Leprosy is an important communicable disease. Despite aggressive control and eradication programs and rapidly developing economies, countries such as India and Brazil continue to be large reservoirs for spread of disease.
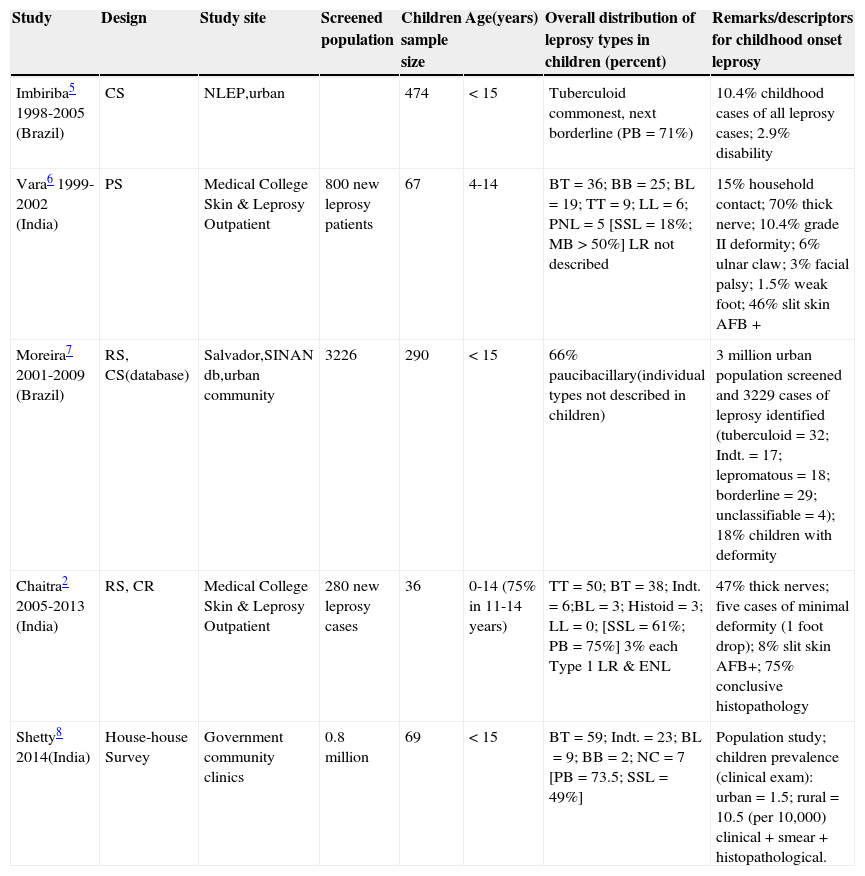
Leprosy amongst children accounts for approximately 10% of all cases in endemic regions. In 2010, the coefficient of detection of leprosy in Brazil was 18.3/100,000 in the general population and 1.3/100,000 in children (< 15 years of age).1 The proportion of children (< 15 years of age) was 12.9% amongst all leprosy cases in a recent prospective study from India.2 53% of the children suffering from leprosy were 10 years old or less in a study from Colombia.3 Leprosy in infants, though rare and often missed, has been reported.4 To understand musculoskeletal (MSK) involvement, it is prudent to review leprosy in pediatric and adolescent subjects (Table 1).2,5–8 Prevalence statistics are confounded by small sample sizes, study site, and methodology.
Selected epidemiological studies of leprosy with special reference to childhood onset disease.
