Review the main aspects of the definition, diagnosis, and management of pediatric patients with sepsis and septic shock.
Source of dataA search was carried out in the MEDLINE and Embase databases. The articles were chosen according to the authors' interest, prioritizing those published in the last five years.
Synthesis of dataSepsis remains a major cause of mortality in pediatric patients. The variability of clinical presentations makes it difficult to attain a precise definition in pediatrics. Airway stabilization with adequate oxygenation and ventilation if necessary, initial volume resuscitation, antibiotic administration, and cardiovascular support are the basis of sepsis treatment. In resource-poor settings, attention should be paid to the risks of fluid overload when administrating fluids. Administration of vasoactive drugs such as epinephrine or norepinephrine is necessary in the absence of volume response within the first hour. Follow-up of shock treatment should adhere to targets such as restoring vital and clinical signs of shock and controlling the focus of infection. A multimodal evaluation with bedside ultrasound for management after the first hours is recommended. In refractory shock, attention should be given to situations such as cardiac tamponade, hypothyroidism, adrenal insufficiency, abdominal catastrophe, and focus of uncontrolled infection.
ConclusionsThe implementation of protocols and advanced technologies have reduced sepsis mortality. In resource-poor settings, good practices such as early sepsis identification, antibiotic administration, and careful fluid infusion are the cornerstones of sepsis management.
Revisar os principais aspectos da definição, diagnóstico e manejo do paciente pediátrico com sepse e choque séptico.
Fontes de dadosUma pesquisa nas plataformas de dados Medline e Embase foi feita. Os artigos foram escolhidos segundo interesse dos autores, priorizaram-se as publicações dos últimos 5 anos.
Síntese dos dadosA sepse continua a ser uma causa importante de mortalidade em pacientes pediátricos. A variabilidade de apresentação clínica dificulta uma definição precisa em pediatria. A estabilização da via aérea com adequada oxigenação, e ventilação se necessário, ressuscitação volêmica inicial, administração de antibióticos e suporte cardiovascular são a base do tratamento da sepse. Em cenários de poucos recursos, deve-se atentar para os riscos de sobrecarga hídrica na administração de fluidos. A administração de drogas vasoativas como adrenalina ou noradrenalina, se faz necessária na ausência da resposta ao volume na primeira hora. O seguimento do tratamento do choque deve seguir alvos como restauração dos sinais vitais e clínicos de choque e controle do foco de infecção. Recomenda-se a avaliação multimodal, com auxílio da ecografia à beira-leito para manejo após as primeiras horas. No choque refratário, deve-se atentar para situações como tamponamento cardíaco, hipotireoidismo, insuficiência adrenal, catástrofe abdominal e foco de infecção não controlado.
ConclusõesImplantação de protocolos e avançadas tecnologias propiciou uma redução da mortalidade da sepse. Em cenários de poucos recursos, as boas práticas, como reconhecimento precoce da sepse, administração de antibióticos e cuidadosa infusão de fluidos, são os pilares do manejo da sepse.
From the dawn of Homo sapiens to the present day, infectious diseases have been a huge challenge for humankind. The World Health Organization estimates that over 60 % of the deaths of children under the age of 5 on the planet are due to infectious diseases.1
Sepsis is a potentially fatal multi-organ failure due to the body's dysregulated response to an infectious process. This dysregulated response may range from an uncontrolled and exaggerated manifestation of proinflammatory activity (e.g., meningococcemia), with intense clinical presentation or a less intense manifestation to the absence of an inflammatory response, as in immunosuppressed patients or those with “immunoparalysis,” where the clinical presentation may be silent or insidious (e.g., central venous catheter-related candidemia in children immunosuppressed by chemotherapy).2–7
In recent decades, several campaigns and recommendations to fight sepsis have been promulgated to facilitate diagnosis, promote early intervention, and reduce child mortality. Consequently, there has been a marked decline in mortality from severe sepsis and septic shock worldwide, with lower rates in developed countries than in poorer nations (19 % vs. 32 %, respectively).8–13
Increased sepsis survival has been attributed to early detection associated with aggressive treatment in a hospital setting, following previously established protocols.2–6 However, sepsis involves complex pathophysiological mechanisms with varied and unspecific clinical presentations, affecting a heterogeneous group of people (newborns, healthy patients, those with comorbidities, among others); thus, a single, simple, and objective definition that encompasses this whole scope has become a tremendous challenge. The current definitions of sepsis and its stages in pediatrics are inaccurate and do not consider this diversity of presentations; thus, they demonstrate low specificity and sensitivity.5,12,14 Consequently, delay in diagnosis is not unusual, and there is a delay in the establishment of adequate treatment that includes well-defined targets in the first hour of care (“the golden hour”).5,6,15 The aim of this study is to review the most important aspects regarding the definition, diagnosis, and management of sepsis in pediatrics; discuss the controversial points of the literature based on the most recent scientific evidence, and critically disclose the authors’ opinion.
Definition of sepsis in pediatricsIn 2005, the International Pediatric Consensus Conference (IPSCC)5 proposed age-adjusted definitions for sepsis and its stages in pediatrics, having systemic inflammatory response syndrome (SIRS) as the central concept. In this perspective, the IPSCC proposed the following as definitions:
- I
Sepsis:
- a
Suspected or proven infection caused by any pathogen or clinical syndrome with high probability of infection, associated with:
Presence of at least two of the following clinical manifestations (requiring abnormal temperature or white blood cell count):
- b
Temperature >38.5°C or <36°C.
- c
Tachycardia or bradycardia (values adjusted to age).
- d
Tachypnea (according to the age range) unrelated to neuromuscular disease or anesthesia.
- e
Elevated or suppressed leukocyte count according to values for each age group.
- a
- II
Severe sepsis: A patient defined as having sepsis according to the above criteria presenting with organ dysfunction (respiratory or cardiocirculatory) or two other dysfunctions.
