To describe the main predictors for excess birth weight in Brazilian children.
Data sourcesSystematic review carried out in the bibliographic databases: PubMed/MEDLINE, Cochrane, Scopus, Web of Science, and LILACS. The research in the gray literature was performed using the Google Scholar database. The bias risk analysis was adapted from the Downs and Black scale, used to evaluate the methodology of the included studies.
Data synthesisUsing the classifications of fetal macrosomia (>4.000g or ≥4.000g) and large for gestational age (above the 90th percentile), 64 risk factors for excess birth weight were found in 33 scientific articles in the five regions of the country. Of the 64 risk factors, 31 were significantly associated with excess birth weight, with excess gestational weight gain, pre-gestational body mass index ≥25kg/m2, and gestational diabetes mellitus being the most prevalent.
ConclusionThe main predictors for excess birth weight in Brazil are modifiable risk factors. The implementation of adequate nutritional status in the gestational period and even after childbirth appears to be due to the quality and frequency of the follow-up of the mothers and their children by public health agencies.
Descrever os principais preditores para o excesso de peso ao nascer em crianças brasileiras.
Fontes dos dadosRevisão sistemática realizada nos bancos de dados bibliográficos: PubMed/MEDLINE, Cochrane, Scopus, Web of Science e LILACS. A pesquisa na literatura cinzenta foi realizada na base de dados Google Acadêmico. A análise do risco de viés foi adaptada da escala de Downs and Black, utilizada para avaliar a metodologia dos estudos incluídos.
Síntese dos dadosUtilizando-se as classificações macrossomia fetal (>4.000g ou ≥4.000g) e grande para idade gestacional acima do percentil 90, foram encontrados 64 fatores de risco para excesso de peso ao nascer em 33 artigos científicos nas cinco regiões do país. Dos 64 fatores de risco, 31 foram significativamente associados a excesso de peso ao nascer, sendo ganho de peso gestacional excessivo, índice de massa corporal pré-gestacional ≥25kg/m2 e diabetes mellitus gestacional os mais prevalentes.
ConclusãoOs principais preditores para o excesso de peso ao nascer no Brasil são fatores de risco modificáveis. O estabelecimento de um estado nutricional adequado no período gestacional e mesmo após o parto parece ser a qualidade e a frequência do acompanhamento dos órgãos de saúde junto às mães e seus filhos.
Birth weight has been extensively investigated since the 1940s,1 mainly because of its intrinsic association with the child's and the mother's health status.2 Directly associated with the newborn's and the mother's nutritional status,3 birth weight is also associated with socioeconomic conditions and the quality of care received during the prenatal period, in addition to influencing the individual's growth and development throughout his/her life.4 Moreover, the fact that the mother is intimately connected to the child through the placenta and the umbilical cord throughout pregnancy causes the nutritional status of the mother–child pair to be potentially influenced by similar factors.5
For a long time, several studies considered low birth weight as the main alteration in the child's nutritional status due to its strong association with infant mortality.6 Low birth weight is also a characteristic considered in the assessment of the Human Development Index (HDI) to classify countries regarding the type of development.7 Developing countries commonly have high rates of low birth weight and, consequently, low HDI.8,9 However, with the rapid change in world populations’ lifestyles, especially changes in diet and physical activity,10 many studies have shown that excess birth weight is also associated with most of the same risk factors for low birth weight.11
In recent years, studies carried out in both developed and developing countries have shown high rates of excess birth weight in their populations.12–15 In Norway, a country with more than five million inhabitants16 and an HDI of 0.944,17 the rate of excess birth weight in 2006 was 20.5%.18 In the United States, with an HDI of 0.91517 and 326.425 million inhabitants,19 the rate of excess birth weight in 2016 was 13.2%.20 Studies carried out in France, Canada, and Spain reported values of excess birth weight of 15.3%, 25.8%, and 16.7%, respectively.21 These same countries had HDIs of 0.888, 0.913, and 0.876 in 2015,17 respectively.
In Brazil, a developing country with more than 200 million inhabitants and an HDI of 0.755,17 the rates of excess birth weight vary between 4.1 and 30.1%, depending on the classification criteria used,14,22–29 and differs considerably depending on the region where the study was carried out.
Currently, excess birth weight has reached alarming levels. The global prevalence of excess birth weight is between 0.5% in India and 14.5% in Algeria.12 The estimate for 2025 is that the world will have 70 million children born with excess weight, an outcome which is already considered by many authors as a serious public health problem.30
The different rates of excess birth weight prevalence, commonly found in countries with high socioeconomic, demographic, and cultural diversity, among others, such as Brazil, highlight the importance for each country to identify the main factors associated with this clinical condition.31 Although several factors associated with excess birth weight are also found in different countries, some factors may be associated with the country's characteristics, and thus cannot be used to explain the same clinical condition in other countries.31
Some studies have shown that excess birth weight is mainly associated with pre-gestational maternal excess weight gain, excess weight gain during pregnancy, diabetes mellitus, hypercholesterolemia, advanced age, and multiparity.4,32–34 However, there is no consensus regarding the main predictors for excess weight at birth specifically for Brazilian children.
It is essential that each country design its public management model based on research data developed with its own population. In this sense, this study aims to identify the main predictors of excess birth weight specifically originating from studies conducted with the Brazilian population.
MethodsThis systematic review followed the criteria of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Checklist (PRISMA).35 The protocol of this systematic review was registered in the CRD's (Centre for Reviews and Dissemination) international prospective register of systematic reviews (PROSPERO) under number CRD 42017070505.
