Melatonin has a protective role in adults with cardiovascular disease, but the effects of melatonin in children with cardiac dysfunction are not well understood. This study was designed to explore the variations in melatonin, myeloperoxidase, and caspase-3 levels in children suffering from heart failure.
MethodsSeventy-two pediatric patients with heart failure and twelve healthy children were enrolled in this study. A modified Ross scoring system was used to evaluate clinical cardiac function. Patients with a score of >2 points were included in the study and were divided into three groups according to severity of heart failure: mild (score: 3–6), moderate (score: 7–9), and severe (score: 10–12). Echocardiographic parameters, laboratory data, and serum levels of melatonin, myeloperoxidase, and caspase-3 were measured and analyzed in all patients.
ResultsCompared with patients with mild and moderate heart failure, patients in the severe heart failure group had significantly decreased left ventricular ejection fraction (p<0.001), and significantly increased serum melatonin levels (p=0.013) and myeloperoxidase levels (p<0.001). Serum melatonin levels were positively correlated with serum caspase-3 levels (p<0.001). The optimal cutoff values of serum melatonin levels for the diagnosis of severe heart failure and primary cardiomyopathy in pediatric patients with heart failure were 54.14pg/mL and 32.88pg/mL, respectively.
ConclusionsSerum melatonin and myeloperoxidase levels were increased in children with severe heart failure. It is likely that increasing melatonin levels may act as a compensatory mechanism in pediatric children with heart failure.
A melatonina possui um papel protetor em adultos com doença cardiovascular, porém os efeitos da melatonina em crianças com disfunção cardíaca não são bem entendidos. O estudo foi projetado para explorar a variação nos níveis de melatonina, mieloperoxidase e caspase 3 em crianças que sofrem de insuficiência cardíaca.
Métodos72 pacientes pediátricos com insuficiência cardíaca e 12 crianças saudáveis foram inscritos no estudo. Um sistema de classificação de Ross modificada foi utilizado para avaliar a função cardíaca clínica. Os pacientes com escore de > 2 pontos foram incluídas no estudo e foram divididos em três grupos de acordo com a gravidade da insuficiência cardíaca: leve (escore: 3-6), moderada (escore: 7-9) e grave (escore: 10-12). Os parâmetros ecocardiográficos, dados laboratoriais e níveis séricos de melatonina, mieloperoxidase e caspase 3 foram medidos e analisados em todos os pacientes.
ResultadosEm comparação aos pacientes com insuficiência cardíaca de gravidade leve e moderada, os pacientes no grupo de insuficiência cardíaca grave apresentaram redução significativa da fração de ejeção do ventrículo esquerdo (p<0,001) e aumento significativo nos níveis séricos de melatonina (p=0,013) e níveis de mieloperoxidase (p<0,001). Os níveis séricos de melatonina foram positivamente correlacionados com os níveis séricos de caspase 3 (p<0,001). Os valores de corte ideais dos níveis séricos de melatonina para diagnóstico de IC e cardiomiopatia primária em pacientes pediátricos com insuficiência cardíaca foram 54,14 pg/mL e 32,88 pg/mL, respectivamente.
ConclusõesOs níveis séricos de melatonina e mieloperoxidase mostraram aumento em crianças com insuficiência cardíaca grave. Especulamos se o aumento nos níveis de melatonina pode agir como um mecanismo compensatório em crianças pediátricas com insuficiência cardíaca.