- III
Septic shock: patient with sepsis with acute circulatory failure, characterized by persistent hypotension (<2 standard deviations for the age range standard) despite adequate volumetric resuscitation and unexplained by other causes.
The IPSCC task force, when creating these definitions, stressed that it was an instrument still under construction that required refinement, yet they were incorporated into the daily practice of pediatric intensive care units (PICUs) worldwide2–4,7,14–16 and formed the basis of concepts for pediatric sepsis diagnosis and treatment recommendations.5,6,15
Despite the fast acceptance in the clinical setting, several studies carried out over the last decade have demonstrated the limitations of the definitions proposed by the ISPCC. The SPROUT (Sepsis Prevalence, Outcomes, and Therapies) study, featuring 7000 children admitted to 128 PICUs in 26 countries, showed poor agreement (46 %) between the presumptive diagnosis of severe sepsis by the attending physician and the diagnosis according to the IPSCC recommendations.17 The clinical diagnosis was performed using a more liberal approach, with lower laboratory alterations, lower mortality, and lower organ failure than that observed in the group that was strictly defined by the consensus criteria.
The main reason to justify the low specificity and sensitivity of the IPSCC criteria in the diagnosis of sepsis in pediatrics is related to SIRS: patients with sepsis may not have SIRS, just as SIRS may be present in patients without infection. In this sense, several other clinical and biological markers have been evaluated in sepsis (e.g., serum lactate, central venous saturation, blood pressure, capillary filling, among others), and none of them showed sufficient accuracy to define and guide the treatment of sepsis in children.6,15,18,19
As a consequence of the dysregulated response to the infection and the established treatment, two forms of death can occur in sepsis: a) refractory shock and b) multiple organ dysfunction syndrome (MODS). One-third of deaths in children with sepsis and septic shock are estimated to occur within the first 72h, in which refractory shock is the leading cause. However, after the third day, MODS, respiratory failure, and neurological failure predominate as the main causes of death.20
Recently new definitions for sepsis and septic shock have been proposed for the adult population: The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3), where some concepts of the Sequential Organ Failure Assessment (SOFA Score) have been incorporated.3,4 These new definitions have been adapted to also be used in the pediatric population. The pediatric SOFA score (pSOFA) is based on the assessment of six systems (respiratory, hepatic, coagulative, cardiovascular, neurological, and renal), with values ranging from zero to 4 points.21 The pSOFA score can range from zero (understood as a normal health condition and no chance of sepsis) up to a maximum of 24 (interpreted as severe septic shock). Therefore, adopting the Sepsis-3 adapted for children, the definitions are as follows:
- I
SEPSIS: suspected or confirmed infection associated with acute increase in the pSOFA score >2 points (within the prior 48h up to 24h of infection).
- II
SEPTIC SHOCK: The same criteria mentioned above in children receiving vasoactive drugs associated with serum lactate level >2mg/dL.
These definitions were retrospectively assessed, analyzing more than 8500 children admitted over a seven-year period to a PICU in the United States. Mortality in the study group was 2.4 %, which is considered very low even for developed countries. In this scenario it was demonstrated that the pediatric Sepsis-3 has great power to predict mortality (AUC=0.94), with little (if any) advantage over other pediatric scores such as PRISM (Pediatric Risk of Mortality) III, P-MODS (Pediatric Multiple Organ Dysfunction Score), and PELOD (paediatric logistic organ dysfunction) 1 and 2. A pSOFA score >8 was the best cutoff point to predict sepsis mortality.21
When the pediatric Sepsis-3 was evaluated in an Indian PICU involving a smaller population but with a much higher sepsis mortality (40 %), a high specificity (identifying the most severe cases of sepsis) was observed, but with a low sensitivity, because it failed to identify 18 % of sepsis episodes diagnosed by the IPSCC.22
In the present authors’ view, Sepsis-3 adapted for pediatrics does not meet the key requirements that ensure accuracy and safety for the identification of sepsis and septic shock in the pediatric population. Obviously, organ dysfunction is a relevant finding in the context of pediatric sepsis. Progression or onset of new organ failure, especially on the second day, has been associated with increased mortality.23 In this context, both pSOFA and PELOD-2 could be used to identify the most severe cases of sepsis (higher risk of death), which require care at referral centers with greater resources. However, considering its low sensitivity, its inclusion in the definition (identification) of sepsis and septic shock is not supported or justified.
Although some pediatric studies have shown that increased serum lactate levels may be a marker of severity and that its decrease may be associated with a good therapeutic response, it is known that several factors affect this oscillation. To date, there is not enough solid scientific evidence to support the use of lactate levels in the characterization of septic shock in pediatrics. Thus, by adopting the criteria proposed by Sepsis-3 in the pediatric population, clinicians run the risk of failing to identify a significant portion of patients with clinically established septic shock but still showing low lactate levels. It is understood that physiological and biological variables are part of this complex equation, but to define sepsis safely and accurately, other elements must be computed.
ManagementPediatric sepsis is a high-risk condition that requires alertness to attain an early and timely diagnosis. Late detection or delayed start of treatment with persistent hemodynamic dysfunction are associated with worse clinical outcomes and increased mortality.24 Rapid and aggressive resuscitation with volume within the first five minutes, antibiotic administration, and early vasoactive agents may be crucial to a successful treatment. It can be observed that this treatment pattern, which can often rapidly change, also makes sepsis management critical for pediatricians.6
The initial management of septic shock is the same as in other life-threatening conditions. It starts with airway stabilization and adequate breathing with extra oxygen supply. Ventilatory support should be sufficient to increase oxygen supply and uptake by the cell. As circulation is the most obviously compromised feature, treatment starts with aggressive volumetric replacement to restore the circulating volume, followed by the correction of negative inotropic factors, increasing cardiac contractility and eventually decreasing the peripheral vascular resistance.
