Identify potential barriers, delays, and missed opportunities in the prevention and diagnosis of childhood TB.
MethodsScoping review according to the PRISMA extension. The definitions considered for the selection followed the acronym PCC where the population (P) is children under 18 years of age with TB disease, the concept (C) refers to missed opportunities for prevention and diagnosis, and context (C) is defined as a diagnosis of TB disease. The authors searched systematically in the databases; VHL/Lilacs, Medline via PubMed, Cochrane, Scopus, and Web of Science, without date or language limitation.
ResultsSeven studies were included. In developed countries, with low disease burden, the main shortcoming is the delay in diagnosing bacilliferous adults in contact with young children. This problem is concentrated in the portion of the population with socioeconomic vulnerability. In underdeveloped countries, with a high burden of disease, the biggest challenge is tracking children who come into contact with bacilliferous patients.
ConclusionsThere are still many missed opportunities in the prevention and diagnosis of childhood TB. The positive legacy of the COVID-19 pandemic should be taken advantage of and the encouragement of scientific development in the management of infectious diseases should be taken.
Despite its ancient origins, tuberculosis (TB) remains a major public health concern. The pediatric population accounts for approximately 11 % of the global disease burden, implying that approximately 1.1 million children suffer from TB every year.1 In 2022, 78,057 new cases of TB were diagnosed in Brazil, and 3.5 % (2703) occurred in children under 15 years of age, proportions recorded in the historical series from 2012 to 2022.2
In 2022, 78.057 new cases of TB were diagnosed in Brazil, with 3.5 % (2703) occurring in children under 15 years of age. This proportion represents a historical number of cases between 2012 and 2022. In the pediatric sample, patients from 0 to 4 years correspond to 37.5 %; from 5 to 10 years represent 22 % and between 10 and 15 years correspond to 40.5 % of cases. Adolescents from 15 to 18 years old are in another group, and the incidence of cases is 6 % of the general population.
Diagnosing TB in children is challenging because of several factors, including the paucibacillary nature of the disease, non-specific symptoms, clinical similarity with other childhood diseases, and difficulty in collecting samples for diagnosis. As per a 2020 estimate, 63 % of children and adolescents under 15 years of age with TB were not notified or did not have access to diagnostic and treatment services; the proportion is even higher (72 %) for children under five.1
The global report of the World Health Organization (WHO), with data updated in 2022, reinforces that the global goals defined by the “End TB” strategy for controlling the number of cases and deaths from the disease are far from being met. A reduction in the numbers diagnosed, in access to essential TB services, and, consequently, an increase in the number of deaths was observed in the last two years.1,3 The coronavirus disease 2019 (COVID-19) pandemic had a negative impact on access to diagnosis and reduced investments and funds destined for essential TB services.3
In addition to TB disease, it is important to identify infected children who have shown no symptoms. Mycobacterium tuberculosis infection without the manifestation of active disease is called latent tuberculosis infection (LTBI), and treating children in this phase, in addition to improving the prognosis and reducing individual morbidity and mortality, also substantially reduces the community transmission of the disease.4-6 Most children were infected through recent household contacts, in the last two years, mainly parents and caregivers. This reflects the fact that every child with TB should be considered a sentinel event and indicative of recent disease transmission.
There are well-established protocols for contact tracing and treatment of LTBI in children.5,6 In Brazil, guidelines structure LTBI surveillance into five pillars: (1) identification of people who are more likely to have LTBI or with higher risks of illness; (2) identification of persons with LTBI; (3) correct indication of treatment (4) notification and (5) monitoring of treatment.6
Despite several advances in recent decades, many difficulties still need to be overcome in the diagnosis of LTBI as well as in the diagnosis of TB disease. This scoping review aimed to systematically map the research conducted in this area and identify the potential barriers, delays, and missed opportunities for the prevention and diagnosis of childhood TB.
