To determine the prevalence of symptoms of asthma, rhinitis, and atopic eczema in adolescents (AD; 13-14 years) living in seven Brazilian cities, by applying the standardized written questionnaire (WQ) of the International Study of Asthma and Allergies in Childhood (ISAAC), and to evaluate the time trend nine years after the last assessment of ISAAC phase 3 (ISP3).
MethodsThe ISAAC-WQ was answered by 20,099 AD from the Northern, Northeastern, Southeastern, and Southern Brazilian regions. Values obtained were compared to those observed in ISP3 using nonparametric (chi-squared or Fisher) tests, and the ratio of annual increment/decrement was established for each of the centers, according to the symptom assessed.
ResultsConsidering the national data and comparing to values of ISP3, there was a decrease in the mean prevalence of active asthma (18.5% vs. 17.5%) and an increase in the frequency of severe asthma (4.5% vs. 4.7%) and physician-diagnosed asthma (14.3% vs. 17.6%). An increase in prevalence of rhinitis, rhinoconjunctivitis, and atopic eczema was also observed.
ConclusionsThe prevalence of asthma, rhinitis, and atopic eczema in Brazil was variable; higher prevalence values, especially of asthma and eczema, were observed in regions located closer to the Equator.
Determinar a prevalência de sintomas relacionados à asma, rinite e eczema atópico em adolescentes (13-14 anos, AD) residentes em sete cidades brasileiras empregando o questionário escrito (QE) padronizado do International Study of Asthma and Allergies in Childhood (ISAAC) e verificar a tendência temporal passados nove anos da última avaliação do ISAAC fase 3 (ISF3).
MétodosO QE ISAAC foi respondido por 20.099 AD (13-14 anos) moradores em centros das regiões Norte, Nordeste, Sudeste e Sul. Os índices obtidos foram comparados aos do ISF3 utilizando-se teste não paramétrico (qui-quadrado ou Fisher) e foi estabelecida a taxa de incremento/decremento anual para cada um dos centros segundo o sintoma avaliado.
ResultadosEm relação ao ISF3, considerando-se os dados nacionais, houve queda da prevalência média de asma ativa (18,5% vs. 17,5%) com elevação da frequência de asma grave (4,5% vs. 4,7%) e de asma diagnosticada por médico (14,3% vs.17,6%). Aumento da prevalência de rinite e rinoconjuntivite e de eczema flexural também ocorreram.
ConclusõesA prevalência de asma, rinite e eczema atópico no Brasil foi variável. Valores mais altos, sobretudo de asma e eczema foram observados nos centros localizados mais próximos ao Equador.
The prevalence of asthma and allergic diseases in children has shown wide variation worldwide and according to some authors, it has increased, especially in developing countries.1–5 Up to a few decades ago this knowledge was limited, for lack of a single, standardized, and validated tool to be used universally, and restricted to studies were performed in small samples, which precluded comparisons between different populations and at different times.
After the International Study of Asthma and Allergies in Childhood (ISAAC), which created a standardized protocol, such comparisons became possible; since then, have been widely performed.6 Before the ISAAC protocol, few studies, using the same tool (written questionnaire [WQ]), were able to evaluate the temporal trend of the prevalence of asthma, rhinitis, and atopic eczema in children. One of these studies assessed Norwegian children aged 7 to 14 years between 1985 and 2008, regarding the prevalence of asthma, rhinitis, and atopic eczema; a trend of increasing asthma and rhinitis prevalence and stabilization for eczema was observed.7 Another study, with a longer follow-up, conducted from 1964 to 2004 in English schoolchildren (7-12 years) observed declining rates of wheezing, with an increase in rhinoconjunctivitis and atopic eczema.8
The mean time between the completion of ISAAC Phase 1 (ISF1) and ISAAC Phase 3 (ISF3) was seven years, and the prevalence rates obtained in all centers that concurrently participated in both phases showed conflicting results, mainly in those centers that presented high values.2 In Brazil, an increase in the prevalence of asthma symptoms in schoolchildren aged 6-7 years from 21.3% in ISF1 to 24.4% in ISF3 and a stable prevalence of rhinoconjunctivitis (12.5% and 12.0%, respectively) and atopic eczema (6.8% and 6.8%, respectively) were observed. Among adolescents (ADs) there was a decrease in prevalence rates of asthma symptoms (22.7% to 19.9%, respectively), rhinoconjunctivitis (16.2% to 15.8%, respectively), and atopic eczema (5.3% to 4.2%, respectively).2
At that time, there was no unanimous explanation for the previously observed findings. This study aimed to determine the variations in the prevalence of asthma, rhinitis, and atopic eczema in Brazilian ADs nine years after ISF3 was completed.