The objective is to maintain the oxygenation, ventilation, circulation, and heart rate within the normal limits, and to restore the patient’s clinical condition by improving perfusion (capillary filling <2s) with full pulses and warm extremities, urinary output >1mL/kg/h, adequate mental status, and normal blood pressure for age. The minimum desired monitoring consists of pulse oximetry, ECG tracing, blood pressure, temperature, hourly diuresis, glucose, and calcium measurements.15
The decisions to intubate and ventilate are clinical: respiratory failure, hypoventilation, altered state of consciousness, or impending death. For volumetric resuscitation and the start of therapy, vascular access should be obtained immediately, and the insertion of a central catheter is preferable for vasoactive drug infusion. Peripheral accesses can also be used for initial volumetric resuscitation and for the administration of some of these sympathomimetic amines. Intraosseous access may be necessary if peripheral access is impossible.25
Volumetric resuscitation and drugs in the first hourFor volumetric resuscitation, a crystalloid solution is used, usually normal saline solution (physiological solution). Eventually, balanced crystalloid solutions (such as Ringer's lactate solution and others) can be used with the same results. These balanced solutions have been recommended as they reduce acidosis, mortality, and post-resuscitation renal injury in pediatric shock.26 Nonetheless, these results are still controversial. Colloids, such as albumin, are used in exceptional and selected cases, such as when large amounts of solution are infused, and it is necessary to restrict volume. The albumin solution, besides not having its benefit confirmed, has a higher cost than the other solutions. No difference was demonstrated between albumin and crystalloid solutions, even in children with dengue shock syndrome.27
Septic shock can progress with volume loss to the third space and capillary leak syndrome. This is an important point in re-evaluating and maintaining the need for volumetric replacement. An infusion of 20mL/kg of saline solution between ten and 20min is recommended. The infusion of 20mL/kg should be repeated until tissue perfusion, oxygen supply, and blood pressure are adequate. Good practice includes the clinical observation of circulatory overload after the infusions. Absence of bullous rales, tachycardia, and tachypnea or the development of a third heart sound (B3) and hepatomegaly allows the fluid infusion to be repeated. Patients with septic shock usually require volumes of up to 60mL/kg or more within the first hour.28
In resource-limited settings that cannot provide advanced airway and circulatory support, children with signs of compensated shock and severe febrile illness should be managed with even greater caution.29 Under these conditions, repeated infusions of 10mL/kg of saline solution every 20min (30mL/kg in the first hour) are suggested, while watching for signs of circulatory overload with each infusion.
In some situations, it will be necessary to use vasoactive drugs in severe shock during fluid resuscitation. Vasoactive agents are most often administered when there is no response to the use of bolus fluids, but they may be used concomitantly. Patients with poor adrenergic response, bradycardia, or who are close to cardiopulmonary arrest should be treated with medium or high-dose epinephrine infusion (0.2–0.5μg/kg/min), and those with hypotension, with norepinephrine (0.05–0.1μg/kg/min), concomitantly and independently of volumetric resuscitation.
Pharmacological support in the first hourHemodynamic management in shock aims at providing a supranormal oxygen supply (above the critical limit) and an increase in mean arterial pressure to a level that allows cardiac output to achieve adequate organic perfusion. Vasoactive drugs should be used judiciously, with a goal-oriented approach. Cold shock (presumably due to low cardiac output) should be treated with epinephrine and warm shock (presumably due to high cardiac output with low systemic vascular resistance [SVR]) should be treated with norepinephrine. Patients with sepsis from the community usually develop cold shock (titrate epinephrine between 0.1 and 0.2μg/kg/min), while those with intra-hospital infection (e.g., catheter-related and fungal infections, among others) tend to evolve with a slower-onset warm shock, with norepinephrine (0.05–0.2μg/kg/min) being recommended in these cases. Consider packed red blood cell transfusions to maintain hemoglobin >7 and central venous oxygen saturation >70 %.15
Shock resistant to the initial managementIf the patient shows no signs of shock reversal with epinephrine up to 0.3μg/kg/min, which should be recognized promptly, norepinephrine is started, especially if the patient is hypotensive, hyperdynamic, or dilated with warm shock, aiming to normalize the infusion and blood pressure. If the patient has cold shock, hypotension with bradycardia, or cardiac dysfunction is the most prevalent picture, epinephrine is titrated at higher doses.
