Evaluate the effectiveness of a children's soap with physiological pH in maintaining cutaneous pH and moisture of the newborn (NB)’s skin after the first bath.
MethodsRandomized, controlled and double-blind clinical trial in a rooming-in of a tertiary maternity hospital in southern Brazil with 204 newborns > 34 gestational weeks. Gestational and obstetric history was evaluated, and newborns were randomized into two groups according to the product applied in the bath: the control group (CG), which used common liquid soap with pH 7.0 and experimental group (EG), which used children's liquid soap with pH 5.8. Evaluation was made immediately before and after bath with skin pH measurement, corneometry and clinical parameters (erythema, scaling and moisture), on the forehead, abdomen and thigh.
ResultsThere was no difference between groups regarding gestational, obstetric and family history (p > 0.05). In CG, skin pH increased in the abdomen and thigh (p < 0.05). In EG there was an improvement in clinical parameters after bathing with: increased moisture, less erythema and less scaling (p < 0.05). On the forehead, there was a significant increase in pH after bathing (p < 0.001) similar in both groups, although no use of soap. There was no difference in corneometry between groups after bathing.
ConclusionChildren's liquid soap with physiological pH maintained the acidic skin pH and moisture of the newborn's skin after the first bath, which reinforces the importance of using products with physiological pH in the hygiene of newborns. Registration number RBR-9ky84vd.
Newborn (NB) skin needs essential care to maintain the integrity of the skin barrier and its protective acid mantle.1,2
Daily hygiene such as the use of soaps and surfactants during the bath can modify the characteristics of a newborn's skin, especially its pH.1,3,4
Due to the importance of the role of soaps and surfactants in the daily hygiene of newborns and the lack of consistent studies on the performance of these products on the barrier function, this study has the purpose of evaluating the effectiveness of a children's liquid soap with physiological pH to maintain cutaneous pH and skin moisture, when compared to a common liquid soap with pH 7.0.
MethodsDesignThis is a randomized, controlled, double-blind clinical trial, approved by the institution's Ethics and Research Committee under opinion number 3.348.559 and registered in the Brazilian Registry of Clinical Trials with the code RBR-9ky84vd.
SettingNewborns admitted to the rooming-in accommodation (RIA) of a tertiary maternity hospital in southern Brazil were evaluated from August 2018 to March 2019.
PatientsIn order to estimate the minimum sample size, it was applied a formula to compare two groups according to quantitative variables, considering the significance level of 5%, type II error of 10%, the magnitude of the effect of 20%, with an indication of 100 participants in each group, giving 90% test power.
NBs over 34 weeks of gestational age were included, whose guardians signed the Informed Consent Form (ICF), and NBs with skin solution of continuity, in phototherapy or with genetic diseases were excluded, totaling 18 NB (Supplementary Material 1).
Patients were selected in a probabilistic manner with electronic randomization performed by www.randomizer.org, where all participants were included in a set of 204 patients, in a single set, with a range of numbers from 1 to 2. Thus, they were allocated into 2 groups, number 1 was represented by the Control Group (CG) and number 2 by the Experimental Group (EG) (Supplementary Material 1).
InterventionNewborn care routine recommended by RIA from birth to bath time was maintained. Evaluations were carried out on the first day of life, after the newborn's thermal and respiratory stabilization (not before 6 hours of life), between 8 and 9 am. Skin conditions (clinical parameters of moisture, scaling, and erythema), pH and corneometry were evaluated immediately before and after the bath. Bath was given to Control Group (CG) with common liquid soap with pH 7.0 and to Experimental Group (EG) with liquid children's soap with a pH of 5.86. Products were packaged in bottles identified as A or B, in a blind manner for the nurse and the researcher.
Standardization of the bathing procedureAverage bath water temperature was maintained at 37 °C and room temperature at 22 °C. Bath was carried out by a trained nursing team to perform the same technique regardless of the soap used:
- 1.
Naked newborns were wrapped in a soft towel.
- 2.
Their face was washed with water from the basin, without soap.
- 3.
