To provide healthcare professional-friendly practical recommendations for early detection of cleft palate-related deformities in newborns and offer an overview of managing these high-prevalent congenital abnormalities.
Source of dataPubMed, SciELO, Lilacs, Cochrane, ScienceDirect, and Scopus databases were reviewed for cleft- and diagnosis-related studies.
Summary of the findingsUnfortunately, the global prevalence of delayed detection of cleft palate-related deformities remains unacceptably high, with over a quarter of cleft palates missed at birth. This delayed identification causes physical and psychological distress for patients and families, including feeding challenges and weight faltering. To improve cleft management, it is essential to adopt routine detailed, in-depth intraoral examination immediately after birth. It is recommended not only to finger-assisted palpate the intraoral structures but also to visually inspect the oral cavity from gingiva to uvula using a wooden tongue depressor and light-assisted examination. With timely diagnosis and referral to specialized care, pediatricians, nurses, speech therapists, and plastic surgeons provide life-changing treatments, including health care maintenance, anticipatory guidance, feeding support, primary surgical reconstruction, and age- and condition-specific protocols.
ConclusionsEncouraging neonatologists and pediatricians, who are the first to examine newborns, to actively investigate the intraoral region for cleft palate-related deformities is instrumental in optimizing therapeutic approaches and prioritizing age-phases in treatment. Their crucial role in early detection and referral can lead to transformative outcomes, impacting not only the future of the newborns by facilitating functional integration into society but also yielding positive effects on families and the health system.
Cleft palate, with or without cleft lip, stands as the most common congenital craniofacial anomaly, constituting a serious burden worldwide.1 The presence of a cleft palate has been associated with preterm birth and underweight.2 Children born with a cleft palate-related deformity also experience difficulty with the complex coordination of sucking, swallowing, and breathing required for adequate feeding.3,4 These babies often have nasal regurgitation, excessive air intake, prolonged feeding time, difficulty creating enough suction to pull milk from a standard bottle or the breast, decreased volume intake, and tire before the oral feed is concluded.3,4 This cleft palate-related physical distress could generate many feeding- and weight-related issues with reduced calorie intake and inadequate nutritional status, especially in the first few days after birth.5 Moreover, it could have a negative impact on babies’ health, including a high risk of lasting growth- and development-related problems, choking with aspiration and secondary pneumonia, and cot death (sudden infant death syndrome).6,7 Feeding-related problems during infancy could also be correlated with amplified risk of cognitive impairment and delayed achievement of major developmental milestones.8 Poor weight gain also renders children with clefts ineligible for surgical candidacy.5 Notably, most of these cleft palate-related problems would truly improve after proper cleft-specialized care with life-changing treatments such as anticipatory guidance, health care maintenance, feeding support, patient-specific preoperative optimization, and primary surgical reconstruction (so-called as palatoplasty or cleft palate repair) provided by pediatricians, clinical nurse specialists, speech and language therapists, and plastic surgeons.9
Importantly, achieving successful management of cleft palate through meticulous, specialized professional-delivered longitudinal care depends on timely, accurate diagnostic evaluation.5,9 A cleft deformity can be diagnosed both before birth through fetal imaging, such as prenatal ultrasound and magnetic resonance imaging of the craniofacial region, or after birth through direct intraoral examination.10,11 However, a high number of delayed diagnoses of cleft palate-related deformities has been published,12-17 as well as observed by the authors in their surgical cleft/craniofacial-focused practices in both high- and low-resource settings.18 Diagnosis of isolated cleft palate (i.e., cleft palate without cleft lip) is difficult antenatally[10,11] and frequently missed, forgotten, or delayed even in the neonatal period,12-17 although a post-natal examination is formally recommended by professional societies across the globe. For newborns with an isolated cleft palate, the lack of proper palatal examination and/or awareness of the cleft could result in a missed opportunity to identify the congenital abnormality at the time of birth and to provide timely feeding support.3,4 Moreover, delaying the proper diagnosis of an isolated cleft palate-related deformity negatively affects the physical and psychological health of both babies and family members.13,14
This review article offers an overview of the challenges and objectives involved in managing cleft palate-related deformities, alongside healthcare professional-friendly practical recommendations for detecting such congenital abnormalities in newborns. The goal is to optimize therapeutic approaches and prioritize age phases in the treatment process. The accompanying schematic drawings and intraoral images displaying the key normal and distorted anatomical components of the palatal region serve as valuable resources for such educational purposes.