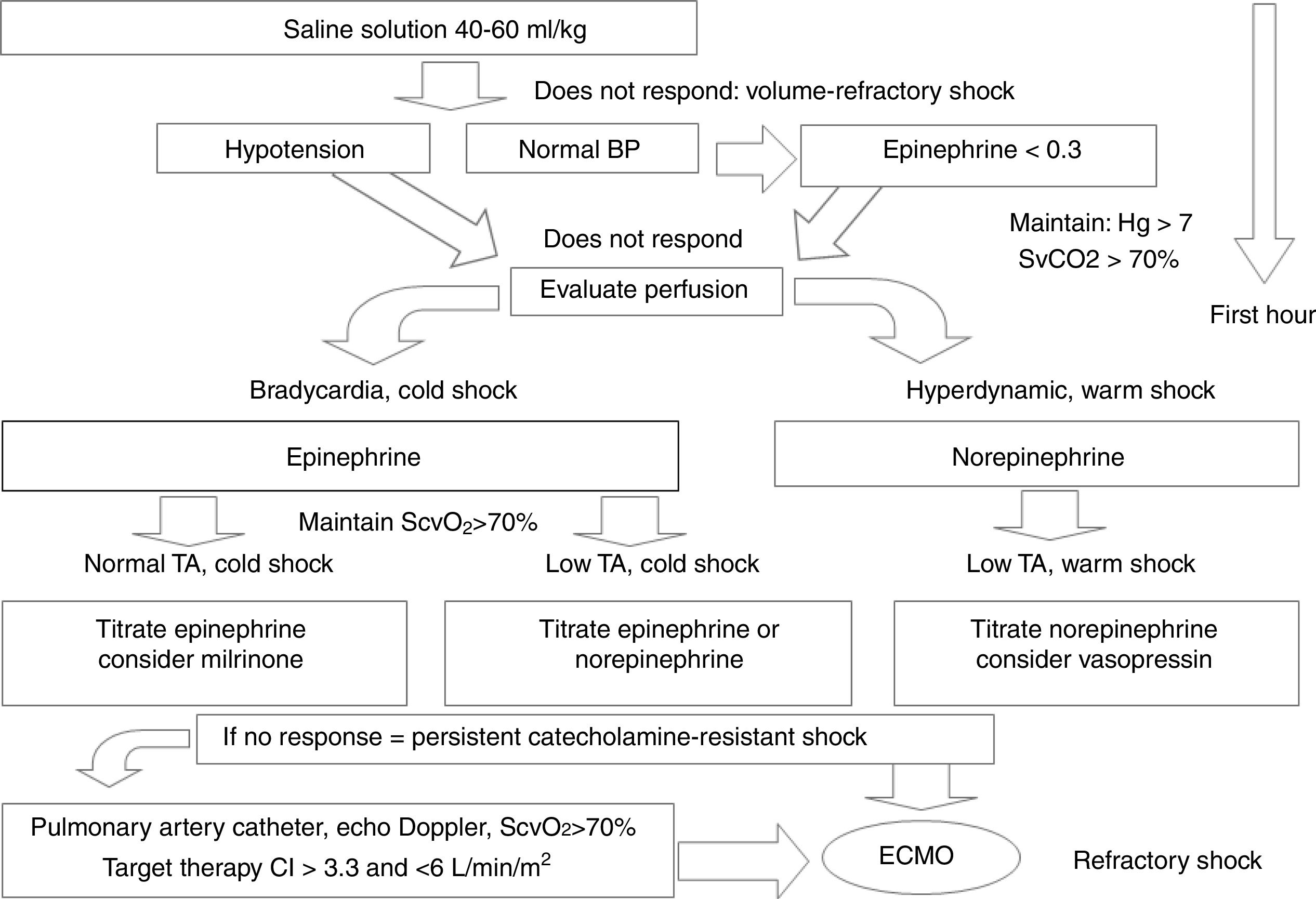
Cold shock is recognized by cold, mottled extremities, poor peripheral perfusion with slow capillary filling (greater than two seconds), weak peripheral pulses, and decreased urine output (less than 1mL/kg/h). Warm shock is recognized by flushing of the extremities, rapid capillary filling (less than two seconds), full or oscillating pulses, but also decreased urine output (less than 1mL/kg/h). Norepinephrine plays an important role in maintaining blood pressure in the renal compartment, where perfusion pressure should be adequate. At the end of this first hour, in patients with volume-refractory warm shock who are resistant to the initial management, an infusion of 0.05μg/kg/min is started and progressively increased by 0.2μg/kg/min. Fig. 1 summarizes the conduct for pharmacological support in septic shock.6
Simplified pharmacological support scheme for children with septic shock. See details in the text.
BP, blood pressure; CI, cardiac index; ECMO, extracorporeal membrane oxygenation.
Modified from Davis et al.6
Although the authors have attempted to classify the septic shock states using a practical approach, it is an evolving clinical condition. Thus, frequently the drug chosen for the start of the therapy may become inadequate and require a new drug or association of drugs. The response of each patient to vasoactive therapy may also be different, and frequent clinical and hemodynamic reassessments should be performed.30
After the first hour, patients with volume-refractory shock who persist in shock despite the use of catecholamines such as epinephrine or norepinephrine are considered to be in catecholamine-resistant shock. In this period, they should be admitted to the PICU, monitored, with continuous measurement of electrocardiographic tracing, heart rate, and temperature; access to a large vessel should have already been obtained and, if a central venous access has already been attained, the central venous pressure should already have been measured through it. Blood pressure measurement should preferably be performed by an invasive method; the authors measure oxygen saturation with a pulse oximeter and urine output through a bladder catheter. A bedside functional ultrasound allows the assessment of volume status and myocardial function. The perfusion pressure [PP=MAP–CVP (mmHg)] is then calculated. The infusion pressure limits are as follows: newborn, 55; infants, 58; preschoolers and schoolchildren, 65.6,31
Cold shock, resistant to catecholamines, low BP (blood pressure), low CO (cardiac output), and high SVR (systemic vascular resistance)In this scenario, the patient with cold shock who is resistant to catecholamines with still low blood pressure, low cardiac output, and high systemic vascular resistance benefits from the inotropic action of epinephrine and its vasopressor action. A good response is expected at doses between 0.2–0.5μg/kg/min. If the heart rate is not too elevated, the patient may benefit from the extra adrenergic stimulus of epinephrine, which can be used up to 1μg/kg/min and, exceptionally, up to 2μg/kg/min. These higher doses usually compromise splanchnic circulation and should only be used in association with vasodilators.
Cold shock, resistant to catecholamines, normal BP, low CO, and high SVRIn this case, the patient has cold shock and is resistant to catecholamines (did not respond to initial fluid or adrenaline infusion up to 0.2μg/kg/min), but blood pressure has normalized; the output is still low (or normal), and systemic vascular resistance is still high. These patients with elevated systemic vascular resistance benefit from the use of vasodilators. At this moment, it is permissible to associate drugs that act directly at the vascular level, aiming to try to counteract this vasoconstriction and improve cardiac output. In those patients who received sympathomimetic amines and in whom clinical improvement has not been observed (especially in those patients with severe hypoxemia with increased pulmonary and/or systemic vascular resistance), milrinone (0.25–0.75μg/kg/min) is used associated with norepinephrine, and epinephrine is withdrawn. When milrinone is contraindicated and/or in cases of SVR resistent increase, the other option is to use a continuous infusion of sodium nitroprusside at 0.5μg/kg/min up to a maximum of 10μg/kg/min.