| Study | Design | Study site | Screened population | Children sample size | Age(years) | Overall distribution of leprosy types in children (percent) | Remarks/descriptors for childhood onset leprosy |
|---|---|---|---|---|---|---|---|
| Imbiriba5 1998-2005 (Brazil) | CS | NLEP,urban | 474 | < 15 | Tuberculoid commonest, next borderline (PB=71%) | 10.4% childhood cases of all leprosy cases; 2.9% disability | |
| Vara6 1999-2002 (India) | PS | Medical College Skin & Leprosy Outpatient | 800 new leprosy patients | 67 | 4-14 | BT=36; BB=25; BL=19; TT=9; LL=6; PNL=5 [SSL=18%; MB>50%] LR not described | 15% household contact; 70% thick nerve; 10.4% grade II deformity; 6% ulnar claw; 3% facial palsy; 1.5% weak foot; 46% slit skin AFB + |
| Moreira7 2001-2009 (Brazil) | RS, CS(database) | Salvador,SINAN db,urban community | 3226 | 290 | < 15 | 66% paucibacillary(individual types not described in children) | 3 million urban population screened and 3229 cases of leprosy identified (tuberculoid=32; Indt.=17; lepromatous=18; borderline=29; unclassifiable=4); 18% children with deformity |
| Chaitra2 2005-2013 (India) | RS, CR | Medical College Skin & Leprosy Outpatient | 280 new leprosy cases | 36 | 0-14 (75% in 11-14 years) | TT=50; BT=38; Indt.=6;BL=3; Histoid=3; LL=0; [SSL=61%; PB=75%] 3% each Type 1 LR & ENL | 47% thick nerves; five cases of minimal deformity (1 foot drop); 8% slit skin AFB+; 75% conclusive histopathology |
| Shetty8 2014(India) | House-house Survey | Government community clinics | 0.8 million | 69 | < 15 | BT=59; Indt.=23; BL=9; BB=2; NC=7 [PB=73.5; SSL=49%] | Population study; children prevalence (clinical exam): urban=1.5; rural=10.5 (per 10,000) clinical + smear + histopathological. |
Studies are of general nature and mostly with mixed children adult population (except for the study by Imbiriba et al.), and are not targeted to musculoskeletal/articular involvement. NLEP, National leprosy eradication program; PS, prospective study; RS, retrospective study; CS, cross sectional analysis; CR, case record; SINAN, Notifiable Diseases Information System Database; db, data base; TT, polar tuberculoid; BT, borderline tuberculoid; BB, borderline/dimorphous; BL, borderline lepromatous; LL, polar lepromatous; PNL, pure neuritic leprosy; Indt., indeterminate; NC, not classifiable; SSL, single skin lesion; MB, multibacillary; PB, paucibacillary; LR, lepra reaction; ENL, erythema nodosum leprosum; AFB, acid fast bacillus.
Several aspects of childhood and adolescent onset disease need to be recognized and may be unique. Leprosy in children is a critical reflection of the extent of transmission (microbe) in the community. Children are believed to be the most vulnerable group to Mycobacterium lepra infection. The incubation period of leprosy is generally long (range of two to seven years) and children need an intense pronged contact with a contagious case (usually in a family) to contract the disease. Several adolescent and young adult cases are likely to have contracted the illness as a child. The entire spectrum of leprosy can also be observed in children, though proportions of types may vary. Tuberculoid, borderline, and indeterminate forms predominate. Significant MSK articular involvement is predominantly found in lepromatous forms and lepra reactions, which are somewhat less observed among pediatric cases.9,10 Single hypo-aesthetic skin lesions, paucibacillary forms and low skin smear positivity (acid fast bacillus) appear to be hallmark of childhood cases.