Eligibility criteriaStudies that evaluated the risk factors for excess birth weight in Brazil were considered eligible, without restriction or limitation of year of publication and language. The classification criteria for excess birth weight were: large for gestational age (LGA), or larger than the 90th percentile,36 and fetal macrosomia (FM; >4000g or ≥4000g),37 regardless of whether there was a reference for the classification.
Regarding the study types, this review included cohort, cross-sectional, and case–control studies, with data originating from primary or secondary sources. The exclusion criteria were as follows: (1) did not consider excess birth weight, (2) did not show data for the classification of FM and LGA, (3) had insufficient data to assess the risk factors associated with excess birth weight, (4) did not assess association, and (5) the full-text article was not available. Review articles, editorials, letters, book chapters, personal opinions, comments, and conference or congress summaries were not considered in this study.
Sources of information and research strategiesDetailed and individualized search strategies were carried out in the following databases: PubMed/MEDLINE, Cochrane, Scopus, Web of Science, and LILACS (Appendix 1). For the search of the first 100 articles in the gray literature, the Google Scholar database was used. The list of references of the included studies was manually revised to evaluate the need to include additional references. The search for the descriptors was performed on June 28, 2017. Duplicate references were removed, and the complete reference list was built using EndNote software, version X7.5.1.1 (Thomson Reuters – Philadelphia, PA, United States).
Study selectionArticle screening followed two selection steps. In the first stage, article selection was carried out individually by three researchers (S.A.C., L.F.S., J.M.) following the inclusion criteria and according to titles and abstracts of all references. Concomitantly, a reviewer (C.K.) analyzed and checked the criteria needed to select the studies.
In the second stage, the same authors read the full-text articles and excluded those that did not meet the inclusion criteria. Two other authors (M.F.M., S.S.B.S.M.) participated in the selection when there were disagreements between the four reviewers.
Data collection processThree authors (S.A.C., L.F.S., J.M.) collected information on the selected articles, such as: author and year of publication, place of data collection, type of institution, study objective, type of study, number of participants, maternal and fetal risk factors, criteria for the classification of excess birth weight, prevalence of excess weight in newborns, and main results of the study (Table 1). After compiling the data and findings from the studies, these were checked by a fourth author (C.K.), aiming to organize the findings of the selected articles. To eliminate doubts, a fifth reviewer (M.F.M.) contributed to define possible disagreements.
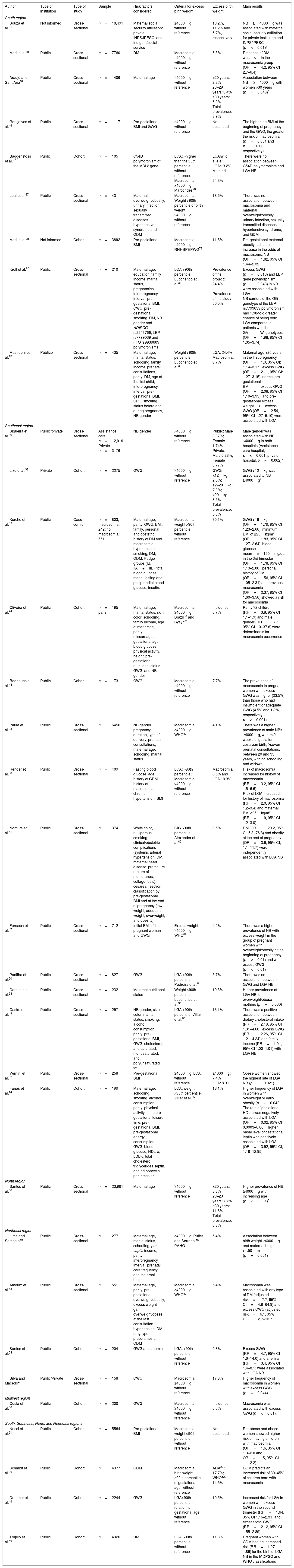
Characteristics of the studies included in this systematic review, according to the region of the country.