The prevalence of heart failure (HF) is rising; it poses an increasing burden in terms of both healthcare costs and mortality, especially when it occurs in young children. Consequently, a better understanding of the best way to evaluate and manage HF is required. While advances in diagnosis and treatment of HF in adults have been made, similar awareness is lacking for pediatric patients with HF.1
Melatonin (N-acetyl-5-methoxytryptamine), a secretory product of the human pineal gland, is well known for its influence on the cardiovascular system. Melatonin has a protective action on the heart that occurs through both receptor-mediated and receptor-independent mechanisms.2 The receptor-mediated mechanism involves the classic melatonin membrane receptors (MT1 and MT2); however, the precise localization of these receptors has not been completely elucidated.3 The receptor-independent mechanism of melatonin occurs through its function as a potent antioxidant and free radical scavenger.4 Melatonin has been shown to reduce hypertension,5 protect the ischemic/reperfused heart,6 and resist the process of atherosclerosis.7 Cardiomyocyte hypertrophy initially occurs as a compensatory response, but eventually becomes pathological and can lead to HF. Melatonin affects hemodynamic overload, nitric oxide (NO) availability, free radicals, and lipid profiles that may also modify cardiomyocyte hypertrophy.8
The association between melatonin and pediatric HF has not been fully understood. The authors performed a study to investigate the circulating levels of melatonin in children with HF.
MethodsThis single-center pediatric study was approved by the Ethics Committee. All patient-derived blood samples were collected after written informed consent was obtained from parents or guardians.
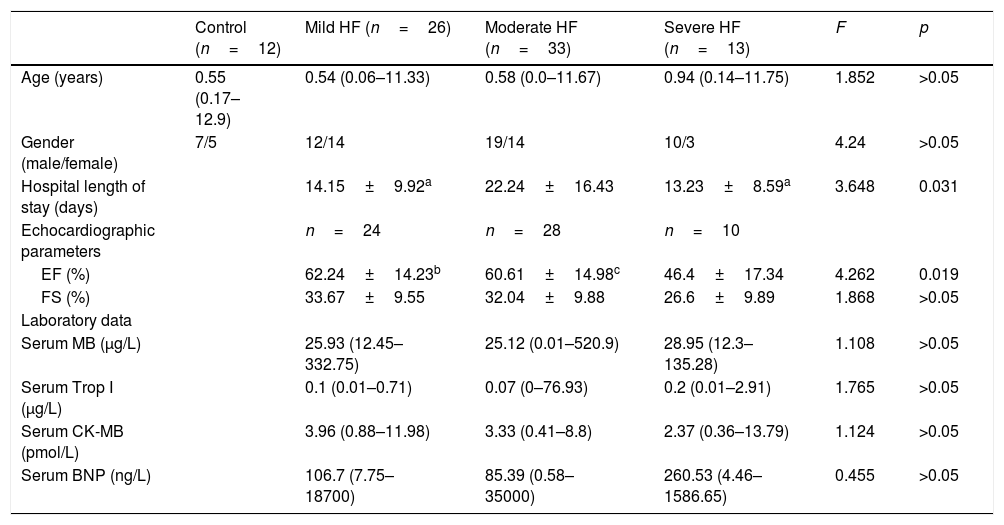
Collection of blood samples and clinic dataBlood samples from 72 children diagnosed with HF and 12 healthy children undergoing routine health examination were collected between December 2014 and December 2015 at the Clinical Examination Center. The median age of children with heart failure was 1 year (range 0–11.75 years), with 44 patients aged less than 1 year (61.1%), 16 patients between 1 and 3 years (22.2%), three patients between 4 and 7 years (4.2%), and nine patients aged >8 years (12.5%). Heart defects included: ventricular septal defects (n=11), atrial septal defects (n=3), tetralogy of Fallot (n=1), patent ductus arteriosus (n=3), complex congenital heart disease (n=20), aorta stenosis (n=1), and single ventricle (n=3). Other diseases included: primary cardiomyopathy (n=20), myocarditis (n=4), arrhythmias (n=4), pneumonia (n=1), and leukemia (n=1). All serum samples were collected between 8:00 and 10:00 am, around the time that the children also underwent clinical examination. Based on the modified Ross criteria for cardiac function,9 HF was divided into mild (score: 3–6), moderate (score: 7–9), or severe (score: 10–12). To analyze the association between relevant clinic data and the incidence of HF in children, data on 72 children with HF were retrospectively collected. Data included each patient's age, gender, length of hospital stay, echocardiographic examination, laboratory values, diagnosis, and heart rates detected when admitted to the hospital. The concentrations of MB, TnI, CK-MB and BNP were measured by chemiluminescent immunoassay (Siemens®, Munich, Germany) by a docimaster in the clinical laboratory of the hospital. The clinical characteristics for all 84 children who were evaluated and the pathologies of the 72 pediatric patients with HF are summarized in Table 1.