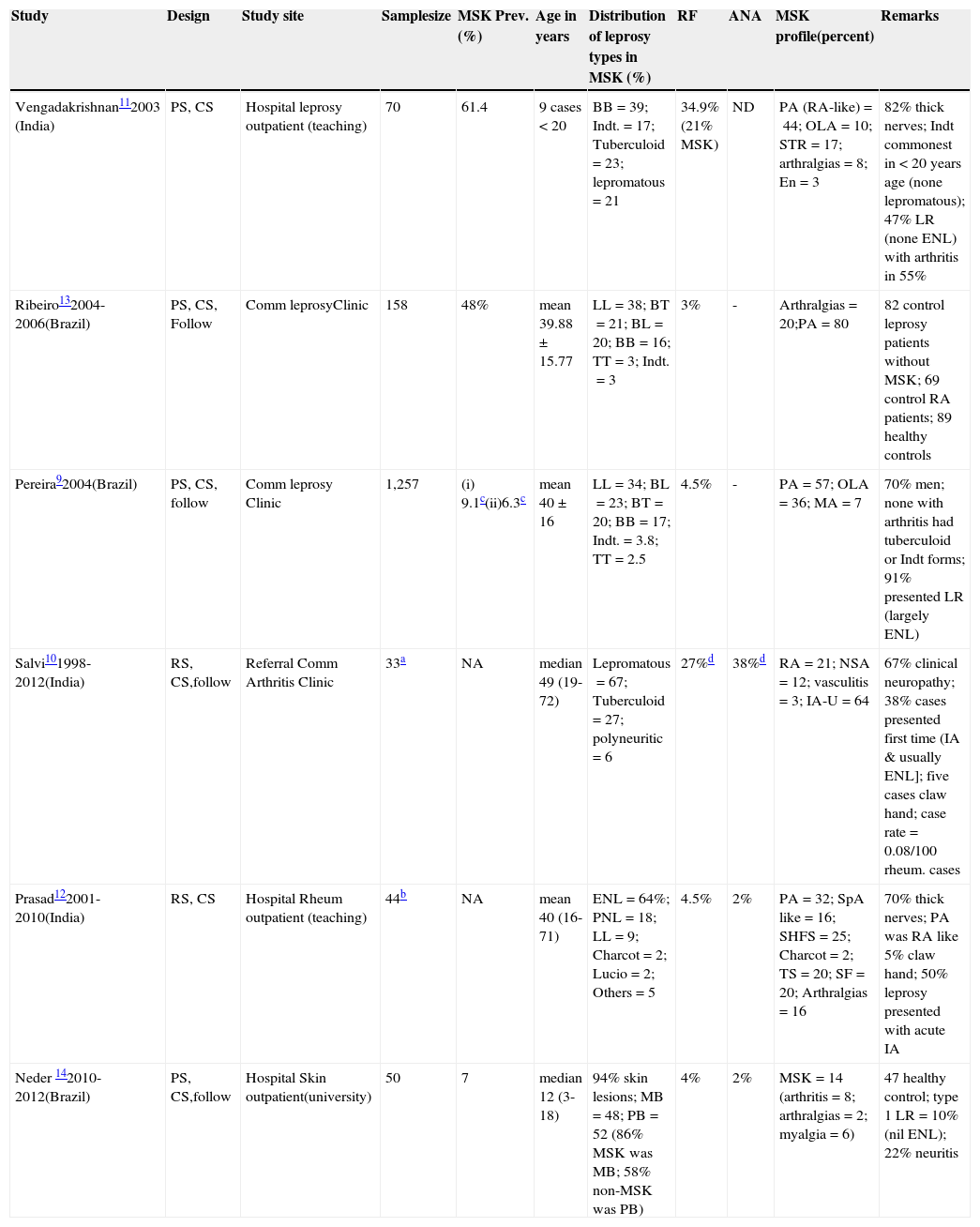
MSK involvement is frequent and variable (Table 2).9–14 The extent is largely influenced by the geographical and endemic factors and study sites; it varies considerably.9,10,13 It is second to cutaneous and neurological manifestations both in non-reactionary and reactionary states. Most of the MSK studies in leprosy are from Brazil and India (Table 2). Overall, MSK data is sparse and woefully dismal in children (Table 2). The antiquity of childhood leprosy was recently established by the discovery of two childhood leprosy cases with bony involvement in ancient skeletal remains.15
Selected studies of leprosy in children & adolescents with reference to musculoskeletal (MSK) articular involvement.