Subjects and methodSeven of the 21 centers participating in the ISF3 in Brazil2,9–11 accepted the invitation to participate in this study, nine years after its completion. The study was supported by FAPESP (Project PPSUS No. 2009-53303-5). The recommended criteria were met in all centers and the ADs were selected as recommended by the ISAAC6,12 protocol. With the exception of Belém (state of Pará, North [N]), all other centers had their ISF3 data approved by the ISAAC International Data Center, and the following were categorized as official centers: Recife (Pernambuco, Northeast [NE]); Maceió (Alagoas, NE); Aracaju (Sergipe, NE); Belo Horizonte (Minas Gerais, Southeast [SE]); São Paulo (São Paulo, SE); and Curitiba (Paraná, South [S]) (Tables 1 and 2).
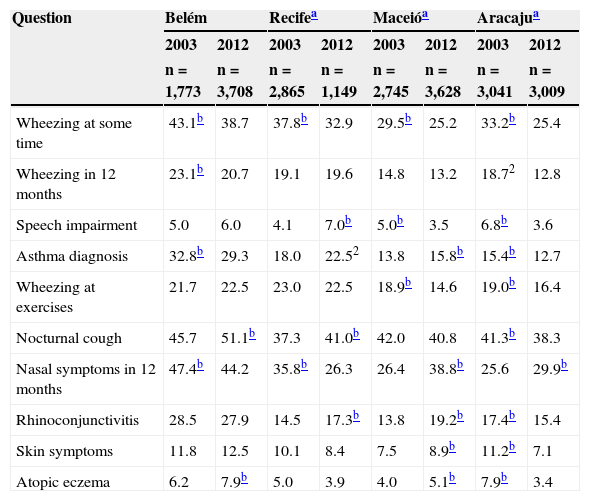
Prevalence of affirmative responses to the questions on symptoms of asthma, rhinitis and eczema of the written questionnaire of the International Study of Asthma and Allergies in Childhood given by adolescent from the centers that participated in the ISAAC phase 3 study and in the current study.
| Question | Belém | Recifea | Maceióa | Aracajua | ||||
|---|---|---|---|---|---|---|---|---|
| 2003 | 2012 | 2003 | 2012 | 2003 | 2012 | 2003 | 2012 | |
| n=1,773 | n=3,708 | n=2,865 | n=1,149 | n=2,745 | n=3,628 | n=3,041 | n=3,009 | |
| Wheezing at some time | 43.1b | 38.7 | 37.8b | 32.9 | 29.5b | 25.2 | 33.2b | 25.4 |
| Wheezing in 12 months | 23.1b | 20.7 | 19.1 | 19.6 | 14.8 | 13.2 | 18.72 | 12.8 |
| Speech impairment | 5.0 | 6.0 | 4.1 | 7.0b | 5.0b | 3.5 | 6.8b | 3.6 |
| Asthma diagnosis | 32.8b | 29.3 | 18.0 | 22.52 | 13.8 | 15.8b | 15.4b | 12.7 |
| Wheezing at exercises | 21.7 | 22.5 | 23.0 | 22.5 | 18.9b | 14.6 | 19.0b | 16.4 |
| Nocturnal cough | 45.7 | 51.1b | 37.3 | 41.0b | 42.0 | 40.8 | 41.3b | 38.3 |
| Nasal symptoms in 12 months | 47.4b | 44.2 | 35.8b | 26.3 | 26.4 | 38.8b | 25.6 | 29.9b |
| Rhinoconjunctivitis | 28.5 | 27.9 | 14.5 | 17.3b | 13.8 | 19.2b | 17.4b | 15.4 |
| Skin symptoms | 11.8 | 12.5 | 10.1 | 8.4 | 7.5 | 8.9b | 11.2b | 7.1 |