| Author | Type of institution | Type of study | Sample | Risk factors considered | Criteria for excess birth weight | Excess birth weight | Main results |
|---|---|---|---|---|---|---|---|
| South region | |||||||
| Souza et al.61 | Not informed | Cross-sectional | n=18,491 | Maternal social security affiliation: private, INPS/IPESC, and indigent/social service | ≥4000g, without reference | 10.2%, 11.2% and 5.7%, respectively | NB≥4000g was associated with maternal social security affiliation for private institution and INPS/IPESC (p<0.01)a |
| Madi et al.55 | Public | Cross-sectional | n=7760 | DM | Macrosomia ≥4000g, without reference | 5.3% | Presence of DM was>in the macrosomic group (OR=4.2, 95% CI 2.7–6.4) |
| Araujo and Sant’Ana59 | Public | Cross-sectional | n=1406 | Maternal age | ≥4000g, without reference | <20 years: 2.8% 20–29 years: 3.4% ≥30 years: 6.2% Total prevalence: 3.9% | Association between NB≥4000g with women >30 years (p=0.048)a |
| Gonçalves et al.45 | Public | Cross-sectional | n=1117 | Pre-gestational BMI and GWG | ≥4000g, without reference | Not described | The higher the BMI at the beginning of pregnancy and the GWG, the greater the risk of macrosomia (p=0.001 and p=0.03, respectively) |
| Baggenstoss et al.27 | Public | Cohort | n=105 | G54D polymorphism of the MBL2 gene | LGA: >higher than the 90th percentile, without reference. Macrosomia >4000g, Marcondes78 | LGA/wild allele: LGA/13.2% Mutated allele: 24.3% | There were no association between G54D polymorphism and LGA NB |
| Leal et al.57 | Public | Cross-sectional | n=43 | Maternal overweight/obesity, urinary infection, sexually transmitted diseases, hypertensive syndrome and GDM | Macrosomia: Weight >90th percentile or birth weight >4000g, without reference | 18.6% | There was no association between macrosomia and maternal overweight/obesity, urinary infection, sexually transmitted diseases, hypertensive syndrome, and GDM |
| Madi et al.25 | Not informed | Cohort | n=3892 | Pre-gestational BMI | Macrosomia ≥4000g, RNHBPEPWG79 | 11.8% | Pre-gestational maternal obesity led to an increase in the odds of macrosomic NB (OR=1.82, 95% CI 1.44–2.32). |
| Kroll et al.28 | Public | Cross-sectional | n=210 | Maternal age, education, family income, marital status, pregnancies, interpregnancy interval, pre-gestational BMI, GWG, pre-gestational smoking, DM, NB gender and ADIPOQ rs2241766, LEP rs7799039 and FTO rs9939609 polymorphisms | LGA >90th percentile, Lubchenco et al.36 | Prevalence of the project: 24.4% Prevalence of the study: 50.0% | Excess GWG (p=0.013) and LEP gene polymorphism (p=0.043) in NB were associated with LGA. NB carriers of the GG genotype of the LEP-rs7799039 polymorphism had 1.98-fold greater chance of being born LGA compared to patients with the GA+AA genotypes (OR=1.98, 95% CI 1.05–3.74). |
| Mastroeni et al.13 | Público | Cross-sectional | n=435 | Maternal age, marital status, schooling, family income, prenatal consultations, parity, DM, age of the first child, interpregnancy interval, pre-gestational BMI, GPG, smoking status before and during pregnancy, NB gender | Weight >90th percentile, Lubchenco et al.36 | LGA: 24.4% Macrosomia: 9.7% | Maternal age <20 years in the first pregnancy (OR=1.9, 95% CI: 1.14–3.17), excess GWG (OR=2.11, 95% CI: 1.27–3.15), normal pre-gestational BMI+excess GWG (OR=2.08, 95% CI 1.10–3.95), and pre-gestational excess weight+excess GWG (OR=2.54, 95% CI 1.27–5.10) were associated with LGA. |
| Southeast region | |||||||
| Siqueira et al.39 | Public/private | Cross-sectional | Assistance care n=12,919, Private n=3176 | NB gender | >4000g, without reference | Public: Male 3.07%; Female 1.74%. Private: Male 6.28%; Female 3.77% | Male gender was associated with NB >4000g in both hospitals (Assistance care hospital, p<0.001; private hospital, p=0.002)a |
| Lizo et al.32 | Private | Cohort | n=2275 | GWG | ≥4000g, without reference | GWG <12kg: 2.6%; 12–20kg: 7.0%; >20kg: 8.5% Total prevalence: 5.3% | GWG >12kg was associated to NB ≥4000ga |
| Kerche et al.42 | Public | Case–control | n=803, macrosomia: 242; no macrosomia: 561 | Maternal age, parity, GWG, BMI; family, personal and obstetric history of DM and macrosomia, hypertension, smoking, DM, GDM, Rudge groups (IB, IIA+IIB), total blood glucose mean, fasting and postprandial blood glucose, insulin. | Macrosomia: weight >90th percentile, without reference | 30.1% | GWG >16kg (OR=1.79, 95% CI 1.23–2.60), minimum BMI of ≥25kg/m2 (OR=1.83, 95% CI 1.27–2.64), blood glucose mean=120mg/dL in the 3rd trimester (OR=1.78, 95% CI 1.13–2.80), personal history of DM (OR=1.56, 95% CI 1.05–2.31) and previous macrosomia (OR=2.37, 95% CI 1.60–3.50) showed a risk for macrosomia |