| Study | Design | Study site | Samplesize | MSK Prev.(%) | Age in years | Distribution of leprosy types in MSK (%) | RF | ANA | MSK profile(percent) | Remarks |
|---|---|---|---|---|---|---|---|---|---|---|
| Vengadakrishnan112003 (India) | PS, CS | Hospital leprosy outpatient (teaching) | 70 | 61.4 | 9 cases < 20 | BB=39; Indt.=17; Tuberculoid=23; lepromatous=21 | 34.9% (21% MSK) | ND | PA (RA-like)=44; OLA=10; STR=17; arthralgias=8; En=3 | 82% thick nerves; Indt commonest in < 20 years age (none lepromatous); 47% LR (none ENL) with arthritis in 55% |
| Ribeiro132004-2006(Brazil) | PS, CS, Follow | Comm leprosyClinic | 158 | 48% | mean 39.88±15.77 | LL=38; BT=21; BL=20; BB=16; TT=3; Indt.=3 | 3% | - | Arthralgias=20;PA=80 | 82 control leprosy patients without MSK; 69 control RA patients; 89 healthy controls |
| Pereira92004(Brazil) | PS, CS, follow | Comm leprosy Clinic | 1,257 | (i) 9.1c(ii)6.3c | mean 40±16 | LL=34; BL=23; BT=20; BB=17; Indt.=3.8; TT=2.5 | 4.5% | - | PA=57; OLA=36; MA=7 | 70% men; none with arthritis had tuberculoid or Indt forms; 91% presented LR (largely ENL) |
| Salvi101998-2012(India) | RS, CS,follow | Referral Comm Arthritis Clinic | 33a | NA | median 49 (19-72) | Lepromatous=67; Tuberculoid=27; polyneuritic=6 | 27%d | 38%d | RA=21; NSA=12; vasculitis=3; IA-U=64 | 67% clinical neuropathy; 38% cases presented first time (IA & usually ENL]; five cases claw hand; case rate=0.08/100 rheum. cases |
| Prasad122001-2010(India) | RS, CS | Hospital Rheum outpatient (teaching) | 44b | NA | mean 40 (16-71) | ENL=64%; PNL=18; LL=9; Charcot=2; Lucio=2; Others=5 | 4.5% | 2% | PA=32; SpA like=16; SHFS=25; Charcot=2; TS=20; SF=20; Arthralgias=16 | 70% thick nerves; PA was RA like 5% claw hand; 50% leprosy presented with acute IA |
| Neder 142010-2012(Brazil) | PS, CS,follow | Hospital Skin outpatient(university) | 50 | 7 | median 12 (3-18) | 94% skin lesions; MB=48; PB=52 (86% MSK was MB; 58% non-MSK was PB) | 4% | 2% | MSK=14 (arthritis=8; arthralgias=2; myalgia=6) | 47 healthy control; type 1 LR=10% (nil ENL); 22% neuritis |
Prev., prevalence; Comm., community; RF, rheumatoid factor; ANA, antinuclear antibody; PS, prospective study; CS, cross sectional analysis; RS, retrospective study; TT, polar tuberculoid; BT, borderline tuberculoid; BB, borderline/dimorphous; BL, borderline lepromatous; LL, polar lepromatous; PNL: pure neuritic leprosy; Indt., indeterminate; MB, multibacillary; PB, paucibacillary; LR, lepra reaction; ENL, erythema nodosum leprosum; IA, inflammatory arthritis; U, undifferentiated; SpA, spondyloarthritis like, dominant lower limb; TS, tenosynovitis; SHFS, swollen hands and feet syndrome; OLA, oligoarticular; SF, swollen feet; En, enthesitis; STR, soft tissue rheumatism; Rheum., rheumatology; RA, rheumatoid arthritis; PA, polyarthritis; NA, not available; MA, mono arthritis; ND, not done; NSA, non-specified arthralgias.
In-depth comparisons of case series in Table 2 are not appropriate because of different methods used and several other confounding issues. The overall pattern of MSK disorders may not differ much in children and adolescents. Several studies9,10,12 have confirmed the non-erosive nature of leprosy associated inflammatory arthritis.