MethodsThis study adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) extension for scoping reviews (2018).7,8
To achieve the proposed objective of the review, the guiding question was: What is the scientific evidence of missed opportunities in the prevention and diagnosis of TB in pediatric patients?
Following the acronym PCC, the population (P) is composed of children and adolescents under 18 years of age with TB disease; the concept (C) refers to missed opportunities such as contact with adults with TB and failure in LTBI investigation or treatment; and the context (C) is defined as the diagnosis of TB disease.
The protocol was registered at https://www.protocols.io (DOI: dx.doi.org/10.17504/protocols.io.x54v9dwwmg3e/v1).
Systematic searches were undertaken between July 2022 and November 2022 in the databases VHL/Lilacs, Medline via PubMed, Cochrane, Scopus, and Web of Science. The following descriptors were used: “tuberculosis”, “missed opportunities” “diagnosis”, “prevention”, “case discovery”, and “gap”.
The final quest in each base is presented below:
- •
VHL/Lilacs: (diagnóstico OR diagnosis OR diagnostic OR "Oportunidade Perdida" OR "Oportunidades Perdidas" OR "Missed Opportunity" OR "Missed Opportunities") AND ("Prevenção de Doenças" OR "Disease Prevention" OR "Prevención de Enfermedades" OR "Prévention des Maladies" OR prevenção OR prevention) AND (tuberculose OR tuberculosis) AND (pediatria OR pediatrics OR pediatría OR pédiatrie OR criança OR child OR niño OR enfant) AND (db:("LILACS" OR "IBECS" OR "BINACIS" OR "CUMED" OR "BIGG" OR "WHOLIS" OR "MULTIMEDIA" OR "BDENF" OR "LIPECS" OR "SES-SP" OR "colecionaSUS" OR "PAHO" OR "SOF" OR "campusvirtualsp_brasil" OR "PAHOIRIS" OR "SMS-SP")
- •
Medline via PubMed / Scopus / Web of Science / Cochrane: (Diagnosis OR "Missed Opportunity" OR "Missed Opportunities") AND ("Disease Prevention" OR Prevention) AND (Tuberculosis) AND (Pediatrics OR Child)
The inclusion criteria were originally published observational studies that included children and adolescents (0–18 years) with TB disease that addressed missed opportunities in the prevention and diagnosis of the disease. The searches were not restricted to any language or date of publication.
Case reports, abstracts, consensuses, letters to the editor, and review articles were excluded. Articles that included adult populations or populations with LTBI were not considered.
Studies were selected after screening the title, abstract, or full text, if necessary, by two independent reviewers. In cases of doubt or disagreement, a third reviewer was consulted.
Thereafter, the full text of all selected studies was read for data extraction. The reviewers aimed to identify and establish the variables presented in the literature as missed opportunities for the diagnosis of TB.
For data summarization, each article was tabulated in accordance with PRISMA guidelines: author, year, number of studied populations included, missed opportunities, frequency of cases in each of the defined missed opportunities, current statistics, and conclusion.