| Oliveira et al.24 | Public | Cohort | n=195 pairs | Maternal age, marital status, skin color, schooling, family income, age of menarche, parity, miscarriages, gestational age, blood glucose, physical activity, height, pre-gestational nutritional status, GWG, and NB gender | Macrosomia ≥4000g, Brazil80 and Sysyn81 | Incidence 6.7% | Parity ≥2 children (RR=3.8, 95% CI 1.1–1.9) and male gender (RR=7.5, 95% CI 1.0–37.6) were determinants for macrosomia occurrence |
| Rodrigues et al.44 | Public | Cohort | n=173 | GWG | Macrosomia ≥4000g, without reference | 7.7% | The prevalence of macrosomia in pregnant women with excess GWG was higher (23.5%) than those who had insufficient or adequate GWG (4.5% and 1.8%, respectively, p<0.001). |
| Paula et al.23 | Public | Cross-sectional | n=6456 | NB gender, pregnancy duration, type of delivery, prenatal consultations, maternal age, schooling, marital status | Macrosomia ≥4000g, WHO82 | 4.1% | There was a higher prevalence of male NBs ≥4000g, with ≥42 weeks of gestation, cesarean birth, ≥seven prenatal consultations, between 20 and 35 years, with no schooling and widows. |
| Rehder et al.53 | Public | Cross-sectional | n=409 | Fasting blood glucose, age, history of GDM, history of macrosomia, chronic hypertension, BMI | LGA: >90th percentile; Macrosomia >4000g, without reference | Macrosomia 8.6% and LGA 19.3% | Risk of macrosomia increased for history of macrosomia (RR=3.2, 95% CI 1.5–6.6). Risk of LGA increased for history of macrosomia (RR=2.0, 95% CI 1.2–3.4) and maternal BMI ≥25kg/m2 (RR=1.9, 95% CI 1.2–3.0) |
| Nomura et al.41 | Public | Cross-sectional | n=374 | White color, nulliparous, smoking, clinical/obstetric complications (systemic arterial hypertension, DM, maternal heart disease, premature rupture of membranes, collagenosis), cesarean section, classification by pre-gestational BMI and at the end of pregnancy (low weight, adequate weight, overweight, and obesity). | GIG >90th percentile, Alexander et al.83 | 3.5% | DM (OR=20.2, 95% CI, 5.3–76.8) and obesity at the end of pregnancy (OR=3.6, 95% CI, 1.1–11.7) were independently associated with LGA NB |
| Fonseca et al.47 | Public | Cross-sectional | n=712 | Initial BMI of the pregnant woman and GWG | Excess weight: ≥4000g, WHO82 | 4.2% | There was a higher prevalence of NB with excess weight in the group of pregnant women with overweight/obesity at the beginning of pregnancy (p<0.01) and with excess GWG (p<0.01) |
| Padilha et al.50 | Public | Cross-sectional | n=827 | GWG | LGA >90th percentile Pedreira et al.84 | 5.7% | There was no association between GWG and LGA NB |
| Carniello et al.54 | Public | Cross-sectional | n=232 | Maternal nutritional status | Weight >90th percentile, Lubchenco et al.36 | 19.3% | Higher prevalence of LGA NB for overweight/obese mothers (p=0.030) |
| Castro et al.49 | Public | Cross-sectional | n=297 | NB gender, skin color, marital status, smoking, alcohol consumption, parity, pre-gestational BMI, GWG, cholesterol, and saturated, monosaturated, and polyunsaturated fat | LGA >90th percentile, Villar et al.85 | 13.1% | There was a positive association between dietary cholesterol intake (PR=2.48, 95% CI 1.31–4.66), excess GWG (PR=2.26, 95% CI 1.21–4.24) and family income (PR=1.01, 95% CI 1.00–1.01) with LGA NB. |
| Vernini et al.52 | Public | Cross-sectional | n=258 | Pre-gestational BMI | ≥4000g, LGA, without reference | ≥4000g: 7.4% LGA: 8.9% | Obese women showed the highest rate of LGA NB (p=0.021). |
| Farias et al.14 | Public | Cohort | n=199 | Maternal age, schooling, smoking, alcohol consumption, parity, physical activity in the pre-gestational leisure time, pre-gestational BMI, pre-gestational energy consumption, GWG, blood glucose, HDL-c, LDL-c, total cholesterol, triglycerides, leptin, and adiponectin per trimester. | LGA: weight >90th percentile, Villar et al.85 | 18.1% | Higher frequency of LGA in women with overweight or early obesity (p=0.042). The rate of gestational HDL-c was negatively associated with LGA (OR=0.02, 95% CI 0.0003–0.88). Higher basal level of gestational leptin was positively associated with LGA (OR=3.92, 95% CI, 1.18–12.95) |
| North region | |||||||
| Santos et al.58 | Public | Cross-sectional | n=23,961 | Maternal age | ≥4000g, without reference | <20 years: 3.8% 20–29 years: 7.7% ≥30 years: 11.8% Total prevalence: 6.8% | Higher prevalence of NB ≥4000g with increasing age (p<0.001)a |
| Northeast region | |||||||
| Lima and Sampaio60 | Public | Cross-sectional | n=277 | Maternal age, marital status, schooling, per capita income, parity, interpregnancy interval, prenatal care frequency, and maternal height. | ≥4000g, Puffer and Serrano,86 PAHO | 5.4% | Association between birth weight ≥4000g and maternal height >1.50m (p=0.001) |