With the body covered, their scalp was washed with soap, which was applied to the nursing professional's hand and then to the newborn's skin.
- 4.
The scalp was rinsed and dried with a soft towel.
- 5.
NB was partially immersed (lower limbs and part of the abdomen) in basin water and their entire body was washed with the soap spread by the hand of the nursing professional.
- 6.
The amount of soap applied throughout the body was standardized at 6 pumps.
- 7.
Soap was rinsed with water that was present in the basin and the newborn was taken out and dried with a soft towel.
In EG, a children's liquid soap with pH 5.86 was used, which does not contain sodium lauryl sulfate (SLS), contains water, coco glucoside, cocoamidopropyl betaine, acrylate/C10–30 alkyl acrylate crosspolymer, sodium benzoate, glyceryl oleate, p-anisic acid, sodium hydroxide, phenoxyethanol, perfume, and citric acid.
CG used common liquid soap with pH 7.0, composed of SLS or sodium laureth sulfate (SLES), cocamide DEA or cocamide diethanolamine, cocoamidopropyl betaine, citric acid, methyl chloroisothiazolinone, sodium hydrochloride, ethylene glycol stearate, fragrance, glycerin, linalool, and water used routinely in the maternity ward.
Because both products are white, creamy and slightly pearly and contain fragrance in a small percentage, it was not possible to differentiate between them without analyzing them in a meticulous and detailed way.
All newborns were followed up for observation of possible reactions to the use of the products used, from intervention to hospital discharge within 48 h.
Study variablesStudy variables included (Supplementary Material 2):
- a)
Maternal, gestational and obstetric data.
- b)
Family history.
- c)
pH values before and after bath.
- d)
Skin clinical parameters before and after bath.
- e)
Corneometry before and after bath.
pH monitoring was measured with cutaneous pH measurement instrument HI 99181 Portable Waterproof Skin pH Meter® (HANNA Instruments). Measurements were taken in three places: frontal region (glabella), abdomen (left flank), and right thigh (medial third).
Measurement sites were based on previous studies,5–7 replacing the arm with the forehead for being a region where soap was not applied.
Clinical parametersClinical observation of newborns' skin was performed immediately before and after bath, being evaluated by the same researcher: erythema, scaling and moisture:3,8,9
- a)
Erythema:
- •
0: Absent
- •
1+: Mild
- •
2+: Moderate
- •
3+: Intense
- •
4+: Intense with erythema and edema
- •
- b)
Scaling:
- •
0: Absent
- •
1+: Fine
- •
2+: Moderate
- •
3+: Intense Crusts
- •
- c)
Hidratação (formação de fissuras):
- •
0: Disseminated with exudation or bleeding
- •
1+: Pronounced single or multiple
- •
2+: Fine cracks
- •
3+: No cracks
- •
Two parameters were analyzed: oil corneometry and water corneometry, in the same regions of pH measurement, which evaluate, respectively, the percentage of oil and water in the skin through the Digital Moisture Monitor for Skin corneometer, Skin Care Digital Analyzer®.
Statistical analysisMeasures of central tendency and dispersion were expressed as means and standard deviation (mean ± SD) for continuous variables with symmetric distribution and in medians, minimum and maximum values for those with the asymmetric distribution. Categorical variables were expressed in absolute and relative frequency. Student's t-tests and Analysis of Variance (Anova) with Duncan's post hoc test were used for symmetric distribution data and the Mann-Whitney test for asymmetric distribution. Estimation of the difference between categorical variables was performed using the Pearson/Yates chi-square test and Fisher's exact test for independent variables and McNemar's test for dependent variables. The minimum level of significance was 5% and the minimum test power was 90%. Statistica Software v10.0 (Statsoft®) was used.
Factors that could influence skin moisture include sex, gestational age, presence of vernix caseosa, hours of life, race, and weight classification for gestational age were controlled in the statistical analysis.
ResultsClinical profile of patientsThere was no difference between groups regarding variables of gestational age (GA), sex, race, route of birth, rupture of amniotic sac, and presence of vernix caseosa (p > 0.05) (Table 1). Groups were similar in terms of maternal diseases during pregnancy, family history of atopy and skin diseases.