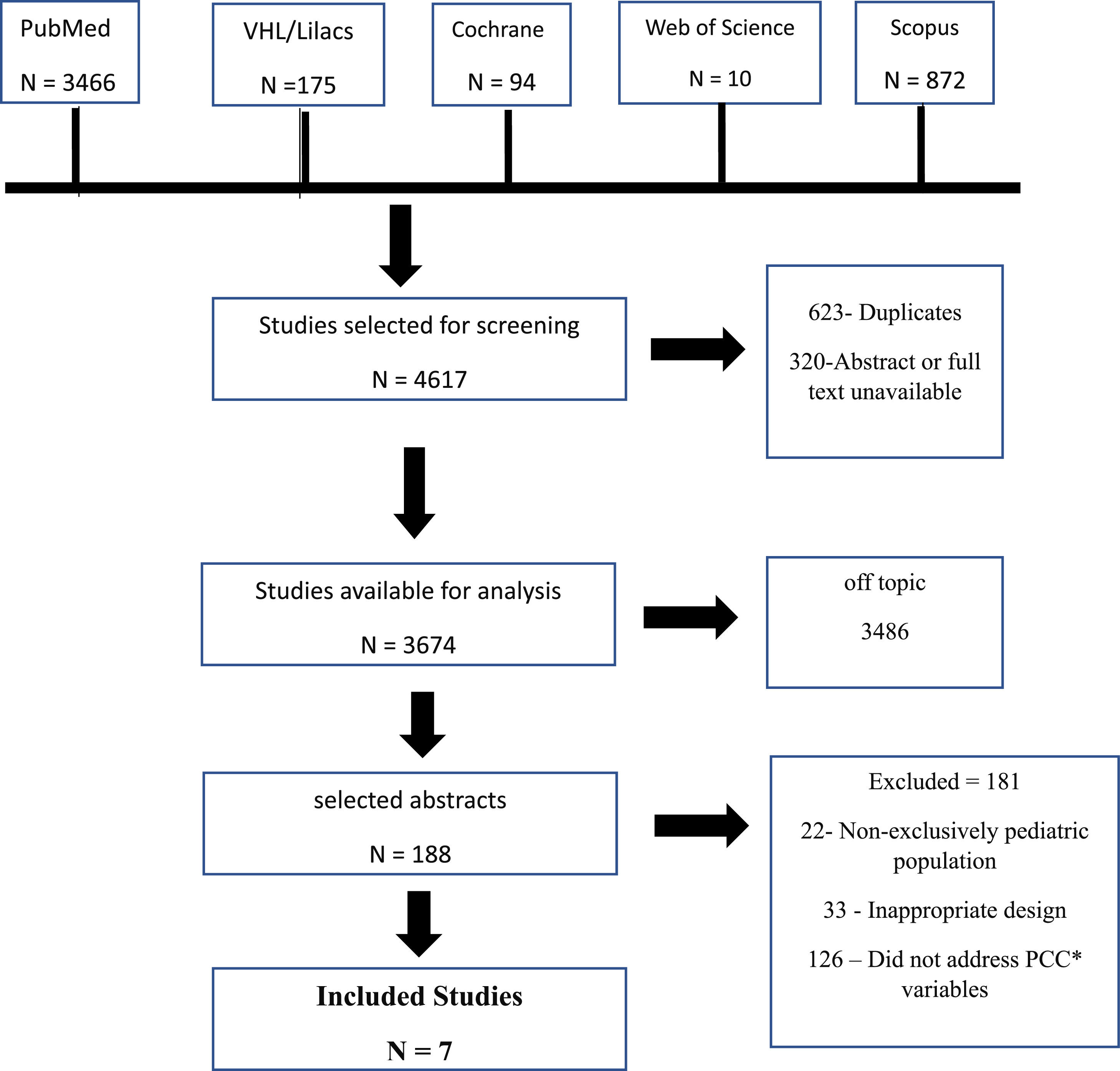
ResultA bibliographic review conducted from July 2022 to November 2022 identified 4617 studies. After screening and analyzing the inclusion criteria, seven articles published between 1992 and 2022 were selected (Figure 1).
Selection of studies evaluating the missed opportunities in the prevention and diagnosis of pediatric tuberculosis. * PCC P (population) = is composed of children and adolescents under 18 years of age with TB; C (concept) = refers to missed opportunities such as contact with adults with TB and failure in LTBI investigation or treatment; C (context): is defined as the diagnosis of TB disease.