| Amorim et al.43 | Public | Cross-sectional | n=551 | Maternal age, parity, pre-gestational overweight/obesity, excess weight gain, overweight/obese at the last consultation, hypertension, DM (any type), preeclampsia, GDM | Macrosomia ≥4000g, WHO82 | 5.4% | Macrosomia was associated with any type of DM (adjusted risk=17.7; 95% CI=4.8–64.9) and excess GWG (adjusted risk=6.1, 95% CI=2.7–13.7) |
| Santos et al.34 | Public | Cohort | n=204 | GWG and anemia | LGA: >90th percentile, without reference | 9.8% | Excess GWG (RR=4.7, 95% CI 1.6–14.0) and anemia (RR=3.4, 95% CI 1.4–8.1) were associated with LGA NB |
| Silva and Macedo40 | Public/Private | Cross-sectional | n=158 | GWG | Macrosomia ≥4000g, without reference | 17.8% | Higher frequency of macrosomia in women with excess GWG (p=0.044) |
| Midwest region | |||||||
| Costa et al.46 | Public | Cohort | n=200 | GWG | Macrosomia ≥4000g, without reference | Incidence: 6.5% | Macrosomia was associated with excess GWG (p<0.01). |
| South, Southeast, North, and Northeast regions | |||||||
| Nucci et al.51 | Public | Cohort | n=5564 | Pre-gestational BMI | Macrosomia: weight >90th percentile, without reference | Not described | Pre-obese and obese women showed higher risk of having children with macrosomia (OR=1.6, 95% CI 1.3–2.0 and OR=1.5, 95% CI 1.1–2.2) |
| Schmidt et al.26 | Public | Cohort | n=4977 | GDM | Macrosomia: birth weight ≥90th percentile of gestational age, without reference | ADA87: 17.7%; WHO82: 14.6% | GDM predicts an increased risk of 30–45% of children born with macrosomia |
| Drehmer et al.48 | Public | Cohort | n=2244 | GWG | LGA>90th percentile in relation to gestational age, without reference | 10.5% | Increased risk for LGA in women with excess GWG in the second trimester (RR=1.64, 95% CI 1.16–2.31) and excess total GWG (RR=2.12, 95% CI 1.55–2.89). |
| Trujillo et al.56 | Public | Cohort | n=4926 | DM | LGA >90th percentile, without reference | 11.8% | Pregnant women with GDM had an increased risk (RR=1.27–1.86) for the birth of LGA NB in the IADPSG and WHO classifications |
INPS/IPESC, National Institute of Social Security/Institute of Social Security of Santa Catarina; WHO, World Health Organization; ADA, American Diabetes Association; DM, Diabetes Mellitus; GDM, Gestational Diabetes Mellitus; SUS, Sistema Único de Saúde (Brazilian Unified Health System); BMI, body mass index; SINASC, Sistema Nacional de Nascidos Vivos (National System of Live Births); PAHO, Pan-American Health Organization; UNESP, Universidade Estadual Paulista; RNHBPEPWG, Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy; SGA, small for gestational age; AGA, appropriate for gestational age; LGA, large for gestational age; GWG, gestational weight gain; OGTT, oral glucose tolerance test; IOM/NRC, Institute of Medicine/National Research Council; IADPSG, The International Association of Diabetes and Pregnancy Study Groups; IB, daily hyperglycemia-glucose tolerance test (GTT) 100g altered and altered glycemic profile (GP); IIA, GTT 100g altered and normal GP; IIB, 100g GTT and altered GP.
Two authors (L.F.S. and J.M.) were in charge of reviewing the methodological quality and the risks of bias according to the scale adapted from Downs and Black38 (Table 2), considering only the studies that fit the inclusion criteria. A third author (C.K.) evaluated and defined any disagreements. The Downs and Black scale aims to evaluate studies not related to randomized clinical trials; it comprises 27 applicable questions/items to assess the quality and biases of articles.38 These criteria assess the quality of data, internal validity (biases and confounding factors), external validity, and the ability of the study to detect a significant effect.
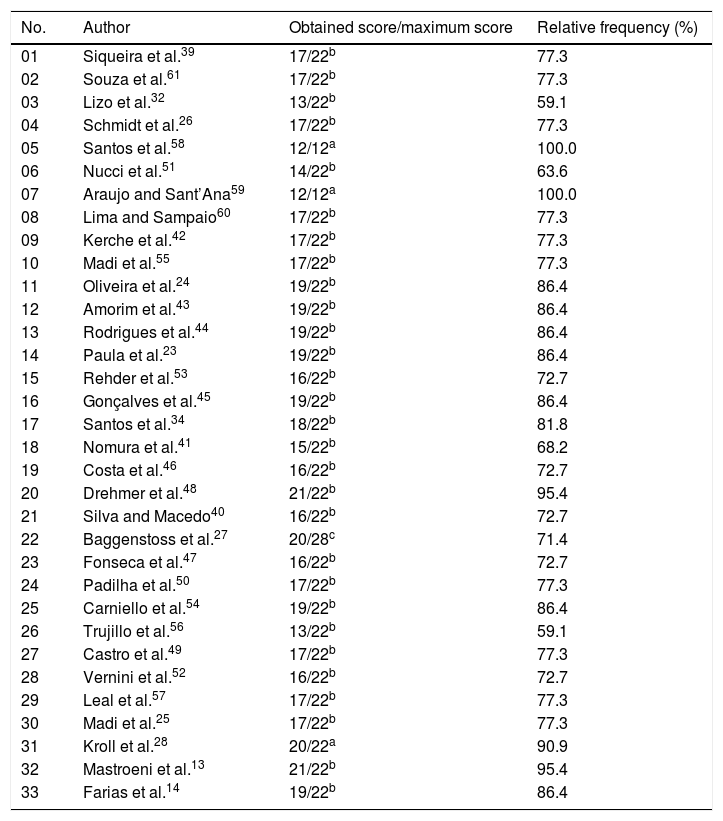
Risk of bias assessment adapted from Downs and Black.38
| No. | Author | Obtained score/maximum score | Relative frequency (%) |
|---|---|---|---|
| 01 | Siqueira et al.39 | 17/22b | 77.3 |
| 02 | Souza et al.61 | 17/22b | 77.3 |
| 03 | Lizo et al.32 | 13/22b | 59.1 |
| 04 | Schmidt et al.26 | 17/22b | 77.3 |
| 05 | Santos et al.58 | 12/12a | 100.0 |
| 06 | Nucci et al.51 | 14/22b | 63.6 |
| 07 | Araujo and Sant’Ana59 | 12/12a | 100.0 |
| 08 | Lima and Sampaio60 | 17/22b | 77.3 |
| 09 | Kerche et al.42 | 17/22b | 77.3 |
| 10 | Madi et al.55 | 17/22b | 77.3 |
| 11 | Oliveira et al.24 | 19/22b | 86.4 |
| 12 | Amorim et al.43 | 19/22b | 86.4 |
| 13 | Rodrigues et al.44 | 19/22b | 86.4 |
| 14 | Paula et al.23 | 19/22b | 86.4 |
| 15 | Rehder et al.53 | 16/22b | 72.7 |
| 16 | Gonçalves et al.45 | 19/22b | 86.4 |
| 17 | Santos et al.34 | 18/22b | 81.8 |
| 18 | Nomura et al.41 | 15/22b | 68.2 |
| 19 | Costa et al.46 | 16/22b | 72.7 |
| 20 | Drehmer et al.48 | 21/22b | 95.4 |
| 21 | Silva and Macedo40 | 16/22b | 72.7 |