Clinical, echocardiographic, and laboratory values, and serum melatonin levels of pediatric patients with heart failure and healthy controls.
| Control (n=12) | Mild HF (n=26) | Moderate HF (n=33) | Severe HF (n=13) | F | p | |
|---|---|---|---|---|---|---|
| Age (years) | 0.55 (0.17–12.9) | 0.54 (0.06–11.33) | 0.58 (0.0–11.67) | 0.94 (0.14–11.75) | 1.852 | >0.05 |
| Gender (male/female) | 7/5 | 12/14 | 19/14 | 10/3 | 4.24 | >0.05 |
| Hospital length of stay (days) | 14.15±9.92a | 22.24±16.43 | 13.23±8.59a | 3.648 | 0.031 | |
| Echocardiographic parameters | n=24 | n=28 | n=10 | |||
| EF (%) | 62.24±14.23b | 60.61±14.98c | 46.4±17.34 | 4.262 | 0.019 | |
| FS (%) | 33.67±9.55 | 32.04±9.88 | 26.6±9.89 | 1.868 | >0.05 | |
| Laboratory data | ||||||
| Serum MB (μg/L) | 25.93 (12.45–332.75) | 25.12 (0.01–520.9) | 28.95 (12.3–135.28) | 1.108 | >0.05 | |
| Serum Trop I (μg/L) | 0.1 (0.01–0.71) | 0.07 (0–76.93) | 0.2 (0.01–2.91) | 1.765 | >0.05 | |
| Serum CK-MB (pmol/L) | 3.96 (0.88–11.98) | 3.33 (0.41–8.8) | 2.37 (0.36–13.79) | 1.124 | >0.05 | |
| Serum BNP (ng/L) | 106.7 (7.75–18700) | 85.39 (0.58–35000) | 260.53 (4.46–1586.65) | 0.455 | >0.05 |
EF, ejection fraction; FS, fractional shortening; MB, myoglobin; Trop I, highly sensitive Troponin-I; CK-MB, creatine kinase-MB; BNP, brain natriuretic peptide. Values are expressed as mean±SD or median (range).
All serum samples were stored in a freezer at –80°C before testing. Melatonin, myeloperoxidase (MPO), and caspase-3 levels were measured using the human melatonin ELISA kit (Arigo, Taiwan), the human MPO ELISA kit (eBioscience® Thermofisher, CA, USA), and the human caspase-3 ELISA kit (Westang Bio-tech®, Shanghai, China), respectively. All assays were performed following the manufacturers’ instructions.
Statistical analysisAll statistical analyses were performed with SPSS software (IBM SPSS Statistics for Windows, version 19.0. NY, USA). All data are shown as mean±SD, with the exception of data that were not normally distributed, which are shown as median (range). For normally distributed variables, between-group comparisons were evaluated using the one-way analysis of variance (ANOVA) test. The least significant difference (LSD) method was utilized to estimate pairwise comparisons. For non-normally distributed variables, intergroup comparisons were assessed using the Kruskal–Wallis H test, and the chi-squared test was used for comparisons. Linear regression analysis was performed to determine the association between serum melatonin levels with ejection fraction (EF) and with levels of caspase-3 and MPO in pediatric patients with HF. Pearson correlation analysis was used to determine whether there was a linear association between serum melatonin concentration and these data mentioned above. To determine the appropriate cutoff value of serum melatonin to diagnose severe HF and primary cardiomyopathy in pediatric patients with HF, the area under the receiver operating characteristic (ROC) curve (AUC), sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and Youden index (J) were analyzed. A p value of <0.05 was considered statistically significant for all tests.