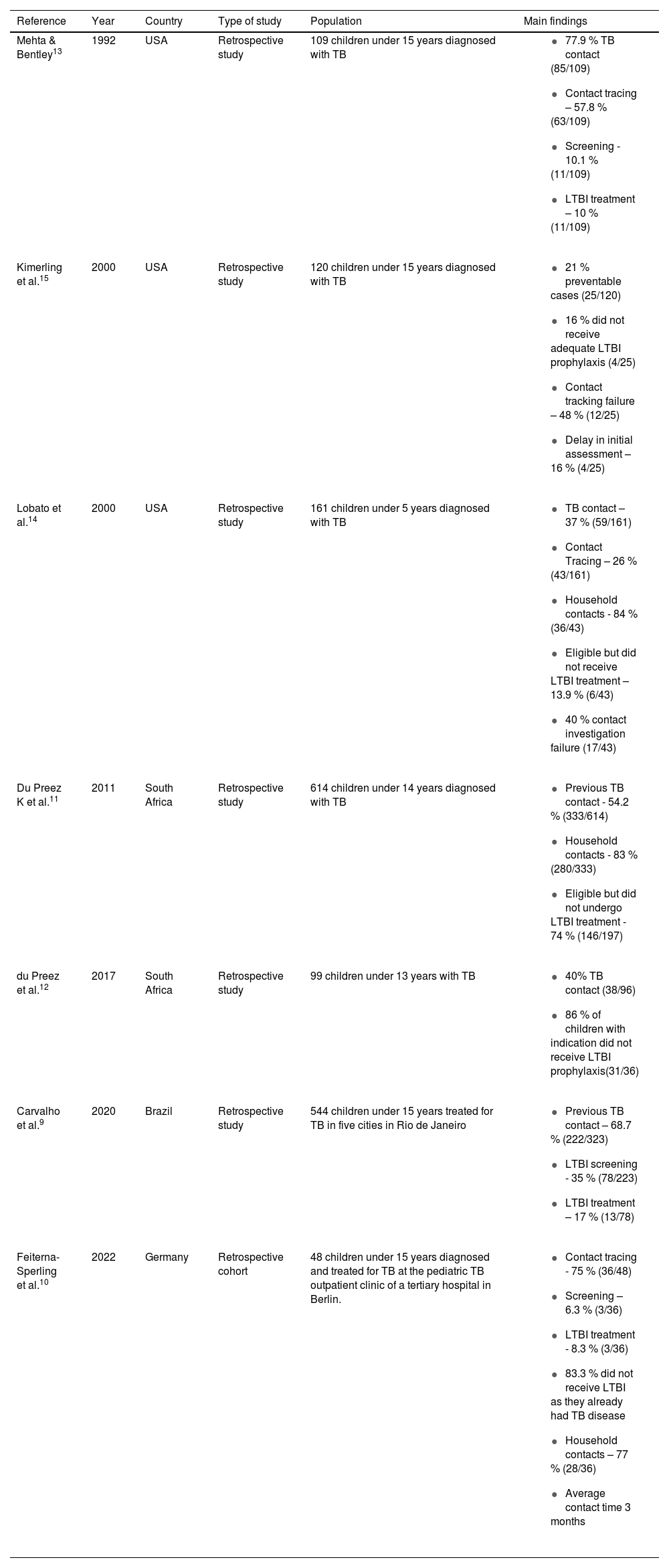
The seven studies summarized in Table 1 cover the year of publication, geographical location, study design, population, and main findings. As seen from the Table, three studies were carried out in the United States, two in South Africa, and one each in Brazil and Germany.
Characteristics of studies that evaluated missed opportunities in the prevention and diagnosis of pediatric TB, scoping review.
| Reference | Year | Country | Type of study | Population | Main findings |
|---|---|---|---|---|---|
| Mehta & Bentley13 | 1992 | USA | Retrospective study | 109 children under 15 years diagnosed with TB |
|
| Kimerling et al.15 | 2000 | USA | Retrospective study | 120 children under 15 years diagnosed with TB |
|
| Lobato et al.14 | 2000 | USA | Retrospective study | 161 children under 5 years diagnosed with TB |
|
| Du Preez K et al.11 | 2011 | South Africa | Retrospective study | 614 children under 14 years diagnosed with TB |
|
| du Preez et al.12 | 2017 | South Africa | Retrospective study | 99 children under 13 years with TB |
|
| Carvalho et al.9 | 2020 | Brazil | Retrospective study | 544 children under 15 years treated for TB in five cities in Rio de Janeiro |
|
| Feiterna-Sperling et al.10 | 2022 | Germany | Retrospective cohort | 48 children under 15 years diagnosed and treated for TB at the pediatric TB outpatient clinic of a tertiary hospital in Berlin. |
|
TB, Tuberculosis; LTBI, latent tuberculosis infection.