Drawing upon the author's experience in treating a high volume of children with cleft deformities in various resource settings (Brazil and Taiwan)18 and the existing cleft-specific literature,10-17 this article also raises a call for action, underscoring the urgency of implementing a detailed in-depth intraoral examination as part of the routine care immediately after birth. In this vital endeavor, healthcare professionals, particularly neonatologists and pediatricians, play a central role as they are often the first to examine newborn babies. As healthcare professionals, the authors are compelled to embrace the critical responsibility in cleft management, undertaking life-changing actions from early diagnosis to a longitudinal rehabilitative process. By achieving timely and successful outcomes for both the babies and their parents, this approach aims to reduce the overall burden of care and seamlessly integrate them into society after functional rehabilitation. Through proactive efforts, the authors can contribute significantly to improving the lives of those affected by cleft deformities and foster inclusive and compassionate healthcare practices.
Literature reviewThis research adheres to the recommendations outlined in the PRISMA statement. To ensure a comprehensive analysis, the authors conducted an extensive literature search on major databases, including PubMed, SciELO, Lilacs, Cochrane, ScienceDirect, and Scopus, utilizing keywords related to “cleft” and “diagnosis” and their synonyms up to May 2023. The search involved combining keywords using the Boolean operators “AND” and “OR.” During the critical appraisal, the authors triaged and selected studies, including original and review articles, systematic reviews, and meta-analyses, based on the information available in the titles and abstracts. The focus of the authors was on identifying the most relevant studies related to the diagnosis of newborns with cleft-related deformities, particularly through intraoral examination immediately after birth. The specific strategy employed for study selection was PCC: Participants = newborns with cleft-related deformity; Concept = diagnosis, especially intraoral examination; Context = care immediately after birth. Furthermore, the authors performed a manual search of the reference lists of the selected articles to identify any additional relevant publications that could contribute to the present research.
Cleft palate spectrumFrom an epidemiological perspective,2,19 the average prevalence of isolated cleft palate has been reported as 0.45 per 1000 live births, with a range from 0.18 to 1.46 per 1000 live births depending on geographic location.20
The cleft deformity has a complex etiology, involving the interaction between environmental, genetic, and epigenetic risk factors,21,22 spanning a wide degree of severity.23 The independent embryological development of the upper lip and alveolar regions from that of the hard and soft palate regions is fundamental to understanding orofacial clefting. The wide spectrum of clinical presentation is the result of its embryological origin, occurring as a failure of fusion of the processes originating the primary palate (comprising the small portion of the palate anterior to the incisive foramen and the alveolus and upper lip regions) and/or the secondary palate (the lack of fusion of the palatal shelves that form the secondary palate, including the hard palate posterior to the incise foramen and the soft palate region) at 4–7 and 8–12 weeks of gestation, respectively.23
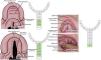
From a classification perspective, children can present with isolated cleft lip, cleft lip with cleft palate, isolated cleft palate (Figure. 1), or its variations. Notably, isolated cleft palate occurs more frequently in females24 and may be associated with other congenital defects, particularly heart disease, and may be part of a syndrome that further complicates children's needs.25,26
(Left) Schematic drawings illustrating cleft palate deformities, encompassing instances featuring (Left, top) solely the soft palate, (Left, bottom) both the soft and hard palates, and (Right) an overt submucous cleft palate deformity. These deformities are categorized using Noordhoff's modified double-numbered Y classification system. Courtesy of Rafael Denadai, M.D.