| 22 | Baggenstoss et al.27 | 20/28c | 71.4 |
| 23 | Fonseca et al.47 | 16/22b | 72.7 |
| 24 | Padilha et al.50 | 17/22b | 77.3 |
| 25 | Carniello et al.54 | 19/22b | 86.4 |
| 26 | Trujillo et al.56 | 13/22b | 59.1 |
| 27 | Castro et al.49 | 17/22b | 77.3 |
| 28 | Vernini et al.52 | 16/22b | 72.7 |
| 29 | Leal et al.57 | 17/22b | 77.3 |
| 30 | Madi et al.25 | 17/22b | 77.3 |
| 31 | Kroll et al.28 | 20/22a | 90.9 |
| 32 | Mastroeni et al.13 | 21/22b | 95.4 |
| 33 | Farias et al.14 | 19/22b | 86.4 |
To assess the risk of bias using the Downs and Black criteria,38 the articles of this systematic review were grouped into three different categories, each with a specific score: (a) first category: articles involving prevalence-type cross-sectional studies, with a maximum score of 12; (b) second category: articles with a cross-sectional and cohort methodological design, with a maximum score of 22; (c) third category: articles involving case–control studies, with intervention and maximum score of 28. To guarantee the proportion of results between the categories, the score obtained from each article was divided by the maximum possible score for each of the three established categories (Table 2).
Association measures usedThis review considered only studies that performed the chi-squared test of proportions or Fischer's exact test to determine the association between excess birth weight and the risk factors. In case of doubt regarding the analysis used in the study, the authors were contacted by e-mail to check if the data were correct. Additionally, the measures of odds ratio, relative risk, and prevalence ratio (PR) were also considered to assess the effect of risk factors and excess birth weight. When a study did not report the p-value for its analyses, the confidence intervals were used to describe whether there was statistical significance. Only categorical variables were considered in this study.
Synthesis of resultsIt was decided not to include meta-analyses in this systematic review due to the heterogeneity of the data between the considered studies, and the different statistical methods used to assess risk in the studies.
Risk of publication biasTo reduce the risk of bias in the study, the selected articles were assessed by considering each risk factor individually, according to the reference category of excess birth weight (>4000g, ≥4000g, >90th percentile or ≥90th percentile).
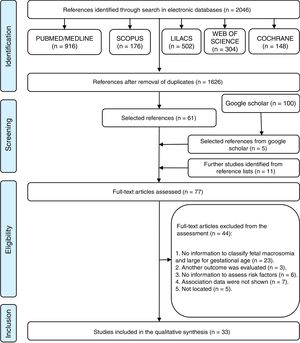
ResultsStudy selectionUsing the selected databases to search for the articles, 2046 articles were identified on the topic of interest. After the removal of 420 duplicated articles, 1626 articles in English, Portuguese, and Spanish were obtained for the analysis. A comprehensive title and abstract analysis eliminated 1565 articles, resulting in 61 articles in the first stage of the study. Based on the analysis of the first 100 results of Google Scholar, five new articles were added, and another 11 articles were added from the references of previously selected articles, totaling 77 articles eligible for the second stage of the review.
In the second stage, all 77 articles were read in full and 44 were excluded from the analysis; 23 of them due to lack of data for the nutritional status classification, three because the articles assessed another outcome, six because they did not provide enough data to assess the risk factors, seven because they did not evaluate the association between the outcome and the predictors, and five because the full-text article was not found (Appendix 2). The flow chart showing the process of identification, inclusion, and exclusion of studies is shown in Fig. 1.
Study characteristicsThe studies used in this review were published in the last four decades (1981–2017) and were carried out in the five regions of Brazil. Most of the studies were carried out in the Southeast (55.0%) and South (39.0%) regions. The total sample included 105,826 newborns, with most of them (60.6%) from cross-sectional studies, and 36.4% from cohort studies. Most of the studies used the scores of FM ≥4000g (42.5%) or LGA >90th percentile (42.5%) to assess the newborns’ nutritional status. The prevalence of fetal macrosomia varied between 1.74%39 and 17.8%,40 whereas the prevalence of LGA varied between 3.5%41 and 30.1%.42 The characteristics of the studies included in this review are shown in Table 1.
Risk of bias in the studiesThe assessment of the methodological quality and risk of bias is shown in Table 2. Of the 33 articles evaluated, a mean score of 79.6% was obtained, with a maximum score of 100.0% and a minimum score of 59.1%. Twenty articles showed values below the mean score and, therefore, were considered as having risk of bias and reduced methodological quality.