The main missed opportunities identified in the studies were failures in screening the contacts of bacilliferous patients, identifying symptomatic infected children in routine consultations, and the institution of LTBI treatment. Failure was also observed in the routine screening of risk groups for the development of active TB, regardless of a known contact history, as in the case of individuals living with HIV.
Exposure to Mycobacterium tuberculosisThe percentage of children with known contact with adults diagnosed with TB was largely diverse (37 % to 85 %). Among the three studies that reported this, over 75 % were classified as household contacts and most children were mainly with parents and caregivers.
The study by Carvalho et al. addressed children diagnosed with TB in Rio de Janeiro, Brazil.9 Among the 544 children, 71 % underwent follow-ups at a basic health unit and 29 % at tertiary reference centers. A high proportion of children (68.7 %) had previous contact with adults with TB. Feiterna-Sperling et al. evaluated 48 children diagnosed with TB treated at a tertiary hospital in Berlin; in 91.7 % of the cases, the child or at least one of the parents had left in another country. In 77 % of the cases, the index case was a family member.10
The South African study by Du Preez et al. (2011), which included 614 children with a confirmed microbiological diagnosis of TB, reported 54.2 % previous contact with an adult with TB, 83 % of whom were family members.11 Another study by the same author in 2017 showed that 40 % of the children had known prior contact with an adult with TB.12
In the American study by Mehta, the index case, the source of childhood TB, was identified in 78 % of 109 cases.13 Notably, 21 % of the childhood TB cases in the study population could have been avoided. In the study by Lobato et al, also undertaken in the USA, the rate of children with previous known contact with an adult with TB was 37 %.14 Among them, 84 % were household contacts, and 44 % had some risk factors associated with the disease, such as alcohol/drug abuse, unemployment, former street dwellers, or health professionals.
Contact tracing and screeningAs regards the investigation of children with known previous contacts, the disease screening rate ranged from 35 % in Brazil to 75 % in Germany. In the German study, children diagnosed with TB disease were identified in two ways: active search for contact tracing or triage (81.3 %) and passively through the patient's voluntary search for the health service to assess symptoms (18.7 %).10 In Germany, the screening of risk groups for TB follows the WHO guidelines for countries with low disease burden, and systematic screening is recommended for individuals living with HIV, immunosuppressed patients or those with chronic diseases, prisoners, health professionals, immigrants from countries with a high TB burden, homeless people, and illicit drug users.
Screening should be performed systematically by identifying symptoms (cough, fever, weight loss, and prostration) and using chest radiography. Confirmation of M. tuberculosis infection can be performed using the tuberculin skin test (TST), or Interferon Gamma Release Assay (IGRA).
The study by Mehta showed that of 109 cases of childhood TB, 63 (57.8 %) were found through contact tracing, 35 (32.1 %) were identified passively through the investigation of symptoms, and 11 (10.1 %) were detected by routine screening.13 In the study by Lobato et al, only 26 % of the 161 children were identified through contact tracing, and 91 % of the infected children referred for screening already had TB disease at the first assessment.14 It is estimated that 40 % of TB disease cases could have been prevented.