Warm shocks, resistant to catecholamines, low BP, high CO, and low SVRIf the shock is still warm and the patient remains hypotensive, hyperdynamic, and with low resistance, it is best to continue to benefit from the norepinephrine’s vasopressor action. At doses above 0.2μg/kg/min, norepinephrine starts to lose its inotropic effect, but its vasopressor effect can be observed at doses up to 5μg/kg/min. The authors gradually increase norepinephrine, and while the patient is hypotensive, epinephrine is maintained at 0.2–0.5μg/kg/min or dobutamine is introduced at doses up to 20μg/kg/min to benefit from its inotropic action. If blood pressure normalization occurs due to the high doses of norepinephrine, epinephrine or dobutamine can gradually be withdrawn and milrinone can be associated.
Refractory shockWhen the shock persists, despite the targeted use of inotropic agents, vasopressors, vasodilators, and maintenance of negative inotropic factors, the case is defined as refractory shock. In this case, an unknown problem, such as pericardial effusion, pneumothorax, hypoadrenalism, hypothyroidism, continuous blood loss, intra-abdominal catastrophe, or the presence of necrotic tissue should be suspected.
Bedside ultrasound plays a fundamental role in this evaluation stage. Fluid responsiveness can be estimated by measuring the inferior vena cava distensibility, cardiac output is measured through left ventricular function, and the diagnosis of pericardial effusion is made through direct visualization in the parasternal long axis or subcostal view.31 A pulmonary arterial catheter may also be used to measure cardiac output, vascular resistance, and pulmonary artery occlusion pressure, and the analysis of the arterial and superior vena cava oxygen saturation may be beneficial to guide therapy in patients with refractory shock. The therapies should be adjusted to maintain mixed venous oxygen saturation (SvO2) above 70 %, cardiac index (CI)>3.3<6.0L/min/m2, and the usual perfusion pressure for age, with the main objective of restoring normal perfusion.
In patients with refractory shock who have not responded to doses of 0.6–1μg/kg/min of norepinephrine, and who are not hypovolemic, the authors have used vasopressin. The initial dose used is 0.0005U/kg/min, gradually increasing to 0.002U/kg/min (optimal dose) and, if necessary, up to a maximum dose: 0.008U/kg/min.32
Hypothyroid syndrome can complicate cases of refractory septic shock. T3 therapy in septic shock is reserved for children with known thyroid dysfunction, children at high risk of hypothyroidism (children with trisomy 21 and children with central nervous system disease), or as rescue therapy in refractory septic shock. The use of T3 as an intravenous infusion at a dose of 0.05 to 0.15μg/kg/hour or T4 (levothyroxine, 0.8–1μg/kg/hour) is recommended.33 As these drugs are seldom available, as an alternative, levothyroxine is administered through a nasogastric tube,whose dose varies according to age and which is re-adjustable according to laboratory variations: from 0 to 3 months: 10–15μg/kg/day; 3–12 months: 6–10μg/kg/day; 1–10 years: 3–6μg/kg/day; and from 10 to 16 years old: 2–4μg/kg/day.34
Extracorporeal membrane oxygenation (ECMO) is an alternative to be considered. In this situation, the veno-arterial ECMO, in addition to providing adequate blood oxygenation, can determine the systemic arterial pressure by regulating the flow released in the arterial portion. As the patient's own cardiac output improves, a gradual reduction in ECMO flow is carried out. The survival expectancy with ECMO in children with septic shock refractory to vasoactive drugs has surpassed 50 %, with a mortality rate below 20 %, thus becoming an alternative worthy of consideration.35
Targets in septic shockSeptic shock can be characterized as an alteration of clinical and hemodynamic signs, including hypo- or hyperthermia, tachycardia or bradycardia, and alteration of mental status and peripheral circulation (vasodilation – warm shock; vasoconstriction – cold shock) that precede hypotension. Shock treatment should aim at maintaining organic perfusion and observing these signs should be the priority in the management of these patients. According to Carcillo et al., hypotension associated with capillary filling time longer than three seconds is associated with a 33 % mortality rate in patients treated in pediatric emergency sectors. Reversing these parameters by following established protocols such as Pediatric Advanced Life Support (PALS) can reduce the chance of death by 40 %, regardless of the initial hemodynamic conditions.36 Thus, despite the current technological advances, physical examination and basic bedside monitoring still play a fundamental role in the care of patients with early sepsis and septic shock, especially in resource-poor settings.
Central venous oxygen saturation (SCVO2) can be measured through a catheter positioned at the entrance to the superior or inferior vena cava junction with the right atrium; its normal value is 70 % in patients without disease producing intracardiac shunt. It indicates how much oxygen remains available after cell consumption, i.e., it represents a balance between oxygen supply and cell consumption. It is a practical, inexpensive, and accessible test in resource-poor countries and has shown benefits, such as reduced organ dysfunction and mortality, and it works as a marker in the treatment of septic shock in children.37,38 Some points should be taken into consideration for its use, as there is a disagreement regarding whether it is useful as a target in the treatment of sepsis in adults.39 First, compared with adults, septic shock in children usually shows low cardiac output, prolonged capillary filling, and peripheral vasoconstriction. In these cases, SCVO2 is initially lower and probably indicates an imbalance between oxygen uptake and supply, besides being a marker of poor prognosis.38 Second, there is no benefit in implementing therapies according to protocols for elevating SCVO2 in those patients with a value close to or above 70 %.40 That is, in patients with high cardiac output and vasodilatory shock, which usually occurs together with hyperlactatemia, SCVO2 may not be a good choice as a therapeutic target.