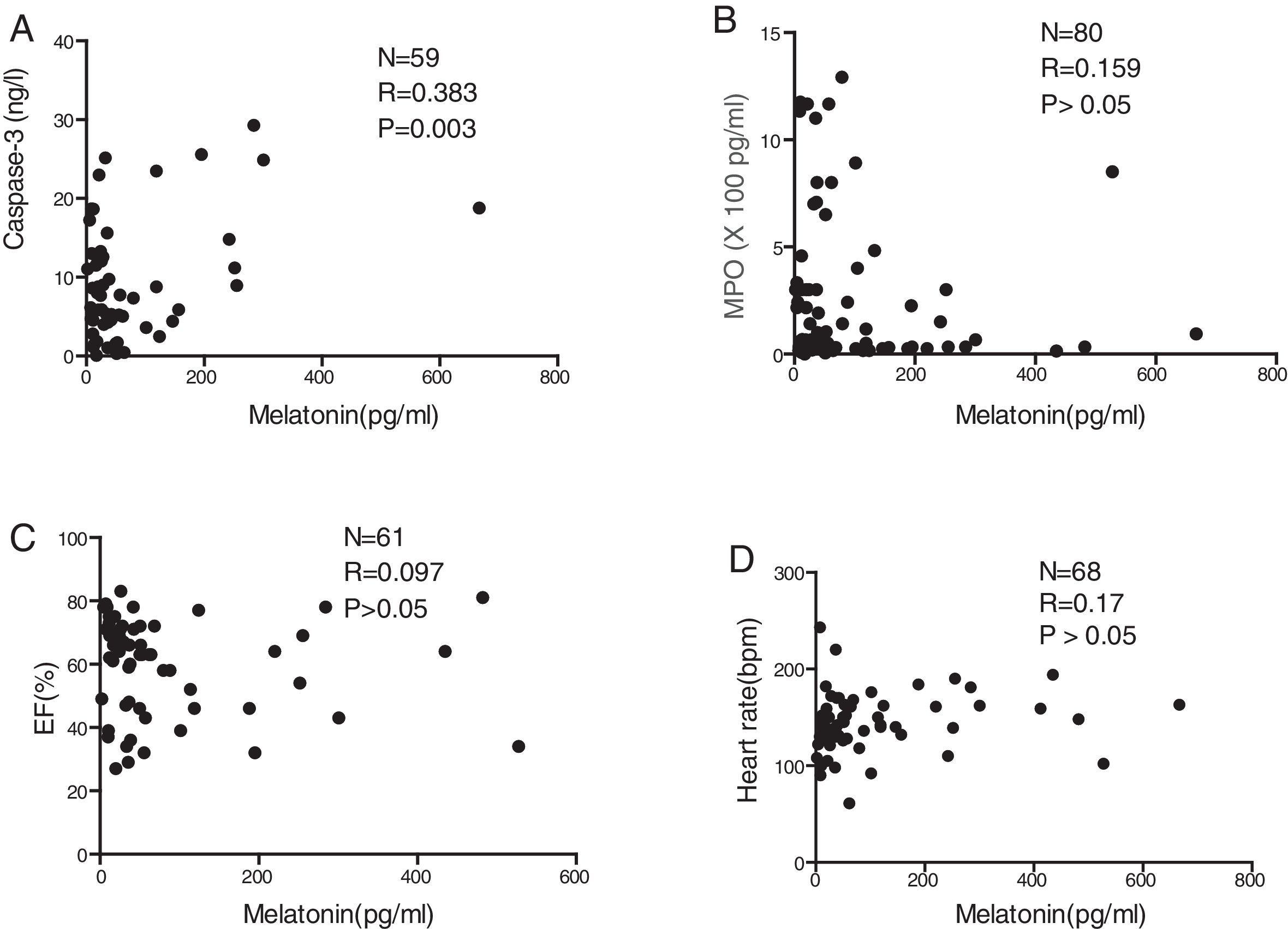
ResultsSerum levels of melatonin, MPO, and caspase-3The EF in the group with severe HF was the lowest among the four groups (p=0.019). Length of hospital stay (p=0.031) was also found to be different between the four groups (Table 1). As shown in Fig. 1A, the median serum melatonin level was 188.3pg/mL, with a range of 10.06–666.7pg/mL in the severe HF group, which was the highest median among the four groups (p=0.031). Fig. 1B plots the serum MPO levels; in the group with severe HF, the median MPO value was 304.200pg/mL, with a range of 61,880–1,402,700pg/mL, which was the highest among the four groups (p<0.001). No significant differences were found in caspase-3 levels among the four groups (p>0.05; Fig. 1C). There was no relationship between circulating melatonin levels and age, gender, hospital length of stay, EF, or HF etiology (all p>0.05; Fig. 1D–H).
Serum levels of melatonin (A), MPO (B), and caspase-3 (C) in patients, by degree of cardiac dysfunction. Serum melatonin levels in pediatrics patients stratified by age (D), gender (E), length of hospital stay (F), ejection fraction (%) (G), and heart failure