In the report by Kimerling et al, including an Alabama cohort, based on contact tracing failure, delay in the initial assessment, or noncompliance with preventive therapy in eligible patients, an identical rate of 21 % of preventable cases was described.15
LTBI treatmentAmong the children with an indication for LTBI treatment, 13.9 % in the US and 86 % in South Africa did not receive the indicated preventive treatment. In the Brazilian study, previous treatment for LTBI was performed in only 17 %.9
In the 2011 South African study, approximately 74 % of children under five years of age eligible for chemoprophylaxis did not receive preventive treatment. Among them, 25 % developed disseminated TB, and 5.1 % died.11 In 2017, another study undertaken by the same authors showed that only 14 % of children eligible for preventive therapy received adequate treatment.12
DiscussionChildren are vulnerable to TB, which is systematically neglected and must be prioritized. To this end, several steps need to be addressed: tracing children who have been exposed to M. tuberculosis (contact tracing), identifying those at greater risk of developing TB by screening risk groups, detecting and treating LTBI in these children, implementing LTBI treatment, identifying sick children through routine screening (childcare), and developing and expanding public health strategies for appropriate fulfillment of these steps.16
Carvalho et al. demonstrated that despite the high rate of known previous contact with TB, only 17 % of children were diagnosed and treated for LTBI, suggesting a failure in the identification and treatment of LTBI.9 A study carried out in eight countries with a high burden of the disease showed that contact tracing helped to detect new cases of TB disease in 12 % of contacts and the prevalence of LTBI was 72 %.4 Therefore, screening household contacts for TB is a high-yield and cost-effective strategy.
In European countries with a low disease burden, contact tracing appears to occur more effectively. In the study by Feiterna-Sperling carried out in Berlin, Germany, 81.3 % of the 48 children were identified through an active search by contact tracing or screening.10 Among them, 83 % did not receive treatment for LTBI because they already met the TB disease criteria at the time of the first assessment.
Although the average time between the diagnosis of the source case and the assessment of the child in contact occurs early, with an average of approximately 18 days, the time between the diagnosis of the source case and the onset of symptoms was on average 3 months. With prolonged exposure and a high bacterial load associated with an increased risk of TB transmission, the biggest challenge in this population is the timely identification of adult cases. Even if it is not possible, in most cases, to detect TB in its latent infection phase, the screening program allows the diagnosis of TB disease in its initial phase, contributing to a better prognosis, reduction of morbidity and mortality, and interruption of the chain of TB transmission.
These challenges are greatest in underdeveloped countries with high TB burdens. The shortage of financial resources hinders the availability of tests that detect M. tuberculosis (tuberculin skin test or IGRA), leads to a shortage of trained professionals for contact tracing, and even results in difficulty in conducting and interpreting imaging tests.
According to Du Preez et al, in two studies carried out in South Africa with an interval of 6 years (2011–2017), the number of children eligible for TB preventive therapy who did not receive chemoprophylaxis remained above 70 %.11,12 WHO has considered the identification and treatment of LTBI the cornerstone of efforts to eliminate TB by 2030. It is estimated that >80 % of adults and children at risk of M. tuberculosis infection do not complete the care cascade.17 A scoping review was conducted in 2021 identifying the following barriers to the care of children evaluated and treated for LTBI; failure to identify children at high risk for LTBI, low availability of tests to diagnose the infection, refusal of parents to perform the test, or adherence to treatment due to the stigma of the disease or fear of adverse effects, and loss to follow-up.18
The unavailability of tests that detect M. tuberculosis is a reality in underdeveloped countries. In the Brazilian study, the tuberculin skin test was performed in 73 % of the patients, and the positivity was 79.6 %.9 According to the WHO recommendation, the test is not essential for the diagnosis of LTBI in high-risk situations, and chemoprophylaxis should be administered to children in contact with TB patients even when the test is not available.17 This guideline is based on the fact that preventive treatment is well tolerated in young children, and due to the paucibacillary nature of the disease in the pediatric population, the risk of inducing resistance to antituberculostatic drugs is minimal. The situation is different in adolescents who generally have multi-bacillary disease, requiring a better investigation to rule out TB disease before LTBI treatment.17
Tuberculosis in adolescence is a topic that is rarely discussed. In the present review, Carvalho et al. report 8.3 % of patients aged 10 to 15 years but do not perform any specific analysis for this subgroup. Feiterna-Sperling reports 33 % of patients aged between 10 and 15 years but also does not report any difference observed in relation to younger children. The literature describes evolution and clinical manifestations like those seen in young adults. The specific characteristics of this age group, such as endocrine-metabolic changes and psychosocial issues, are generally not valued.