Leprosy is predominantly managed by dermatologists. It is likely that only a proportion of patients with significant MSK affection is attended to by a rheumatologist (Table 2). Childhood leprosy with significant arthritis appears to be infrequent. The author visited the source database of a case series report (Table 2).10 One case report found was that of a 19-year-old male (past history of skin psoriasis at 10 years of age) who was evaluated for an acute febrile onset of rheumatoid arthritis (RA)-like polyarthritis (seronegative for rheumatoid factor [RF]), atypical skin lesions, and a few suspicious nodules, and finally diagnosed erythema nodosum leprosum (ENL); around 1,700 rheumatic referral patients (children and adolescents) had been evaluated during the study period (1998-2013). A significant proportion of leprosy associated inflammatory arthritis examined by rheumatologists in leprosy clinic based study9 was reported to closely resemble RA or spondyloarthritis (SSA).
It is against this perspective that the recent study14 by Neder et al. holds merit. Despite a relatively small sample size, it was a well-designed study. Both dermatologists and pediatric rheumatologists were involved. The study provided some important insights. Unlike others (Table 2), that study was truly focused on MSK and arthritis in children and adolescents suffering from leprosy. The prevalence of MSK articular disorder (median duration 12 months) was 14%. Five patients, predominantly borderline leprosy, showed a chronic asymmetric polyarthritis (hands). Despite severe articular pain, none of the children were diagnosed with MSK pain syndromes (like fibromyalgia). A significant functional impairment was observed. Lepra reactions (only Type 1) and significant neuropathy (often silent) were significantly (p<0.05) observed in the MSK group. Although paucibacillary forms were predominant, MSK patients were mostly diagnosed with multibacillary leprosy. The prevalence of RF and antinuclear antibodies (ANA) was low (Table 2), and except for immunoglobulin-M (IgM) anti-cardiolipin antibody (cases=8, controls=6), several other autoantibodies (AAb) were absent or insignificant (< 2%).
In a broad sense, based on personal experience and literature review, MSK articular involvement in leprosy may be classified into the following categories: (i) inflammatory arthritis, usually acute and commonly observed during lepra reactions, it can mimic RA (juvenile idiopathic arthritis in the case of children) or SSA; (ii) inflammatory swollen hands and or feet (similar to ‘remitting seronegative symmetrical synovitis with pitting edema’ syndrome); (iii) neuropathic arthritis or Charcot's joints, generally observed as chronic arthritis; (iv) septic arthritis; (v) non specific arthralgias and myalgias; (vi) soft tissue rheumatism affection including tenosynovitis and enthesitis; (vii) inflammatory multisystem involvement similar to collagen vascular disease – including vasculitis, myositis, purpura fulminans, Lucio's phenomenon, cryoglobulinemia, digital vasculitis/gangrene; and (viii) co-existing chronic forms of arthritis which include RA, SSA, osteoarthritis, and other rheumatological disorders, which are often difficult to totally differentiate from leprosy associated arthritis.
Patients may have an overlap of categories or express different MSK disorders over time. Several studies10,12,16 from rheumatology clinics have unequivocally demonstrated that a significant proportion of leprosy patients may present for the first time with acute severe inflammatory arthritis, often a component of lepra reaction, and are mistakenly treated for prolonged periods with anti-rheumatic drugs (with potentially disastrous consequences). Articular involvement is generally ignored in children and adolescents with leprosy, and differential diagnosis of chronic polyarthritis includes juvenile idiopathic arthritis, acute leukemia, and childhood-systemic lupus erythematosus.14 To summarize, leprosy is the great mimic of the MSK-articular system and can present with protean manifestations requiring a high index of clinical suspicion to make a correct and timely diagnosis.10,12
It is well known that leprosy patients can be flooded with antibodies. From a rheumatological perspective, it is important to recognize false positive AAb; these include RF, ANA, antibody to anti-citrullinated cyclic peptides (a-CCP), antibody to anti-neutrophilic cytoplasmic antigens (ANCA), and antiphospholipid antibodies (APL)/anti-cardiolipin antibodies (ACL). The frequency of seropositive RF (Table 2) has varied considerably, which is due to assay methods, patient selection, and other reasons. In a controlled leprosy study,17 35% and 55.8% of patients tested seropositive for RF and ANA, respectively; 15.8% patients were seropositive for both AAb. There was no correlation between RF/ANA and arthritis (68% prevalence) in the latter study.17 The frequency of seropositive RF was reported11 to vary considerably in different leprosy types (lepromatous > borderline > tuberculoid > Indeterminate).