| Atopic eczema | 6.2 | 7.9b | 5.0 | 3.9 | 4.0 | 5.1b | 7.9b | 3.4 |
| Question | Belo Horizontea | São Pauloa | Curitibaa | General | ||||
|---|---|---|---|---|---|---|---|---|
| 2003 | 2012 | 2003 | 2012 | 2003 | 2012 | 2003 | 2012 | |
| n=3,088 | n=2,642 | n=3,161 | n=2,433 | n=3,628 | n=3,530 | n=20,301 | n=20,099 | |
| Wheezing at some time | 47.3 | 45.5 | 44.6 | 43.7 | 40.7 | 39.8 | 38.8 | 35.5b |
| Wheezing in 12 months | 17.8 | 19.7 | 18.7 | 21.3b | 18.9 | 17.6 | 18.5 | 17.5b |
| Speech impairment | 4.8 | 5.0 | 2.9 | 4.4b | 3.1 | 4.5b | 4.5 | 4.7 |
| Asthma diagnosis | 9.8 | 17.3b | 10.4 | 13.6b | 9.2 | 13.1b | 14.3 | 17.6b |
| Wheezing at exercises | 18.6 | 21.7b | 17.0b | 12.1 | 19.1 | 19.9 | 19.4 | 18.3b |
| Nocturnal cough | 37.4 | 39.3 | 33.3 | 45.4b | 34.7 | 42.4b | 38.2 | 43.0b |
| Nasal symptoms in 12 months | 26.1 | 34.1b | 27.4 | 49.9b | 39.2b | 31.6 | 31.8 | 37.2b |
| Rhinoconjunctivitis | 14.5 | 18.3b | 12.2 | 24.5b | 17.2 | 18.8 | 16.2 | 20.6b |
| Skin symptoms | 9.1 | 8.3 | 12.7b | 8.6 | 6.3 | 8.8b | 9.6 | 9.1 |
| Atopic eczema | 5.2 | 5.4 | 3.6 | 6.6b | 3.7 | 5.7b | 5.0 | 5.6b |
Speech impairment, wheezing so intense that was able to prevent two consecutive words from being said in the last 12 months; Wheezing at exercise, wheezing during exercise in the past 12 months; Nocturnal cough, coughing at night without having a cold in the last 12 months; Nasal symptoms in 12 months, sneezing, runny nose or nasal stuffiness at some time in the last 12 months; Rhinoconjunctivitis, nasal problem with itching and watery eyes in the last 12 months; Skin symptoms, itchy rash that appeared and disappeared in the last 12 months; Atopic eczema, itchy rash that appeared and disappeared in the last 12 months and in characteristic locations (skinfolds, among others).
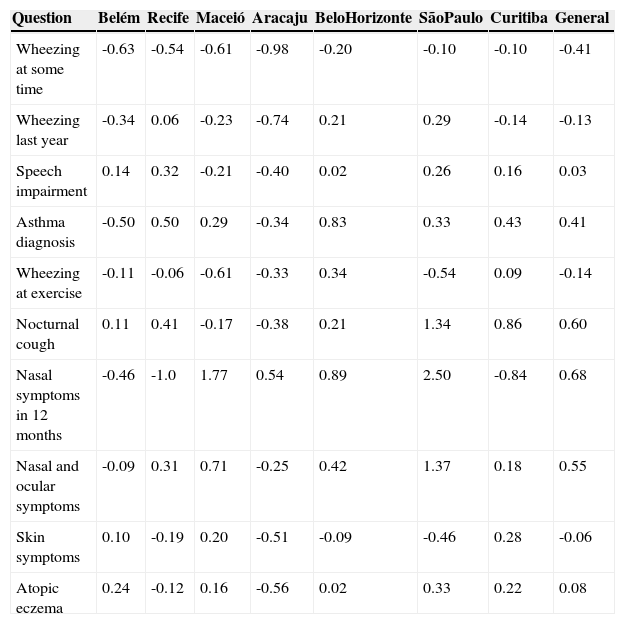
Percentage of variation per year in the prevalence of asthma, rhinitis and eczema symptoms in adolescents who answered the International Study of Asthma and Allergies in Childhood (ISAAC) written questionnaire in centers that participated in ISAAC phase 3 and the current study.