Central venous pressure (CVP) can also be measured at the same catheter that measures SCVO2. Alone, it has limited value because it does not accurately reflect intravascular volume due to the influence of positive mechanical ventilation pressure and right ventricular dysfunction. However, it can be used to measure and monitor perfusion pressure (PP=MAP - CVP), aiming to improve renal, peripheral, and tissue oxygenation as a whole.41 Two studies have shown improved survival with the combination of adequate PP and SCVO2 >70 %.37,38
Serum lactate is the product of anaerobic respiration under conditions where there is no adequate perfusion in the tissues. Its decrease or clearance after therapy is implemented (10 % in relation to the baseline value) has been associated with increased survival in adults.42,43 As explained before, this represents a patient profile showing vasodilatory shock with high cardiac output and hyperlactatemia. In them, lactate clearance may be used as a therapeutic response and prognostic marker. However, for the vast majority of pediatric patients, this test will show normal values upon arrival and cannot be used for this purpose.37 It is believed that for the vast majority of pediatric sepsis cases, lactate has value as a prognostic marker, and not as a monitoring factor or therapeutic target.
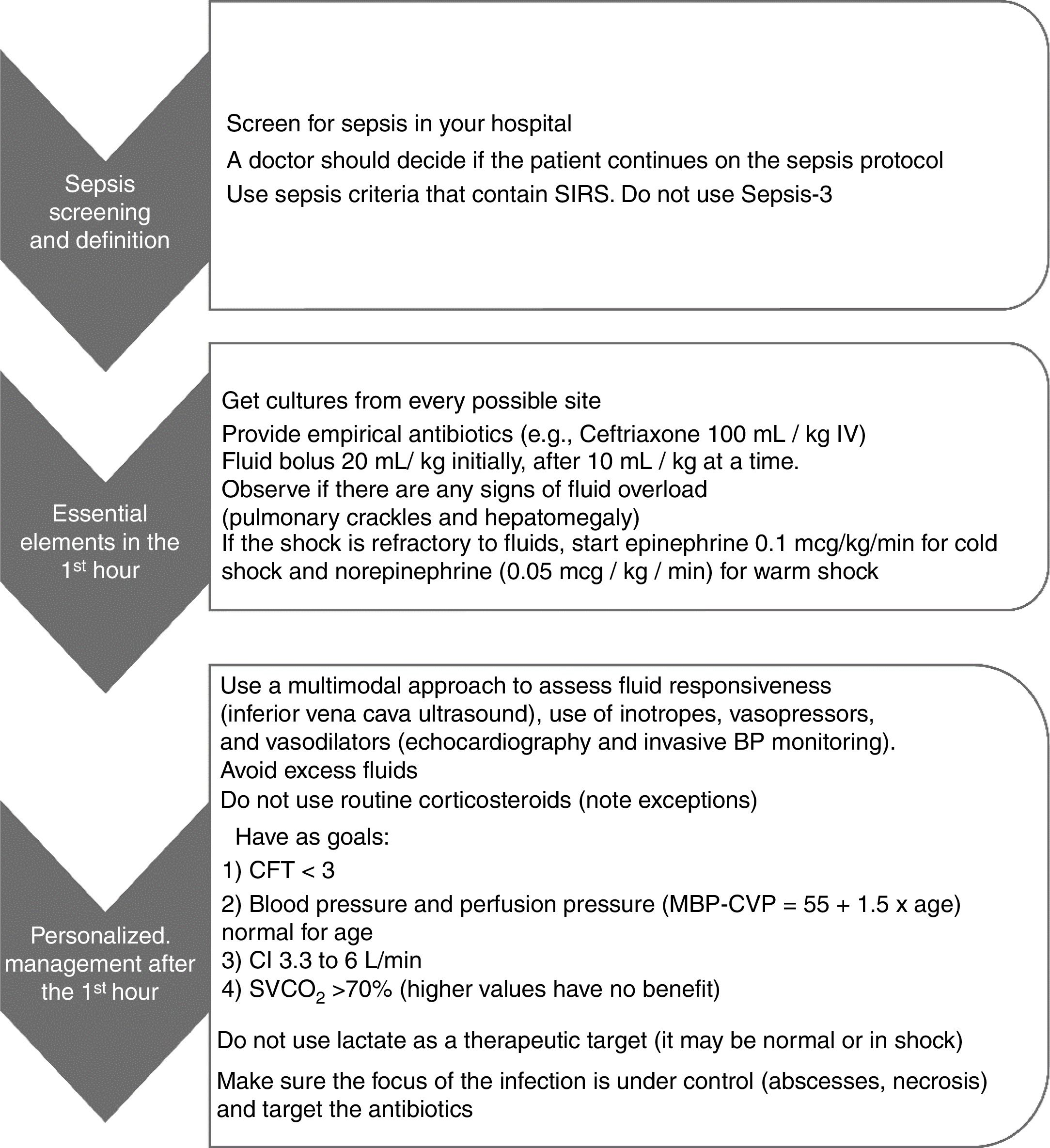
Hemodynamic decompensation in sepsis involves a complex interaction between vascular tone, myocardial dysfunction, and hypovolemia, which often make clinical reasoning guided only by the bedside physical examination difficult. In patients with volume refractory shock (>40mL/kg), the decision to provide more fluids, inotropes, or vasopressors was traditionally guided by the clinical examination, classifying the shock as “warm” or “cold”.31 However, clinical observation is not always accurate in defining the type of shock or estimating the cardiac index; moreover, their characteristics may change during the course of disease treatment.31,44 Multimodal monitoring has emerged to increase accuracy in this decision-making. It uses clinical parameters, invasive pressure monitoring, and bedside ultrasound examination for that purpose.31 In the study by Ranjit et al., cardiac and inferior vena cava ultrasound assessment performed by an intensivist at the bedside, associated with invasive blood pressure monitoring, accounted for a change in conduct in 87.5 % of the study patients. In this study, physical examination alone was not reliable in defining the type of shock (high vs. low cardiac index; vasoconstricted vs. vasodilated).31 It is believed that this individualized approach, guided by reliable, easy-to-perform bedside examinations and pre-established goals, is the best decision-making strategy for this more complex patient profile. Fig. 2 summarizes the best practices and targets for septic shock.