Children born with clefts face higher mortality rates compared to the normal population, with significant differences within the spectrum of cleft deformity.27,28 Specifically, the mortality rate for isolated cleft palate was found to be 68.1 per 1000 cleft births, significantly higher than the overall mortality rate for all types of clefts, which was 36 per 1000 cleft births.28 Children with isolated cleft palate have a 15 times higher risk of mortality compared to normal infants (death rate of 4.1 per 1000 live births).28 The prompt recognition of a cleft lip deformity, which visibly alters the appearance, at birth instigates further examination of the palatal region and potentially linked structural anomalies. This facilitates a comprehensive approach to early holistic management of potential issues, thereby contributing to a reduction in the mortality rate during the initial months after birth.28,29 However, an isolated cleft palate diagnosis is often overlooked or forgotten.10,11 The absence of external appearance-altering markers or visible signs makes the identification of an isolated cleft palate less obvious and potentially overlooked entirely both during the antenatal and postnatal periods.10-18 This, coupled with other factors discussed throughout the subheadings of this article, underscores the paramount importance of timely and precise detection as well as therapeutic care for isolated cleft palate-related deformities.
There are different types of isolated cleft palate, including complete and incomplete forms, which involve both the hard and soft palate regions or only the soft palate (Figure. 1), respectively. Another distinction exists between overt and occult submucous cleft palates (Figure. 1). The overt submucous cleft palate presents with a classic triad of intact overlying mucosa, which includes a bifid uvula, a bony notch or defect in the posterior portion of the hard palate, and a translucent zone (zona pellucida) in the midline of the soft palate.30 Conversely, the occult submucous cleft palate lacks this classic triad and is therefore more challenging to diagnose.30
Furthermore, a specific variation of isolated cleft palate arises from the failure of proper tongue positioning due to congenital obstruction from an underdeveloped mandible (small lower jaw, mandibular hypoplasia, or micrognathia).31,32 This condition is known as the Pierre Robin sequence and can clinically manifest as a wide U-shaped cleft of the palate, accompanied by a range of feeding- and breathing-related issues, which may vary from mild disturbance to life-threatening distress.31,32 The high prevalence of associated anomalies in the cardiovascular (18.4 %), musculoskeletal (11.2 %), central nervous (7.1 %), urinary (6.1 %), and eye (6.1 %) system justifies a thorough screening for other congenital anomalies in children with Pierre Robin sequence.32
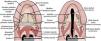
Despite the various types, all cleft palate-related deformities share the same anatomic abnormality (Figure. 2) characterized by the insertion of the palate muscles onto the hard palate rather than the midline soft palate raphe, resulting in velopharyngeal dysfunction. In the submucous cleft palate, the insufficient median fusion of the palatal muscles, hidden under the mucosa of the soft palate, may appear anatomically intact (Figure. 1), making its proper identification more challenging.
Schematic drawings illustrating the (Left) normal anatomy of the palate and (Right) the abnormal anatomy in an isolated cleft palate deformity. (Right) The cleft muscle mass (including the levator muscle) runs almost parallel with the cleft margin before it inserts aberrantly into the posterior border of the hard palate. The anterior tendinous fibers of the tensor attach to the lateral aspect of the posterior edge of the hard palate. The aberrant positioning of the cleft muscle mass, along with an abnormal fusion with the tendon of the tensor muscle, is believed to impair the function of the tensor muscle in assisting with Eustachian tube function. The aberrantly inserted cleft muscle mass results in ineffective contraction and an inability to close the palate against the posterior pharyngeal wall. Courtesy of Rafael Denadai, M.D.