Ribeiro et al.13 (Table 2) demonstrated a lower prevalence of a-CCP (2.6%) and IgM RF (1.3%). A Mexican adult leprosy study18 reported significant a-CCP in 5.9% patients and RF in 16.8% patients; polar lepromatous (LL) patients had higher a-CCP and RF levels than polar tuberculoid (TT) patients. The low seropositivity of a-CCP can usefully differentiate between RA and leprosy associated inflammatory arthritis.
ANCA, a marker of vasculitis, was reported in leprosy19 and p-ANCA (31% lepromatous, 16% borderline, nil tuberculoid) had a higher frequency than c-ANCA (5% lepromatous only). An Indian study20 (children included in borderline tuberculoid [BT] group) demonstrated a wide spectrum of AAb, including ANA, double-stranded DNA (dsDNA), and ANCA in different leprosy types. The high prevalence of ANCA antibodies (62.5% c-ANCA) in the latter study was intriguing.
An association of APL antibodies with leprosy is well documented. Several patients have presented with typical phenotypes of arterial thrombosis and, uncommonly, anti-phospholipid antibody syndrome has been mistaken for Lucio's phenomenon and vice versa.21
A large-sample genome-wide association study from China demonstrated an unequivocal association of NOD2 locus, HLA-DRB1, LRRK2, TNFSF15 (tumor necrosis factor (TNF)–like molecule), and PARK2 with leprosy.22 The pathogenesis of articular involvement in leprosy is still not fully clear. It is evident that an intense immune-mediated inflammation driven by the unique genetic configuration and cytokine milieu in a susceptible host is at the core of inflammatory rheumatic syndromes and lepra reactions in leprosy. Several pro-inflammatory cytokines play a critical role (Th1 cytokines in type-I reactions, and Th2 cytokines and tumor necrosis factor-alfa in ENL). Direct infiltration of the synovium and peripheral sensory neuropathy leads to destructive arthritis (Charcot's or neuropathic joints). Some molecular mechanisms of immune inflammation appear to be common to infections and autoimmune disorders. Serum concentration of pro-inflammatory myeloid-related proteins (MRPs) 8 and 14 were recently reported to be elevated in patients with juvenile idiopathic arthritis (> 40-fold in systemic onset type) and infections (almost seven-fold in leprosy type-II reactions) as compared to healthy controls.23
Early diagnosis is critical. Skin histopathology is diagnostic, but somehow neglected in clinical practice. Recently, a new serological test for detection of antibodies to the M. leprae-specific phosphoglycolipid-1 was validated, but has not yet been used in routine practice.24 Other new tools validated in pediatric leprosy patients include detecting specific nucleic acid sequences by gene probes and amplification techniques (polymerase chain reaction [PCR]), immunocytochemistry, and in situ hybridization (using skin tissue).25
A recent retrospective study26 of 99 patient records (several children and adolescents included) of leprosy with ENL concluded that in at least two patients, ENL was the direct cause of death. Although not described in detail, almost 70% of the cases appeared to have suffered from significant extra-cutaneous features that included fever, neuritis, arthralgias, arthritis, tenosynovitis, osteitis, dactylitis, orchitis, lymphadenopathy, epistaxis, and proteinurea (>70% had neuritis; each other feature was recorded in <15% cases).
Although leprosy is endemic in some parts of the world, it continues to be a global problem. An important contributory factor is the large number of migrants seeking shelter in developed countries. The diagnosis may be a greater challenge in non-endemic countries because of low awareness. When everything is considered, childhood onset leprosy should ring alarm bells that all is not well with the prevention and eradication programs.
Conflicts of interestThe author declares no conflicts of interest.
Please cite this article as: Chopra A. Rheumatic and other musculoskeletal manifestations and autoantibodies in childhood and adolescent leprosy: significance and relevance. J Pediatr (Rio J). 2014;90:431–6.
See paper by Neder et al. in pages 457–63.