Synthesis of resultsTable 3 shows the risk factors and their association with the assessed outcome. There were 67 risk factors found for excess birth weight in the five regions of the country. Of these, 31 risk factors were significantly associated with the outcome (Table 3). Risk factors were grouped according to five main characteristics: (a) biological, (b) socioeconomic, (c) other risk factors, (d) risk factors not associated with excess birth weight, and (e) region of the country (South, Southeast, North, Northeast, and Midwest).
Risk factors associated with excess birth weight in Brazil.
| Variables | Outcome | OR, RR, and PR (95% CI) | Adjustment variables | p-valuea | Region | Author |
|---|---|---|---|---|---|---|
| Gestational weight gain | ||||||
| >12kg | ≥4000g | <0.001 | SE | Lizo et al.32 | ||
| Excess | ≥4000g | RR=2.80 (0.80–7.70) | 0.070 | SE | Oliveira et al.24 | |
| Excess | ≥4000g | PR=6.90 (2.90–16.90) | NE | Amorim et al.43 | ||
| Excess | ≥4000g | <0.001 | NE | Rodrigues et al.44 | ||
| 9–12kg | ≥4000g | OR=1.30 (0.70–2.40) | 0.030 | S | Gonçalves et al.45 | |
| 13–16kg | ≥4000g | OR=1.10 (0.60–2.30) | 0.030 | S | Gonçalves et al.45 | |
| ≥17kg | ≥4000g | OR=1.70 (0.80–3.40) | 0.030 | S | Gonçalves et al.45 | |
| Excess | ≥4000g | 0.010 | MW | Costa et al.46 | ||
| Excess | ≥4000g | 0.044 | NE | Silva and Macedo40 | ||
| Excess | ≥4000g | OR=1.75 (0.76–4.04) | 0.260 | SE | Fonseca et al.47 | |
| >16kg | >90th percentile | OR=1.79 (1.23–2.60) | 0.020 | SE | Kerche et al.42 | |
| Excess | >90th percentile | RR=4.70 (1.60–14.00) | 0.009 | NE | Santos et al.34 | |
| Excess 2nd trimester | >90th percentile | RR=1.64 (1.16–2.31) | S, SE, N, NE | Drehmer et al.48 | ||
| Excess | >90th percentile | RR=2.12 (1.55–2.89) | S, SE, N, NE | Drehmer et al.48 | ||
| Excess | >90th percentile | OR=0.95 (0.48–1.86) | Smoking, parity, number of prenatal consultations, nutritional assistance | 0.891 | SE | Padilha et al.50 |
| Excess | >90th percentile | PR=2.26 (1.21–4.24) | Maternal age, family income, pre-gestational BMI, GWG, cholesterol | 0.011 | SE | Castro et al.49 |
| Excess | >90th percentile | 0.013 | S | Kroll et al.28 | ||
| Excess | >90th percentile | OR=2.11 (1.27–3.15) | Schooling, family income, smoking during pregnancy, age of first child, pre-gestational BMI, glycated hemoglobin | S | Mastroeni et al.13 | |
| Pre-gestational BMI | ||||||
| Pre-obese | >90th percentile | OR=1.61 (1.30–2.00) | S, SE, N, NE | Nucci et al.51 | ||
| Obese | >90th percentile | OR=1.53 (1.08–2.17) | S, SE, N, NE | Nucci et al.51 | ||
| ≥25kg/m2 | >90th percentile | OR=1.83 (1.27–2.64) | 0.003 | SE | Kerche et al.42 | |
| Overweight/obesity | >90th percentile | 0.020 | SE | Nomura et al.41 | ||
| ≥25kg/m2 | >90th percentile | PR=1.88 (1.05–3.36) | 0.033 | SE | Castro et al.49 | |
| Obesity | >90th percentile | 0.021 | SE | Vernini et al.52 | ||
| <25kg/m2 | >90th percentile | 0.677 | S | Kroll et al.28 | ||
| Overweight | >90th percentile | OR=1.00 (0.54–1.79) | Schooling, family income, smoking during pregnancy, age of first child, GWG, glycated hemoglobin | S | Mastroeni et al.13 | |
| Obesity | >90th percentile | OR=1.15 (0.56–2.36) | Schooling, family income, smoking during pregnancy, age of first child, GWG, glycated hemoglobin | S | Mastroeni et al.13 | |
| ≥25kg/m2 | >90th percentile | 0.042 | SE | Farias et al.14 | ||
| Overweight/obesity | ≥4000g | RR=3.70 (1.80–9.20) | 0.010 | SE | Oliveira et al.24 | |
| Overweight/obesity | ≥4000g | PR=2.80 (1.00–7.80) | NE | Amorim et al.43 | ||
| Overweight | ≥4000g | OR=3.40 (0.40–26.10) | 0.001 | S | Gonçalves et al.45 | |
| Obesity | ≥4000g | OR=6.70 (0.90–52.50) | 0.001 | S | Gonçalves et al.45 | |
| Obesity | ≥4000g | 0.037 | SE | Vernini et al.52 | ||
| Obesity | ≥4000g | OR=1.20 (1.44–2.32) | Hyperglycemic Disorder | <0.010 | S | Madi et al.25 |
| BMI ≥25kg/m2on the last consultation | ≥4000g | PR=4.90 (2.00–12.50) | NE | Amorim et al.43 | ||
| BMI during pregnancy | ||||||
| ≥25kg/m2 | >90th percentile | RR=1.90 (1.20–3.00) | SE | Rehder et al.53 | ||
| Overweight/obesity | >90th percentile | 0.030 | SE | Carniello et al.54 | ||