It is crucial to conduct a methodical evaluation of the fetal craniofacial structure, assessing its size, shape, and integrity in different planes during prenatal high-resolution ultrasound examinations. Professional societies (e.g., International Society of Ultrasound in Obstetrics and Gynecology; American Institute of Ultrasound in Medicine; Royal College of Obstetricians and Gynaecologists; Society of Fetal Medicine; the Asia and Oceania Federation of Obstetrics & Gynecology; and Brazilian College of Radiology and Image Diagnosis) have recommended the assessment of the craniofacial region during the second-trimester anomaly scan (screening at 18–22 weeks gestation).33 Additionally, in recent years, there has been a recommendation to examine the fetal craniofacial structure during the first-trimester sonography examination (screening at 11–13+6 weeks' gestation).34 In clinical scenarios where a cleft abnormality is identified, a detailed scan should be performed to search for any additional anomalies, particularly subtle facial, central nervous system, heart, or extremity malformations.31,35 While offering invasive testing to all pregnant women might not be appropriate, invasive prenatal diagnostic techniques could be considered in specific situations to obtain further insights.29,35
In this context, the prenatal diagnosis of a facial anomaly may have an impact on the rate of abortions, as studies have shown differences in abortion-related laws, cultural beliefs, and religious perspectives worldwide.36 While in utero diagnosis of clefts can lead to parental psychological distress,37,38 the authors of this article address this concern by providing regular surgical prenatal counseling.18 During these counseling sessions, up-to-date and relevant information are offered to parents expecting children with cleft-related deformities.18 This comprehensive approach covers a wide range of topics, from coping strategies and fetal anomaly types to postpartum management, feeding approaches, surgical protocols, and the child's life course following reconstructive surgery.18 Potential difficulties in feeding is also addressed, as well as with speech, and hearing, and assist in developing a postnatal care plan while supporting parents in decision-making conflicts. By providing comprehensive educational information, comfort, and support, the authors have observed a considerable reduction in parental anxiety and guilt, thus relieving the psychosocial burden. Parents gain a better understanding of the available treatments and the potential successful outcomes for their infants. The prenatal diagnosis of a cleft, followed by specialized surgical counseling, has also led to decreased rates of hospitalization in pediatric units for newborns, shorter hospitalization durations, and fewer feeding difficulties. This ultimately results in a reduced need for feeding tubes.18
Despite the potential positive impact of in-utero diagnosis of cleft deformities, it is important to note that not all clefts, especially isolated cleft palate, have been diagnosed during pregnancy.10-18 The prenatal detection rate of cleft-related abnormalities can vary significantly based on factors such as the type and severity of the deformity, gestational age, presence of associated anomalies, oligohydramnios, and maternal obesity, sonographer experience, and the use of specific examination techniques and technology.10,11,33,34
A cleft lip (with or without cleft palate) is more likely to be detected during prenatal examinations when there is a visible loss of lip integrity on one or both sides or the presence of a premaxillary protuberance.10,11 When a cleft lip is found, it is essential to define whether there is any cleft palate. However, diagnosing a cleft palate (either midline cleft of the hard and soft palates or soft palate only) in the absence of an affected lip (cleft of the fetal lip) can be challenging and is often missed.10,11 Submucous cleft palate, in particular, is even more frequently missed in prenatal diagnoses.39
Advances in fetal 3D/4D ultrasound and magnetic resonance imaging have the potential to enhance the possibility of detecting isolated cleft palate prenatally, but these advanced imaging techniques do not guarantee a definitive diagnosis, as evidenced by systematic reviews.33,40 In fact, a normal result from these imaging methods does not exclude the possibility of a cleft palate. Furthermore, restricted accessibility and the high cost of sophisticated imaging equipment are challenges faced in various low-resource settings globally, leading to limited availability and long waiting lists for these advanced imaging services.41 Consequently, many children will be born without a defined prenatal diagnosis of cleft palate, as further discussed in the following subhead.
Postnatal diagnosisAlthough there is no international standard, conducting a complete physical examination is considered a good practice in the guidelines for postnatal and newborn care.42,43 Various specialty societies and health organizations(e.g., World Health Organization; American Academy of Pediatrics; National Health Service Litigation Authority; Clinical Negligence Scheme for Trusts; Royal College of Paediatrics and Child Health; National Health Service, England; Ministry of Health, Brazil; and Brazilian Society of Pediatrics)[42,43] recommend that newborn physical examinations should be performed within the first 24 to 72 h after birth. The preferred time for this examination is within the first hour of skin-to-skin contact and before the neonate reaches 24 h of age. The primary purpose of this initial examination is to confirm or exclude any conditions suspected during the prenatal period and to assess the overall health of the neonate. The examination helps identify any additional clinical features that may necessitate specialty referral and further screening tests, including imaging exams.
