemic vasculitis.18 IgA from the serum of IgAV patients binds to human endothelial cells in vitro, supporting the presence of IgA AECAs.19 A review of IgAV pathogenesis indicated that ACEAs might bind to autoantigens on endothelial cells, which could induce cross-talk between IgA, neutrophils, and endothelial cells and ultimately lead to neutrophil infiltration and vascular damage.8 The present study found higher neutrophils and IgA values in patients with IgAV than that in the control group. However, no statistical differences were noted between the IgAV group and purpura group for serum neutrophils. In addition, IgA was not as an independent predictor for IgAV based on logistic regression analysis, which may be related to the limitation of patient sample size. Thus, a possible association between neutrophils, IgA, and endothelial injury should be explored further.
SDC-1, a major component of the dense inner layer of glycocalyx, which also is a sensitive indicator of endothelial dysfunction.20 SDC-1 can be cleaved and released into the serum by various mediators, such as cytokines or reactive oxygen species (ROS) in inflammatory conditions; the ectodomain of SDC-1 is shed from endothelial cells, suggesting that SDC-1 may be associated with inflammatory diseases. Liu and Akkoyunlu21 found an increase in serum SDC-1 level in systemic lupus erythematosus (SLE) mice, and SDC-1 may be associated with B-cell differentiation and autoreactive antibody production in SLE. A prospective observational study, conducted by Ikeda et al.22 found that serum SDC-1 levels are associated with severity and mortality in patients with sepsis.
Luo et al.23 tested the serum SDC-1 levels of 119 children with Kawasaki disease (KD) and of 43 healthy children as normal controls. The serum SDC-1 levels of the KD patients were significantly higher than those of the healthy control group, suggesting that SDC-1 is a potential target for coronary artery protection in KD patients. Ohnishi et al.24 confirmed this conclusion. Furthermore, elevated serum SDC-1 levels have been reported in patients with cardiovascular diseases, including acute coronary syndrome25 and heart failure26; thus, serum SDC-1 levels are associated with inflammatory disease and with endothelium damage.
SDC-1 is expressed on the basolateral surface of intestinal epithelial cells and plays an irreplaceable role in maintaining the intestinal epithelial barrier function.27 The extracellular domain of SDC-1 is shed by proteolytic cleavage near the plasma membrane, releasing soluble SDC-1 into the blood.28 Increased serum SDC-1 levels coinciding with infection, inflammation, and tissue injury of the gastrointestinal tract occur may be related to decreased intestinal epithelial tight junctions and mucosal barrier dysfunction.12 Intestinal epithelial SDC-1 levels were found to be associated with colitis disease activity and histopathological changes in an animal experiment.29 Yablecovitch et al.30 found that the serum SDC-1 levels of IBD patients were significantly higher than those of the healthy control group and decreased after treatment. And serum SDC-1 might be correlated with mucosa inflammation and Mayo score in ulcerative colitis.17 Additionally, serum SDC-1 is considered a biomarker for intestinal damage in children with celiac disease.16 Thus, serum SDC-1 levels appear to be associated with intestinal mucosal barrier damage.
IgAV, a common vasculitis, produces inflammatory changes and endothelial damage; SDC-1 may be a potential predictor of IgAV. However, the association of SDC-1 with IgAV and IgAV with gastrointestinal involvements is unconfirmed. The present study demonstrated that SDC-1 values increased significantly in IgAV patients. Moreover, the significantly increased SDC-1 in the IgAV-GI group (compared to the IgAV-NGI group) might impair gut integrity, affect SDC-1 metabolism, and be associated with gastrointestinal involvements in IgAV. These results revealed a possible association between serum SDC-1 levels and IgAV.
There are several limitations that should be addressed. First, the small sample size may weaken the findings, and few IgAV patients with renal involvements had been included in this study; thus, the study result may require a test and verification in more IgAV patients. Second, changes in serum SDC-1 levels are also found in other diseases, but the conclusions of this study pertain to patients with suspected IgAV associated with purpura rash at the early stage. The authors sought to improve the diagnostic specificity of SDC-1 in IgAV; however, comparable control groups of patients with rash symptoms in other diseases are needed. Third, no follow-up data were available. Indeed, the association of SDC-1 level with recurrence and long-term prognosis of IgAV also needs to be evaluated in prospective follow-up studies. Last, the controls were not age-matched with IgAV patients. This may be related to the fact that children who require physical examination are generally older. However, conclusions were drawn mainly from a comparison of the IgAV and Purpura groups, and the two groups matched in age.
ConclusionThe results of this pilot study suggest that SDC-1 is a possible, novel diagnostic marker for vascular endothelium injury and mucosal damage in IgAV patients, considering the paucity of biomarkers in IgAV. Additionally, SDC-1 is potentially valuable for differentiating IgAV from other diseases with purpura. Further prospective studies with a larger number of patients are required to confirm these findings. Furthermore, the prevention of SDC-1 shedding may represent a novel therapeutic strategy in IgAV.
FundingKey Research and Development Project of Zhejiang Province (No. 2021C03064).
The author would like to thank Editage, a division of Cactus Communications, for their useful comments and language editing, which have greatly improved the manuscript. The authors also appreciate Prof. Jianfeng Liang, for his help with statistical methods.