A USA study conducted in 1992 identified 21 % of TB disease cases in children in the studied population that could have been avoided considering failure or delay in contact tracing, incomplete evaluation/workup, and loss of follow-up.13
Two North American studies conducted in 2000 were analyzed. In one referring to the population of Alabama, an identical rate of 21 % of preventable cases was described.15 In the other 37 % of the patients (72.8 %) had known prior contact with a TB carrier.14 However, due to failure to investigate the source cases, delay in investigating contacts, incomplete assessment due to the unavailability of tuberculin skin testing and radiological examination, or inadequate/missing treatment for LTBI, it is estimated that 40 % of TB cases could have been prevented. Evaluating the time elapsed since the first study, it was observed that the challenges encountered in the 11990s remain in force in coping with TB in children even today.
A systematic review and meta-analysis conducted in 2022 analyzed interventions in the TB care cascade. Relatively simple interventions, such as education, counseling, and incentives, could substantially reduce the burden of the disease. In practice, this translates to updating clinical guidelines, integrating care, providing tools and resources to improve case detection, training staff, providing TB educational materials for patients, and involving laypeople in service delivery.19 These measures sensitize health professionals and raise attention and strong suspicion for the diagnosis of TB in children in routine pediatric consultations, which is essential for early diagnosis.
A 2023 systematic review estimated the number of children screened to detect a single case of TB disease (NNS).20 Most studies have been conducted in countries with a high disease burden, and mainly screening for symptoms was undertaken. The estimated NNS in healthcare settings (109) was lower than the estimated NNS in the community (1117) and school settings (464). Screening in child health services, such as outpatient clinics and wards, is a potential opportunity to increase the diagnosis of childhood TB.20
The North American studies identified racial and economic barriers to TB care. One study observed that all patients who presented with missed opportunities were black,15 while the other demonstrated that 44 % of the source cases of childhood TB had social risk factors, such as unemployment or substance abuse.14 Despite being a disease present in all social spheres, the negative impact of TB on those with greater socioeconomic vulnerability is undeniable, considering overcrowding, poor ventilation, food insecurity (malnutrition), and indoor and outdoor air pollution. Therefore, increased attention and public health policies aimed at this population are required.
Summary of evidenceThere are still many missed opportunities for preventing and diagnosing childhood TB.
In developed countries with a low disease burden, the main shortcoming is the delay in diagnosing bacilliferous adults who are in contact with young children. In this context, the problem is mainly concentrated in the portion of the population with greater socioeconomic vulnerability, which includes Afro-descendants and immigrants.
In underdeveloped countries with a high disease burden, the greatest challenge is tracking children who are in contact with adults with TB. Closing persistent gaps in LTBI management and the early diagnosis of TB are paramount. The main difficulties encountered were notification of the index case, identification of contacts and patients with LTBI, recommendation and adherence to preventive treatment, available workups, high suspicion of professionals, and early diagnosis.
To intervene in this process, it is necessary to improve public health policies through financial investment, the availability of new technologies, professional training, and the awareness of parents and guardians. The positive legacy of the COVID-19 pandemic should take advantage of community mobilization, and rapid scientific development in the management of infectious diseases should be encouraged.
LimitationsThis study has some limitations. A quality assessment of the articles was not performed because of the heterogeneity of the studies.
The time interval of publication of the included articles was long (1992 to 2022). Thus, the data presented may have been influenced by the incidence of the disease and the heterogeneity of the protocols in force at different times.
Most articles did not present sophisticated statistical analyses and described only the percentage of missed opportunities. It is necessary to conduct new original studies with greater methodological rigor so that it is possible to carry out a systematic review.