Characteristics of newborns and maternal history in control and study groups.
| Characteristics | Control group (n = 102) | Study group (n = 102) | p |
|---|---|---|---|
| Sex | |||
| Male | 46 (45.1%) | 56 (54.9%) | 0.20a |
| Female | 56 (54.9%) | 46 (45.1%) | |
| Race | |||
| White | 87 (85.3%) | 89 (87.2%) | 0.91b |
| Mixed race | 14 (13.7%) | 12 (11.8%) | |
| Black | 1 (1.0%) | 1 (1.0%) | |
| Gestational age (weeks) | 38.7 ± 1.6 | 38.8 ± 1.4 | 0.68c |
| Gestational age Classification | |||
| Term | 89 (87.3%) | 91 (89.2%) | 0.82a |
| Preterm | 13 (12.7%) | 11 (10.8%) | |
| Delivery type | |||
| Vaginal | 44 (43.1%) | 43 (42.2%) | 1.00a |
| C-section | 58 (56.9%) | 59 (57.8%) | |
| Ruptured sac | 48 (47.1%) | 54 (52.9%) | 0.48a |
| Ruptured sac time (hours) | 3.7 (0.5–168.0) | 4.0 (0.3–24.0) | 0.18d |
| Vernix caseosa | 79 (77.5%) | 87 (85.3%) | 0.20a |
| Maternal diseases | |||
| Arterial pressure disorders | 12 (11.8%) | 23 (22.6%) | 0.06a |
| Gestational diabetes mellitus | 16 (15.7%) | 25 (24.5%) | 0.16a |
| Hypothyroidism | 13 (12.7%) | 11 (10.8%) | 0.82a |
| TORCHS | 6 (5.9%) | 5 (4.9%) | 1.00a |
| Atopy and skin diseases | |||
| Atopy | 73 (71.6%) | 65 (63.7%) | 0.29a |
| Skin diseases | 37 (36.3%) | 31 (30.4%) | 0.45a |
| Atopic Dermatitis | 11 (10.8%) | 9 (8.8%) | 0.81a |
| Xerosis | 9 (8.8%) | 7 (6.9%) | 0.79a |
Newborns were evaluated with 17.2 ± 5.8 h of life in both groups (p = 0.75).
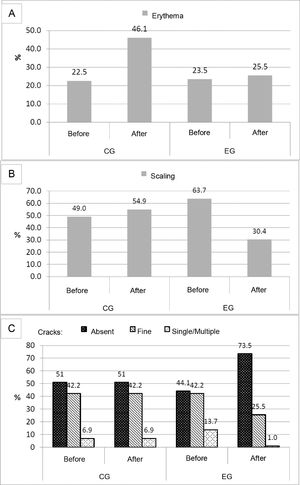
Evaluation of clinical cutaneous parametersErythema was more frequent in CG after bath (p < 0.001), which did not occur in EG (p = 0.86). When comparing erythema before and after bath, there was an increase in the intensity of erythema after a bath in CG (p < 0.001) and a decrease in EG (p = 0.04) (Fig. 1A).
Distribution of frequency of erythema, scaling and degree of moisture level in newborns before and after a bath in control and study Groups.
CG, control group; EG, experimental group.
Distribution of frequency of erythema, scaling and degree of moisture level in newborns before and after bath in control and study groups. A, Erythema. CG before versus after: p < 0.001; EG before versus after: p = 0.86. Pearson/Yates chi-square test: between groups after: p < 0.01. B, Scaling. McNemar test: CG before versus after: p = 0.56; EG before versus after: p < 0.001/Pearson's chi-square test/Yates: between groups after: p < 0.01. C, McNemar test: CG before versus after: p = 1.00; EG before versus after: p < 0.001; Pearson's chi-square test: between groups after: p < 0.01.