| Question | Belém | Recife | Maceió | Aracaju | BeloHorizonte | SãoPaulo | Curitiba | General |
|---|---|---|---|---|---|---|---|---|
| Wheezing at some time | -0.63 | -0.54 | -0.61 | -0.98 | -0.20 | -0.10 | -0.10 | -0.41 |
| Wheezing last year | -0.34 | 0.06 | -0.23 | -0.74 | 0.21 | 0.29 | -0.14 | -0.13 |
| Speech impairment | 0.14 | 0.32 | -0.21 | -0.40 | 0.02 | 0.26 | 0.16 | 0.03 |
| Asthma diagnosis | -0.50 | 0.50 | 0.29 | -0.34 | 0.83 | 0.33 | 0.43 | 0.41 |
| Wheezing at exercise | -0.11 | -0.06 | -0.61 | -0.33 | 0.34 | -0.54 | 0.09 | -0.14 |
| Nocturnal cough | 0.11 | 0.41 | -0.17 | -0.38 | 0.21 | 1.34 | 0.86 | 0.60 |
| Nasal symptoms in 12 months | -0.46 | -1.0 | 1.77 | 0.54 | 0.89 | 2.50 | -0.84 | 0.68 |
| Nasal and ocular symptoms | -0.09 | 0.31 | 0.71 | -0.25 | 0.42 | 1.37 | 0.18 | 0.55 |
| Skin symptoms | 0.10 | -0.19 | 0.20 | -0.51 | -0.09 | -0.46 | 0.28 | -0.06 |
| Atopic eczema | 0.24 | -0.12 | 0.16 | -0.56 | 0.02 | 0.33 | 0.22 | 0.08 |
Speech impairment, wheezing so intense that was able to prevent two consecutive words from being said in the last 12 months; Wheezing at exercise, wheezing during exercise in the past 12 months; Nocturnal cough, coughing at night without having a cold in the last 12 months; Nasal symptoms in 12 months, sneezing, runny nose or nasal stuffiness at some time in the last 12 months; Skin symptoms, itchy rash that appeared and disappeared in the last 12 months; Atopic eczema, itchy rash that appeared and disappeared in the last 12 months and in characteristic locations (skinfolds, among others).
In all participating centers, ADs (13-14 years old) were selected from public and private schools. Data on number of schools and students enrolled were provided by the respective Departments of Education of each center, and a minimum of 1,000 students were assessed. The study was approved by each Research Ethics Committee, and all subjects signed an informed consent; data collection iniciated in 2011 and was completed in 2012. The general project was approved by Universidade Federal de São Paulo/Hospital São Paulo Research Ethics Committee (n. 1345/09).
After defining the sample in each of the cities, the ISAAC WQ, previously translated into Brazilian Portuguese and culturally validated,13–15 was completed by ADs in their classrooms (n=20,099), which yielded a high rate of properly filled out questionnaires. Data were manually transferred to the database provided by the general coordinators of ISAAC protocol.
From the ISAAC-WQ asthma module, the following questions were considered: wheezing at some time; wheezing in the last 12 months (current asthma); wheezing severe enough to limit speech in the last 12 months (severe asthma); physician-diagnosed asthma (asthma at some time in life); wheezing at exercises; and nocturnal cough.2,13
From the ISAAC-WQ rhinitis module, the following questions were considered: sneezing, runny nose and nasal obstruction at some time in the past 12 months (rhinitis), and nasal problems associated with itchy and watery eyes in the last 12 months (rhinoconjunctivitis).2,14
From the ISAAC-WQ eczema module, the following questions were considered: skin rash that appeared and disappeared in the last 12 months (eczema) and this characteristic rash in places such as skinfolds and buttocks (atopic eczema).2,15
The values obtained were compared to those previously published in ISF39–11 and expressed as an annual percentage of change (Table 2). Data analysis was performed with non-parametric tests, chi-squared test, or Fisher's exact test and 5% was established as the rejection level for the null hypothesis.
ResultsTable 1 summarizes the percentage data on the prevalence of symptoms of asthma, rhinitis, and eczema of ADs from seven Brazilian centers that participated in the ISF3, as well as the current values, obtained nine years later. Comparative analysis between the values obtained in the two studies was performed in each center; significantly higher values were identified.
Considering the general data, a significant decrease was observed in the prevalence of wheezing at some time in life, wheezing in the previous year (active asthma), and wheezing associated with exercise during this period (Table 1). Conversely, there was a significant increase in the prevalence of physician-diagnosed asthma, nocturnal cough, nasal symptoms without having a cold (rhinitis), rhinoconjunctivitis, and atopic eczema (Table 1). Regarding the annual percentage of change, a decrease of 0.41%/year was observed for the prevalence of wheezing at some time in life and an increase of 0.6%/year for the reporting of nocturnal cough (Table 2). For nasal symptoms, there was an increase of 0.68%/year for rhinitis and 0.55%/year for rhinoconjunctivitis, as well as a 0.08% annual increase for atopic eczema (Table 2).