Unfortunately, despite the recommendations for meticulous physical examination of every newborn, the diagnosis of an isolated cleft-related deformity is still commonly missed worldwide.12-18 There have been reports of significant delays in the diagnosis of isolated cleft palate, ranging from days or weeks to several years (Table 1).12-17 Many neonates are discharged from the birth hospital without a proper diagnosis of isolated cleft palate.12-18,30 As a result, these children later present to healthcare providers with serious feeding difficulties and inadequate caloric intake, leading to failure to thrive.12-18,30
Studies reporting delayed diagnosis of isolated cleft palate. 12-17
Notably, parents can only be properly informed about their neonate's cleft palate-induced issues after an accurate diagnosis is defined. Qualitative research has shown that some healthcare professionals, both within the hospital and the medical community, lacked sufficient knowledge or awareness of cleft palate and its clinical consequences.14 Parents reported that a timely and accurate diagnosis could have prevented nutritional problems, the use of feeding tubes, and prolonged hospital stays or readmissions.14 Many parents expressed feelings of frustration and anxiety due to the delay in diagnosis, as it affected their ability to properly feed their babies. Consequently, this led to poor parental satisfaction with healthcare services, loss of trust in healthcare professionals, and potential legal actions.13
In this setting, the authors strongly advocate for a heightened level of suspicion focused on cleft palate abnormalities among all healthcare professionals, including nurses, neonatologists, and pediatricians, who are involved in neonatal care. The early postpartum period, which is the most critical phase after birth, presents a golden opportunity for timely recognition and diagnosis of intraoral abnormalities. Healthcare professionals with appropriate training should begin by examining the heart and lungs, followed by a systematic head-to-toe evaluation, carefully looking for signs of birth trauma and congenital anomalies. In the craniofacial region, external examination (assessing head size, shape, symmetry, mandible development, ears, external auditory canals, skin, cranial bones, sutures, fontanelles, and eyes) is more accessible than intraoral examination in the small and confined oral cavity of a newborn baby (including the palate, gingiva, tongue, and mucosal structures).
When considering postnatal screening for potential cleft-related deformities, an isolated cleft palate may be missed unless a thorough intraoral examination is conducted (Figure. 3). During the intraoral examination, an assistant can hold the infant in the proper position. It is essential not only to palpate the intraoral structures by gently inserting a clean finger but also to visually inspect the oral cavity using a single-use wooden tongue depressor (or a sterile disposable 1 ml syringe) and a light-assisted visual examination to exclude any congenital anomalies, such as epulis, neonatal tooth, Epstein pearls, vascular anomaly, ankyloglossia, and cleft palate-related deformity. For the palatal region, a comprehensive examination along its complete length, from the anterior to posterior direction (i.e., gingiva to uvula regions), is necessary to differentiate normal anatomy from overt deformities or occult abnormalities (indicative of a submucous cleft palate). It would be better to first perform a complete intraoral inspection and then proceed with an organized physical examination to ensure the intactness of the gingival margin, hard palate, and soft palate, as well as the existence and proper positioning of the uvula.
Intraoral images displaying the spectrum of isolated cleft palate deformity, highlighted by a gray arrow to indicate the escalating severity of abnormalities. Tongue depressors assist in gently retracting the tongue, providing a broader intraoral view. Simultaneously, direct lighting helps to illuminate the intraoral space, ensuring adequate brightness and contrast to observe the specific details of the palatal structures. Courtesy of Rafael Denadai, M.D.