Before a bath, there was a greater frequency of scaling in EG compared to the CG (p = 0.04). When scaling before and after the bath was compared, it decreased in EG (p < 0.001) and remained the same in CG (p = 0.56) (Fig. 1B).
Moisture remained the same before and after a bath in CG (p = 1.00). In EG, there was an improvement in moisture after bath, with fewer cracks (p < 0.001) Comparison of moisture between groups showed a better degree of moisture in EG (p < 0.01) (Fig. 1C).
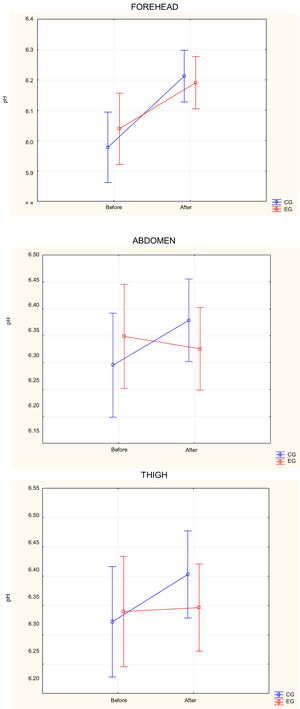
pH assessmentNo difference in pH measurements was observed in each anatomical region comparing EG and CG before bathing (p > 0.05). In the frontal region, there was an increase in pH in both groups after bath (p < 0.001), but with no statistically significant difference between them, even without using soap in this location (Fig. 2).
Distribution of pH averages on the forehead, abdomen and thigh of newborns before and after bathing in the control and experimental groups.
CG, control group; EG, experimental group.
Distribution of pH averages on the forehead, abdomen and thigh of newborns before and after bathing in the control and experimental groups. Anova, Duncan's post-hoc test between groups. For the forehead; Before: p = 0.38; After; p = 0.85; Comparison between before and after: CG: p < 0.001; EG: p < 0.001. For the abdomen: Before: p = 0.42; After; p = 0.43; Comparison between before and after: CG: p = 0.02; EG: p = 0.49; For the thigh: Before: p = 0.77; After; p = 0.35; Comparison between before and after: CG: p = 0.03; EG: p = 0.84.
In the abdomen and thigh, there was an increase in pH after bathing in CG (p = 0.02 and 0.03, respectively), while in EG there was no significant variation (p = 0.49 and 0.84, respectively) (Fig. 2).
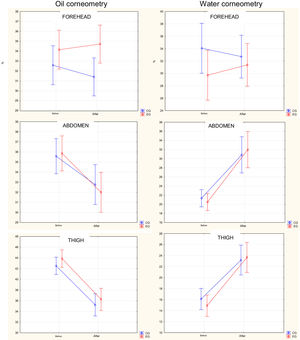
Assessment of corneometryThere was no statistically significant difference in the parameters of water and oil corneometry before or after bath between the two groups (Fig. 3).
Distribution of percentage of oil and water corneometry on the forehead, abdomen and thigh of newborns before and after bathing in the control and experimental groups.
CG, control group; EG, experimental group.
Distribution of percentage of oil and water corneometry on the forehead, abdomen and thigh of newborns before and after bathing in the control and experimental groups. Anova, Duncan's post-hoc test between groups. In the left column are the values of oil corneometry and in the right are the values of water corneometry. Oil corneometry on the Forehead: Before: p = 0.25; After; p = 0.02; Comparison between before and after: CG: p = 0.34; EG: p = 0.64; Abdomen: Before: p = 0.83; After; p = 0.56; Comparison between before and after: CG: p = 0.03; EG: p < 0.001; Thigh: Before: p = 0.30; After; p = 0.44; Comparison between before and after: CG: p < 0.001; EG p < 0.001. Water corneometry on the forehead: Before: p = 0.14; After; p = 0.61; Comparison between before and after: CG: p = 0.57; EG: p = 0.48; Abdomen: Before: p = 0.71; After; p = 0.61; Comparison between before and after: CG: p < 0.001; EG: p < 0.001; Thigh: Before: p = 0.47 After; p = 0.77; Comparison between before and after: CG: p < 0.001; EG p < 0.001.