DiscussionWhen the prevalence rates observed in different centers participating in this study are analyzed, nine years after the end of ISF3, a distinct and variable behavior can be observed. In general, a decline in the prevalence of active asthma and an increase in the prevalence of rhinitis, rhinoconjunctivitis, as well as atopic eczema were observed (Tables 1 and 2).
Regarding asthma and related symptoms, it was observed that the decrease in the prevalence of active asthma was partly due to the decrease observed in Belém and Aracaju, despite the significant increase that occurred in São Paulo. Moreover, there was a significant increase in the prevalence of physician-diagnosed asthma, which ranged between 14.3% and 17.6% in all centers, except Belém and Aracaju. Conversely, the number of severe events (speech impairment) remained unchanged, as opposed to nonspecific symptoms such as nocturnal cough, which increased (Tables 1 and 2).
What happened during this time interval that would explain these changes? When analyzing the period of 18 years since the ISF1 data were obtained, it can be observed that the Human Development Index (HDI) in Brazil increased, going from 0.724 in 1993/199416 to 0.807 in 2012.17 This increase was observed in all centers involved in the study; however, it was more pronounced in the NE and N regions. There was no significant correlation between HDI and the prevalence of active asthma, rhinoconjunctivitis, and atopic eczema in both 2003 and 2012 (data not shown).
These data are corroborated when considering the fact that the Gross Domestic Product presented the same behavior, going from US$ 3,040 in 1994 to US$ 11,630 in 2012.18,19 Although the country economic status did improve, to infer that such improvement is responsible for the changes in the prevalence rates observed herein is a fragile assumption.
Moreover, after the 2000s, asthma treatment improved, as the creation of the National Asthma Control Plan (Plano Nacional de Controle da Asma - PNCA) started providing drug therapy for patients with severe asthma20 and was extended in 2005 to patients with mild or moderate asthma.21
In December of 2010, the rules for financing and implementation of the Basic Component of Pharmaceutical Care were approved, providing the asthma and rhinitis program with medications from the National Reference List of the Basic Component of Pharmaceutical Care.22 Certainly, these facts allowed the creation of care programs for patients with asthma, which may explain the increased prevalence of medical diagnosis. As these patients have access to treatment, greater control of the disease was expected, as evidenced by a reduction in the frequency of severe exacerbations, as well as in nonspecific symptoms observed in part of the Ads included in the present study. With the introduction of medical guidelines and consensuses on asthma, there was a greater dissemination of knowledge on the disease; thus, the term “asthma” started to be more often employed by physicians and patients, replacing euphemisms such as bronchitis and tracheobronchitis.23 Another consequence was the asthma treatment standardization, although it is not always fully based on national or international23,24 guidelines.
Regarding rhinitis and rhinoconjunctivitis, an increase in the prevalence of both was observed in most centers involved in the study. After excluding the economic factor, another component that could be involved in this increase is environmental pollution. A previous study by the present authors (ISF3) evaluated the association between the prevalence of asthma, rhinitis, and eczema and levels of photochemical pollutants in Brazilian centers that had air pollution control (Sao Paulo, Santo André, Curitiba, and Porto Alegre). Although a characteristic pattern between the evaluated symptoms and a specific air pollutant was not detected, an association was observed between greater exposure to photochemical pollutants and high prevalence or risk of symptoms of asthma, rhinitis, and atopic eczema.25 Of the centers that participated in this study, only São Paulo and Curitiba have had air pollutant monitoring since the ISF3 was performed, which allowed for the observation, in both centers, of an improvement of air quality in recent years.26–29
In conclusion, the present study found that the prevalence of active asthma has possibly reached its highest level and has stabilized. Would this fact be due to the reduction in levels of air pollution? If so, how can the increased prevalence of rhinitis, rhinoconjunctivitis, and atopic eczema be explained? Possible explanations for these findings still require further studies that are targeted for these primary outcomes.
FundingFAPESP-PPSUS (Process n: 2009/53303-5).
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Solé D, Rosário Filho NA, Sarinho ES, Camelo-Nunes IC, Barreto BA, Medeiros ML, et al. Prevalence of asthma and allergic diseases in adolescents: nine-year follow-up study (2003-2012). J Pediatr (Rio J). 2015;91:30–5.
Study conducted at the Discipline of Allergy, Clinical Immunology and Rheumatology, Department of Pediatrics, Escola Paulista de Medicina, Universidade Federal de São Paulo, São Paulo, SP, Brazil.