Summary of best practices and targets in septic shock. Adapted from Ames et al.41
SIRS, systemic inflammatory response syndrome; BP, blood pressure; CFT, capillary filling time; MAP, mean blood pressure; CVP, central venous pressure; CI, cardiac index; SVCO2, central venous oxygen saturation.
Inflammatory markers are tests that can be used in clinical practice for diagnosis, risk stratification (prognosis), and monitoring of antibiotic response therapy (and its rational use in determining treatment time) in sepsis. The use of these biomarkers must be cautious and always interpreted in a clinical context. Below, the main biomarkers used in pediatric sepsis are described.
C-reactive protein (CRP) is one of the most widely used biomarkers in pediatrics. Its usefulness in diagnosis is limited, mainly because of the low sensitivity in differentiating cases of severe sepsis and common bacterial infections in an isolated measurement.45 The most important use of CRP is in the follow-up of sepsis patients. Its 60 % drop on the fourth day of the disease is associated with a favorable prognosis, and maintenance close to the initial values indicate a poor therapeutic response.46
Serum ferritin, in addition to representing the body's iron stores, is an acute phase protein that increases in the presence of inflammatory cytokines. Since 2007, it has been used as a prognostic marker in pediatric sepsis, estimating mortality or unfavorable outcomes, either alone or in association with CRP.18,47 Moreover, its follow-up during the episode of septic shock with poor evolution may indicate the presence of hyperferritinemic sepsis, often indicating specific therapies such as corticosteroids, immunoglobulin, plasmapheresis, or even immunosuppression.48 Its cutoff value for mortality remains a subject of study, but it is believed to be lower than that found in the pioneering study by Garcia et al. (500ng/mL).18 There is evidence that it occurs in resource-poor settings with a high prevalence of iron-deficiency anemia.49
Procalcitonin, compared to CRP and ferritin, has a higher diagnostic power of bacterial sepsis in pediatrics. Values below 0.5ng/mL are suggestive of inflammation without infectious etiology and values >2ng/mL, of bacterial sepsis. Just like CRP, it can also be used to monitor disease progression and is useful in deciding to discontinue antibiotic therapy, without showing increased therapeutic failure.50 Its high cost in some centers is still the main factor limiting the dissemination of its use in pediatrics.
Choice of antibiotic therapyThe American College of Critical Care Medicine/Pediatric Advanced Life Support (ACCM/PALS) protocol advises the administration of antibiotic therapy within the first hour of sepsis treatment.6 Adherence to this protocol, which includes measures such as sepsis detection, obtaining venous access, volume resuscitation, administration of antibiotics, and, when necessary, start of vasoactive drugs, have been associated with improved patient care quality and reduced mortality.51–53 However, some authors have questioned whether administering antibiotics up to the first hour of treatment initiation in adequately resourced settings would really make any difference. In an observational study, this delay was not associated with increased mechanical ventilation time, hospital length of stay, or mortality. However, in this same study, there was no increase in benefits with an early administration.54 Overall, it is believed they should be administered as early as possible in pediatric sepsis, ideally within the first hour. Another key point is that culture tests should be collected from all suspected foci of infection, preferably before antibiotic therapy is started. These collections should not significantly delay the initiation of antimicrobial therapy.
The choice of the antibiotic agent in sepsis should be targeted according to the local epidemiology, focus of infection, and culture results. However, this information is not always defined at the initial assessment. Empirically, for community-acquired infections, the present authors have chosen a monotherapy regimen with a third-generation cephalosporin (ceftriaxone 100mg/kg/day intravenously).The association of antibiotics does not seem to have any benefit in improving outcomes in previously healthy patients with no risk factors.55 Additionally, adverse effects are less frequent when monotherapy is used.56 This regimen may not be optimal if the patient is under risk of community-acquired, methicillin-resistant staphylococcus infection, multidrug-resistant Gram-negative microorganisms, or if patients have a history of immunosuppression or neutropenia.55,57 For patients with hospital-acquired infection or those in the PICU in refractory shock, due to the high risk of infection by methicillin-resistant staphylococcus and Pseudomonas spp., a combination of vancomycin combined with beta-lactam (piperacillin+tazobactam) is used, or fourth-generation cephalosporin (cefepime) with anti-pseudomonas action. In these patients, the place of hospitalization and the high severity influence the antibiotic regimen decision.
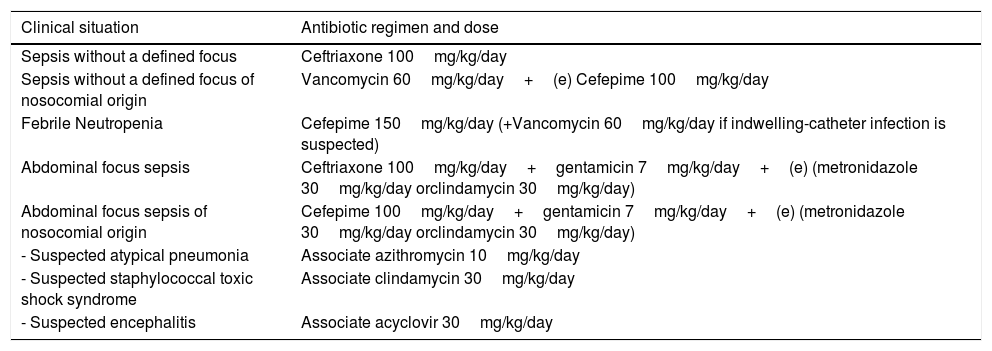
Overall, a maximum treatment time of seven days is used for patients with good evolution and without an etiological agent defined by culture tests. In patients with immunosuppression, neutropenia, or difficulty resolving the focus of infection (e.g., empyema, necrosis, or abscess) the treatment may take longer, usually 10–14 days. A proposal for intravenous empirical antibiotic therapy in the different clinical conditions is shown in Table 1.