etiology (H). VSD, ventricular septal defects; ASD, atrial septal defects; TOF, tetralogy of Fallot; PDA, patent ductus arteriosus. **p<0.01 compared with severe heart failure group.
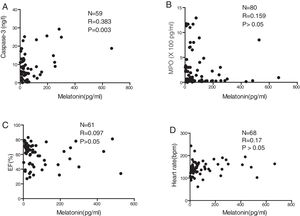
There was a significant positive correlation between serum melatonin level and serum caspase-3 level (p=0.003; Fig. 2A). In contrast, serum melatonin levels were not correlated with MPO (p>0.05; Fig. 2B), EF (p>0.05; Fig. 2C), or the heart rates of patients detected at admission to the hospital (p>0.05; Fig. 2D).
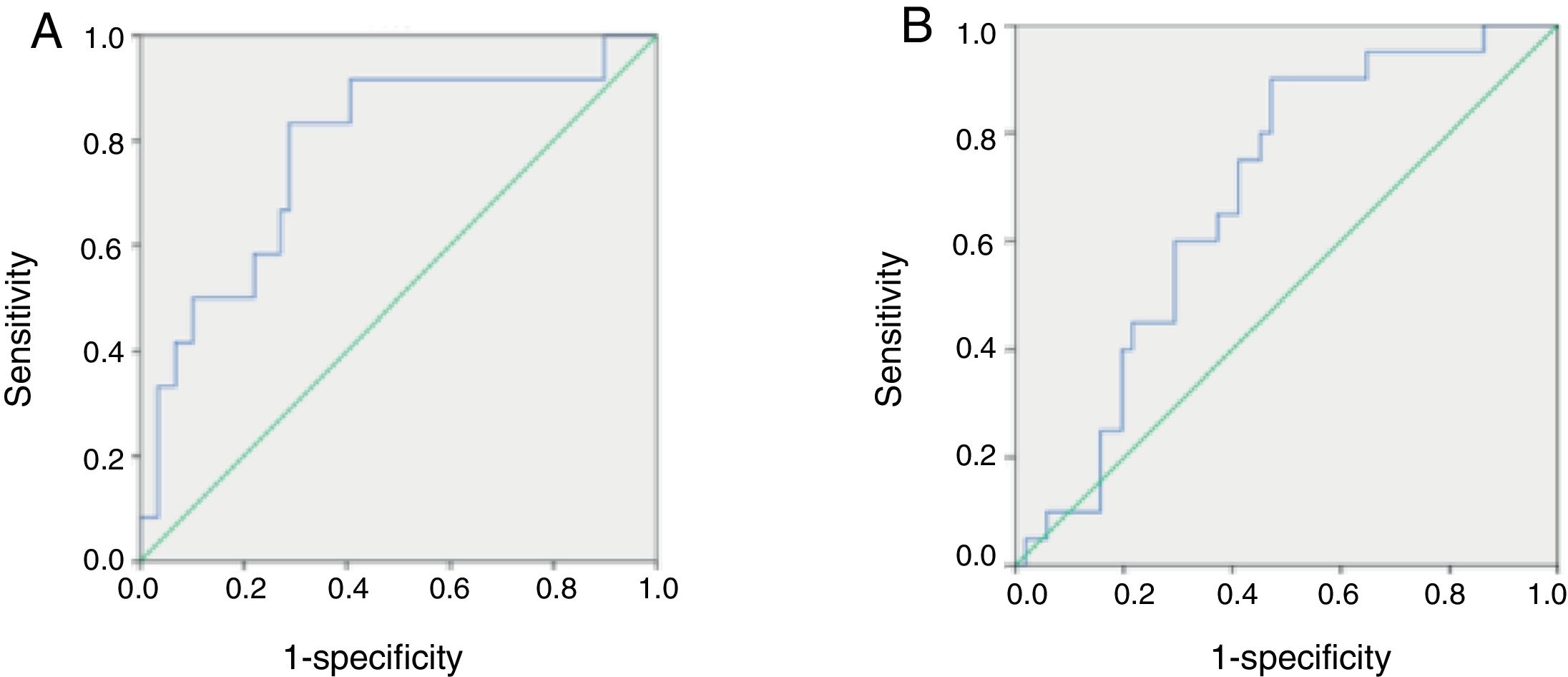
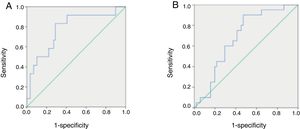
ROC curves of serum melatonin concentrations in pediatric patients with HFSerum melatonin concentrations ranging from 1.1851 to 667.6936pg/mL were used to generate ROC curves and define the optimal value of serum melatonin to diagnose severe HF in pediatric patients. The AUC, sensitivity, specificity, PPV, NPV, and J value were assessed. Among all pediatric patients with HF, a cutoff value of 54.1404pg/mL yielded the highest J (0.545), with sensitivity of 0.833, specificity of 0.712, PPV of 0.37, and NPV of 0.94, indicating that this may be the optimal cutoff value for diagnosing severe HF (Fig. 3A).
Comparison of receiving operator characteristic (ROC) curves for the diagnostic performance of melatonin in identifying severe heart failure (HF) (A) and primary cardiomyopathy (B) in pediatric patients with HF. (A) Area under the curve (AUC)=0.780 for melatonin (p=0.002). The maximal cut-off value was 54.1404pg/mL for melatonin (sensitivity=0.833, specificity=0.712, PPV=0.37, NPV=0.94, and J=0.545). (B) AUC=0.683 for melatonin (p=0.017). The maximal cut-off value was 32.8805pg/mL for melatonin (sensitivity=0.900, specificity=0.529, PPV=0.43, NPV=0.93, and J=0.429). PPV, positive predictive value; NPV, negative predictive value; J, Youden index.
ROC curves for concentrations of circulating melatonin were used to define the optimal value of serum melatonin to diagnose primary cardiomyopathy in pediatric patients with HF. Among all HF children, a cutoff value of 32.8805pg/mL yielded the highest J value (0.429), with sensitivity of 0.9, specificity of 0.529, PPV of 0.43, and NPV of 0.93, indicating that this may be the optimal cutoff value for diagnosing primary cardiomyopathy in these patients (Fig. 3B).