Notable signs of a submucous cleft palate include a very wide or split (bifid) uvula, translucency of the mucosal tissue along the middle of the soft palate, and/or a notch in the back of the hard palate (Figures. 1 and 3). Digital palatal examination to search for the absence of the posterior nasal spine or notching of the posterior hard palate can also be helpful in investigating a submucous cleft palate. Transillumination of the palatal region, which reveals midline translucency and discontinuity of the velar musculature, is another useful maneuver for screening an undiagnosed submucous cleft palate. Since reflux medication has sometimes been mistakenly used to address cleft palate-related milk regurgitation, healthcare professionals should consider milk flow from the nostrils during or after feeding as a red flag for the presence of a cleft palate-related deformity.
Healthcare professionals involved in newborn care must be aware of the anatomical and clinical aspects of these intraoral-specific conditions to properly identify each disorder and facilitate a prompt referral for early assessment by a specialist focused on these disorders. To enhance transparency and communication between healthcare providers and family members, the healthcare professional should ensure that all parts of the postpartum physical examination are considered, and they should record all the findings in the infant's medical record and convey this information to the parents. Importantly, if the intraoral examination is not performed or partially executed for any reason, it should also be detailed in the child's health record and explained to the parents to ensure proper evaluation of the whole palate in the near future.
To ensure the health, safety, and improved quality and equity of care for patients with cleft palate-related deformities, healthcare providers (including neonatology, pediatric, and nurse staff in training or senior positions) should receive continuous and effective training, education, and updates on the importance of identifying intraoral abnormalities soon after birth, as well as the typical treatment pathway.
Postdiagnosis specialized careProper coordination between different professionals is the foundation of delivering holistic cleft care, ensuring that key outcome parameters (such as intact palate, normalized speech and hearing functioning, nasal airway patency, natural oral functioning, good dental and periodontal health, and normal psychosocial development) can be achieved.44 All newborn babies diagnosed with a cleft palate-related abnormality must be referred to an experienced plastic surgeon specialist in cleft care, as recommended by the authors of this article,18 for a comprehensive cleft-focused evaluation and management. Ensuring a timely referral with a dynamic interprofessional collaborative approach is lifechanging and would not only transform the future of babies with a cleft (in terms of feeding,44 speech,45 hearing,46 sleep,47 psychosocial,48 and dentofacial49 developments) but also have a positive impact on families, the health system, and society.18,44
Professionals from various disciplines, including pediatric primary care, pediatric subspecialists, speech therapy, genetics, nutrition, nursing, plastic surgery, dentistry, otolaryngology, and psychology, could assess the child during longitudinal follow-up.18,44 However, not all children with isolated cleft palate require all of these providers. Children with syndromic cleft palate or those who present with associated anomalies may necessitate more escalated care (i.e., the process of recognizing patient-specific needs and timely and effectively communicating [referring to] this to a specialist who is in a position to implement definitive treatment) than children with nonsyndromic isolated cleft palate. Referral and treatment variations may be appropriate based on the specific condition and needs of each individual child. Adopting a needs-based approach with age- and disorder-focused priorities for clinical arrangements with different specialties could reduce the burden of unnecessary appointments and related issues.18,44 This management protocol process advocated and adopted by the authors of this article requires the appropriate identification of patient-specific needs and well-structured coordination, communication, and cooperation among disciplines, along with appropriate interventions.18
Wholly discussing the nuances of the postdiagnosis pathway of a cleft palate-related deformity was beyond the scope of this article. Briefly, the primary focus of cleft palate-related care is on providing information through open communication and education to parents, facilitating the exploration of their feelings and anxieties related to the cleft. Additionally, helping parents understand the condition, treatment options, and comprehensive care required for their child is paramount.18,44,50 It is common for many parents to experience a sense of guilt or responsibility for their child's cleft condition.51 Assuring parents that the cleft deformity is not their fault and explaining the complex etiology of cleft palates can be helpful in alleviating feelings of guilt and providing emotional support.51 Feeding is a primary concern for parents of infants with clefts.51 An initial plan is formulated to manage feeding and monitor growth and development over the early months of the child's life.18,44,50,52 Furthermore, parents are provided an explanation of the treatment pathway and are offered support throughout the primary surgical procedures.18,44,50
Pediatricians play a critical role in the comprehensive care of children with cleft palate-related deformities, collaborating with other specialists and providing essential medical support to ensure the best possible outcomes for these patients.50,53 Firstly, they play a vital role in ensuring timely diagnosis and referral to cleft-focused specialists and disorder-specific specialists for conditions such as heart or kidney abnormalities that may be part of a syndrome or occur independently. Pediatricians also provide ongoing health care maintenance for these children, with a focus on preventive measures such as immunizations, monitoring growth and development, and addressing any concerns that parents may have. They offer age-specific anticipatory guidance, which involves providing information about healthy lifestyles and practices that promote injury and disease prevention. In addition, pediatricians are responsible for acute care, such as treating infections, and act as a connection between the patient and specialty cleft-related care. This ensures that the appropriate timing and order of specific cleft palate-related treatments are followed and encourages compliance with the treatment protocol.