Proposed antibiotic therapy regimen for pediatric patients with sepsis.
| Clinical situation | Antibiotic regimen and dose |
|---|---|
| Sepsis without a defined focus | Ceftriaxone 100mg/kg/day |
| Sepsis without a defined focus of nosocomial origin | Vancomycin 60mg/kg/day+(e) Cefepime 100mg/kg/day |
| Febrile Neutropenia | Cefepime 150mg/kg/day (+Vancomycin 60mg/kg/day if indwelling-catheter infection is suspected) |
| Abdominal focus sepsis | Ceftriaxone 100mg/kg/day+gentamicin 7mg/kg/day+(e) (metronidazole 30mg/kg/day orclindamycin 30mg/kg/day) |
| Abdominal focus sepsis of nosocomial origin | Cefepime 100mg/kg/day+gentamicin 7mg/kg/day+(e) (metronidazole 30mg/kg/day orclindamycin 30mg/kg/day) |
| - Suspected atypical pneumonia | Associate azithromycin 10mg/kg/day |
| - Suspected staphylococcal toxic shock syndrome | Associate clindamycin 30mg/kg/day |
| - Suspected encephalitis | Associate acyclovir 30mg/kg/day |
This table refers to an antibiotic regimen suggested by the authors themselves. Dose and regimen may vary according to clinical condition, patient age, and local microbiology.
The routine use of corticosteroids is not recommended in patients with catecholamine-refractory septic shock, despite their potential theoretical benefits, such as improvements in the cardiovascular system and anti-inflammatory actions.58 Current data in the literature are inconsistent to justify their use.59 The basal cortisol value and the adrenocorticotropic hormone (ACTH) response test are not sufficient to diagnose adrenal insufficiency. High or low basal cortisol values are known to be associated with increased mortality in sepsis, and ACTH axis response failure after hormonal stimulation is also a predictor of poor response to exogenous corticosteroid use.60,61 However, many patients can show alterations in these tests without the corresponding clinical repercussion.62 Additionally, important side effects such as hyperglycemia, bleeding, hypernatremia, and suppression of the adaptive cell immune response have been previously reported after corticosteroid use, which cannot be ignored.59,63 It is suggested that only patients with catecholamine-refractory shock who are at risk of adrenal insufficiency or adrenal axis failure due to purpura fulminans, Waterhouse-Friderichsen syndrome, chronic use of corticosteroids, congenital adrenal hyperplasia, hypothalamus/pituitary axis disease, and intubation with etomidate use may benefit from hydrocortisone infusion, which should be started optimally after the collection of basal cortisol level.6 The present authors use 4mg/kg hydrocortisone as loading dose and, thereafter, 2mg/kg 8/8h dose for a maximum of seven days or until vasoactive drug infusion is discontinued.
Mechanical ventilationMechanical ventilation (MV) provides adequate oxygenation and improved tissue perfusion, mainly due to decreased respiratory work in patients with septic shock. Some aspects should be taken into consideration when patients with sepsis or septic shock develop ventilatory failure:
- I)
As a general rule, invasive mechanical ventilation is chosen. The possibility of bronchoaspiration and the hemodynamic instability generated by shock increase the chance of complications if the patient’s airway is not secure. Moreover, in invasive MV it is possible to determine the appropriate tidal volume, avoiding hyperventilation and the consequent decrease in venous return. In very selected cases, such as resource-poor settings, a noninvasive ventilation mode or a high-flow cannula can be successfully used.64
- II)
Although some patients require immediate intubation, such as in cases of coma or apnea, in most cases there is time and it is recommended that volume resuscitation be initiated and, if indicated, also the peripheral infusion of vasoactive drugs prior to the intubation procedure.6 These patients are at high risk of deterioration due to hypoxemia, the action of drugs used in the rapid sequence intubation (RSI), and decreased preload, which generates hemodynamic instability during the intubation procedure and the start of mechanical ventilation.
- III)
The use of atropine as premedication in cases of bradycardia, and ketamine for sedation in RSI are recommended for patients with septic shock. As long as there are no contraindications to their use, this regimen seems to promote better cardiovascular status maintenance.65
- IV)
In resource-poor settings, where there is a high prevalence of diseases such as dengue or malaria, volume resuscitation should be carefully performed, and the clinician should look for signs of early ventilatory failure caused by pulmonary edema.66
ECMO support has been increasingly used with success in pediatrics. In patients with sepsis, the indications are usually ventilatory failure and/or refractory shock.6 Survival rates in patients submitted to ECMO for circulatory instability in septic shock may reach 75 %.35 Historically, these rates were only reached in the neonatal age group.67 The increasing number of centers and the experience of the existing ones in Latin America has contributed to the attaining of these rates in pediatrics.68 ECMO has shown in recent years to be a cost-effective treatment, provided that it is performed by well-trained centers that follow international protocols. The justification for providing resources in this type of treatment is that it considers a population with high mortality, above 80 % when not using extracorporeal support, and in many cases, it enables survival with few sequelae.
ConclusionsIn spite of the great progress in the treatment of sepsis in recent years, with the implementation of protocols in most centers and advanced technologies, as well as management improvements in PICUs, it remains a condition with high morbidity and mortality. A more precise and adapted definition is required for the pediatric population. This would help in early detection, definition of disease stages, and identification of specific therapies for each disease evolution stage. Although these markers are not available yet, good practice should be encouraged in all scenarios, with the implementation of the best evidence-based and resource-adjusted protocols that have shown to be decisive factors in controlling and reducing sepsis mortality.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Garcia PC, Tonial CT, Piva JP. Septic shock in pediatrics: the state-of-the-art. J Pediatr (RioJ). 2020;96(S1):87–98.