DiscussionHF is a serious syndrome and is the terminal phase of many pediatric cardiovascular diseases, especially primary cardiomyopathy or congenital heart disease.10 However, the molecular mechanism is not fully understood. Cardiovascular diseases have been associated with temporal rhythmicity and seasonal affective disorder.11 Hypertension, myocardial ischemia, arrhythmia, angina, and sudden death due to HF often have a higher incidence in the morning, especially between 6:00 am and 12:00 pm.12 Cardiovascular diseases have also been shown to vary in severity with seasonal alterations.13 Melatonin is secreted by the pineal gland with diurnal rhythmicity. Light exposure, especially during daytime, inhibits the secretion of melatonin.14 Nonetheless, the role of melatonin in the rhythmicity-associated pathophysiology of HF in pediatric patients remains unclear.
Research has demonstrated that lower melatonin levels are observed in the New York Heart Association (NYHA) class III subgroup of adult patients with HF.15 A separate study revealed that serum melatonin levels in HF patients suffering from hypertensive cardiomyopathy were lower than in individuals without HF.16 The present data contradicts that which is observed in adults, and shows that circulating levels of melatonin were significantly higher in children with more severe HF. The data suggest that there may be a separate mechanism in pediatric patients with HF that affects melatonin levels. Melatonin plays a protective role in cardiovascular diseases as an antioxidant and powerful radical scavenger.17 It is likely that the increased melatonin concentration in pediatric patients with severe HF may be a compensatory mechanism; this speculation warrants further investigation.
Numerous studies have suggested that excessive oxidative stress is linked to apoptosis of myocardial cells18 and the pathological process that leads to HF.19 In the present study, MPO levels were increased in patients with severe HF, indicating that excessive stress from reactive oxygen species is involved in the pathological development of pediatric HF. Though no significant differences were found in the serum levels of caspase-3 among the groups, the circulating levels of melatonin were found to be positively associated with serum caspase-3, suggesting a possible role of melatonin in the apoptotic process leading to pediatric HF. Previously studies have demonstrated that serum melatonin concentrations varied with age, with younger children having higher melatonin levels under healthy conditions; however, the present study did not find a relationship between melatonin and age, gender, hospital length of stay, EF, heart rate detected at admission, or HF etiology. This difference from previous studies may be due to the fact that serum samples in the previous studies were taken from healthy children, whereas the present study examined children with pediatric HF.
The plasma levels of brain natriuretic peptide (BNP) or N-terminal pro-brain natriuretic peptide (NT-proBNP) have been proven to be useful in the diagnosis, prognosis, and management of children with heart failure.20 A negative correlation has been found between plasma concentrations of BNP or NT-proBNP and ages in infants and children with cardiac dysfunction.21,22 In the present study, such a correlation between the serum levels of melatonin and ages in children with HF was not detected. However, these values of melatonin in diagnosing children with HF are far better than BNP or NT-proBNP. In adults, melatonin has been suggested not only as a diagnostic biomarker, but also as a potential therapeutic option for the cardiovascular diseases.17 Thus, learning the levels of melatonin in cardiac children is needed to demonstrate the true benefit of melatonin in the management of children with HF.
In conclusion, the circulation levels of melatonin and MPO were elevated in pediatric patients with severe heart failure. Additionally, serum melatonin levels were correlated with serum caspase-3 levels. Further studies are needed to investigate the role of melatonin in children with HF.
Ethical approval and informed consentThis study was approved by the Ethics Committee. All patient-derived blood samples were collected after written informed consent was obtained from parents or guardians.
Conflicts of interestThe authors declare no conflicts of interest.
The authors would like to thank Fengchuan Jing and Danyi Peng for providing information and for the support during this study.
Please cite this article as: Wu Y, Si F, Luo L, Yi Q. Serum levels of melatonin may contribute to the pathogenesis of heart failure in children with median age of 1 year. J Pediatr (Rio J). 2018;94:446–52.