As far as the authors know, this is the first Brazilian study that compared changes in skin pH, corneometry and clinical parameters of skin moisture determined by the action of two types of soaps in a newborn's bath.
It was observed that the children's liquid soap specific for NBs caused less erythema, dryness, scaling and less alteration of cutaneous physiological pH, when compared to the common liquid soap.
There are few scientific publications on this topic in literature.3,10,11 Mendes et al. demonstrated that children's bar soaps have a more alkaline pH when compared to liquid soaps, which promotes disruption of the stratum corneum and dryness.12,13 For this reason, preference for liquid cleaners that perform a smoother cleaning, without damaging the skin barrier or significantly altering its pH, especially in NBs who have thinner and more sensitive skin and less natural moisture factor (FHN).14–19
The soap used in EG is a detergent-free cleaner intended for newborn skin. It does not contain sodium lauryl sulfate (SLS), only nonionic and amphoteric surfactants and has a pH of 5.86. It consists of water, coco glucoside, cocoamidopropyl betaine, phenoxyethanol, acrylate/C10–30, acrylate crosspolymer, sodium benzoate, glyceryl oleate, p-anisic acid, sodium hydroxide, phenoxyethanol, perfume, and citric acid. The soap used in CG, on the other hand, is a common liquid soap, of general use, with pH 7.0 and consists of SLS or sodium laureth sulfate (SLES), cocamide DEA or cocamide diethanolamine, cocoamidopropyl betaine, citric acid, methylchloroisothiazolinone, sodium hydrochloride, ethylene glycol stearate, fragrance, glycerin, linalool, and water.
Common liquid soaps, such as the soap used in CG, do not have identification on the packaging for use on children. In addition, it contains SLS in its formulation, a surfactant that removes the skin's protective lipid layer. This component is generally absent in synthetic cleaners indicated for sensitive skin,14 which probably determined the higher frequency of erythema and scaling in CG.
In the present study, an increase in pH was observed after the bath in the frontal region, in which cleaning was carried out only with water, without using any type of soap. This data corroborates what was found by Garcia Bartels et al. who observed lower pH values in the group that used liquid soap when compared to the group that used only water for bathing.11 This can be explained by water's buffering potential, which can increase the pH by up to 2 points,5 leading to protein denaturation of keratin in the stratum corneum (SC), by removing its lipids and altering water retention capacity. As a result, skin becomes xerotic and cracked. These deleterious effects occur due to factors such as osmolarity, pH, hardness and temperature or only by the extraction or dilution of the NMF. For these effects and for their inability to remove lipophilic residues properly, water-only baths are not indicated.20,21
Tarun et al., studied soaps and mention that only 4 out of 64 evaluated had product pH information on the label. Of these, two had physiological pH, and two neutral pH.22 Similar data to that observed in the Brazilian study by Mendes et al., where only two of the 90 soaps evaluated mentioned the pH on the packaging. It was noticed that even the products that contained phrases in the packaging such as "neutral pH", "balanced pH", or "dermatologically tested", had a pH above 5.0, which can confuse consumers.13
It is important to reinforce the inherent risk in the use of unsuitable products for the child age group, especially for NBs, whose skin barrier is still immature, with thinner, more sensitive skin and greater power to absorb irritating or potentially allergenic substances.13,18,23
A limiting factor in the present study was that the evaluation was performed in a single bath of NB. Assessment of several baths over a longer period could verify the alteration of these parameters in the long term.
Based on all these facts, the authors conclude that baths with children's liquid soap maintained the moisture and physiological pH of newborns’ skin and decreased erythema and scaling. Common liquid soap and water-only use raised the pH. The effects of these altered parameters are not known in the long term, but it is ideal that the physiological skin conditions are maintained.
The authors thank Farmadoctor pharmacy for blinding soap bottles, the nursing team for their crucial participation in carrying out the baths, prof. Dr. Monica Lima for her valuable contribution to statistical analysis and to Johnson & Johnson of Brazil for translating the article into English.