Nutritional management of infants with cleft palate presents a significant challenge.54 Prior to introducing oral feeding, several factors need to be considered, including the type of cleft, the presence of other craniofacial anomalies (e.g., micrognathia), extra craniofacial anomalies, tongue positioning, oral reflexes, and the infant's ability to coordinate suckling. To ensure adequate nutrition and weight gain in infants with cleft palate-related deformities, specific maneuvers, and positioning with assisted breast/bottle feeding and devices (e.g., shaped cups and modified bottles to help draw milk from the bottle with minimal pressure or sucking effort) are commonly recommended by experienced specialists. These interventions aim to eliminate the need for negative intraoral pressure generation during the feeding of expressed breast milk or formula.4,5
Early surgical reconstruction is essential for isolated cleft palate.55-58 Age at cleft palate surgery is a critical predictor of outcome.57,58 Unfortunately, missed diagnoses and delayed referrals leading to late reconstruction12–18 impact final repair quality and speech outcomes.57,58 Older age at surgery (beyond the timeframe of the primary surgery protocol) requires more revisions and extensive speech therapy compared to timely procedures.57,58 Younger age at cleft palate repair is positively linked to better psychosocial and speech functioning.58
The World Health Organization recommends that the burden of care should be decreased. However, the increased number of cleft palate repair-related complications and speech-directed procedures has a negative impact on the burden of care.18,44-48 This leads to a higher number of clinical appointments, treatment episodes, revisionary reconstructive interventions for fistula (abnormal communication between the oral and nasal cavities), and velopharyngeal insufficiency (an anatomical or structural abnormality that prevents complete closure of the velopharyngeal port during speech). This, in turn, results in emotional and psychological hardship, as well as elevated direct and indirect costs.18,44-48 To optimize cleft care, various therapeutic protocols have been proposed.18 Significantly, the protocol followed by the authors in the current article,18,44,56,59,60–62 which involves conducting primary cleft palate surgery (modified double-opposing z-plasty method) at 9 months of age, has demonstrated favorable outcomes when compared to other published protocols. The authors’ approach effectively reduces the burden of care by minimizing morbidity, as evidenced by a decreased need for grommet tube insertions. Furthermore, it positively influences maxillary growth by mitigating scar-induced disturbances and results in a lower occurrence of revisionary surgical interventions, particularly with a very low correction rate for fistula and velopharyngeal insufficiency.18,44,56,59,60–62
ConclusionFailure to diagnose an isolated cleft palate can lead to feeding difficulties, a fussy baby, and poor weight gain. Since prenatal identification of an isolated cleft palate remains challenging, a comprehensive intraoral examination as part of the full newborn physical check can significantly contribute to the accurate diagnosis and timely referral of babies with isolated cleft palate-related deformities, ensuring appropriate early intervention. The authors hope that this article will guide healthcare professionals in their journey to deliver high-quality cleft care, a challenging yet rewarding endeavor.
Authors’ contributionsRafael Denadai and Lun-Jou Lo conceived the idea for the manuscript, reviewed the literature, and drafted the manuscript.