| Overweight/obesity | >90th percentile | 0.340 | S | Leal et al.57 | ||
| ≥25kg/m2 | >4000g | RR=2.00 (0.90–4.00) | SE | Rehder et al.53 | ||
| Obesity at the moment of delivery | >90th percentile | OR=3.60 (1.10–11.70) | Smoking, diagnosis of arterial hypertension, DM, GWG, pre-gestational BMI, BMI at the end of pregnancy, classification of maternal nutritional status by pre-gestational BMI and at the end of pregnancy | 0.040 | SE | Nomura et al.41 |
| BMI ≥25kg/m2at the beginning of pregnancy | ≥4000g | <0.010 | SE | Fonseca et al.47 | ||
| Association of pre-gestational BMI and GWG | ||||||
| Low/normal weight and excess GWG | >90th percentile | OR=2.08 (1.10–3.95) | Schooling, family income, smoking during pregnancy, age of first child, glycated hemoglobin | S | Mastroeni et al.13 | |
| Overweight and appropriate GWG | >90th percentile | OR=0.46 (0.13–1.64) | Schooling, family income, smoking during pregnancy, age of first child, glycated hemoglobin | S | Mastroeni et al.13 | |
| Overweight and excess GWG | >90th percentile | OR=2.54 (1.27–5.10) | Schooling, family income, smoking during pregnancy, age of first child, glycated hemoglobin | S | Mastroeni et al.13 | |
| Obesity and appropriate GWG | >90th percentile | OR=1.94 (0.72–5.25) | Schooling, family income, smoking during pregnancy, age of first child, glycated hemoglobin | S | Mastroeni et al.13 | |
| Obesity and excess GWG | >90th percentile | OR=1.54 (0.58–4.08) | Schooling, family income, smoking during pregnancy, age of first child, glycated hemoglobin | S | Mastroeni et al.13 | |
| Diabetes mellitus | ||||||
| Present | >90th percentile | 0.050 | SE | Kerche et al.42 | ||
| Present | >90th percentile | OR=20.2 (5.30–76.80) | Smoking, diagnosis of arterial hypertension, DM, GWG, pre-gestational BMI, BMI at the end of pregnancy, maternal nutritional status classification by pre-gestational BMI and at the end of the pregnancy | <0.001 | SE | Nomura et al.41 |
| Present | >90th percentile | 0.580 | S | Kroll et al.28 | ||
| Present | >90th percentile | OR=1.08 (0.47–2.51) | S | Mastroeni et al.13 | ||
| Present | ≥4000g | OR=4.20 (2.70–6.40) | <0.050 | S | Madi et al.25 | |
| Present | ≥4000g | PR=8.90 (4.10–19.40) | SE | Amorim et al.43 | ||
| Presence of GDM | ||||||
| ≥90th percentile | ADA, RR=1.29 (0.73–2.18) | Ethnicity, maternal height, pre-gestational BMI, GWG, and NB gender. | S, SE, N, NE | Schmidt et al.26 | ||
| ≥90th percentile | WHO, RR=1.45 (1.06–1.95) | Ethnicity, maternal height, pre-gestational BMI, GWG, and NB gender. | S, SE, N, NE | Schmidt et al.26 | ||
| ≥90th percentile | 0.100 | S | Leal et al.57 | |||
| >90th percentile | 0.050 | SE | Kerche et al.42 | |||
| ≥90th percentile | IADPSG, RR=1.40 (1.15–1.70) | S, SE, N, NE | Trujillo et al.56 | |||
| ≥90th percentile | WHO, RR=1.67 (1.30–2.15) | S, SE, N, NE | Trujillo et al.56 | |||
| ≥90th percentile | ADA, RR=1.50 (0.95–2.34) | S, SE, N, NE | Trujillo et al.56 | |||
| ≥4000g | PR=12.0 (6.0–24.2) | NE | Amorim et al.43 | |||
| History of DM | ||||||
| Any | >90th percentile | 0.262 | SE | Kerche et al.42 | ||
| Family | >90th percentile | 0.073 | SE | Kerche et al.42 | ||
| Personal | >90th percentile | OR=1.56 (1.05–2.31) | 0.003 | SE | Kerche et al.42 | |
| Obstetric | >90th percentile | <0.001 | SE | Kerche et al.42 | ||
| History of GDM | ||||||
| >90th percentile | RR=0.40 (0.10–2.60) | SE | Rehder et al.53 | |||
| >4000g | RR=0.90 (0.10–6.60) | SE | Rehder et al.53 | |||
| Groups of Rudge IB, IIA+IIB | >90th percentile | 0.030 | SE | Kerche et al.42 | ||
| Total blood glucose mean ≥120mg/dL | >90th percentile | OR=1.78 (1.13–2.80) | 0.000 | SE | Kerche et al.42 | |
| Fasting blood glucose (mg/dL) | ||||||
| ≥90 | >90th percentile | 0.069 | SE | Kerche et al.42 | ||
| ≥90 | >90th percentile | RR=1.10 (0.70–1.70) | SE | Rehder et al.53 | ||
| 80.0–175.0 | ≥4000g | RR=1.70 (0.50–4.80) | 0.380 | SE | Oliveira et al.24 | |
| ≥90 | >4000g | RR=0.90 (0.40–2.00) | SE | Rehder et al.53 | ||
| Postprandial blood glucose ≥130mg/dL | >90th percentile | 0.012 | SE | Kerche et al.42 | ||
| Maternal age group (years) | ||||||
| >35 | >4000g | RR=1.00 (0.50–2.20) | SE | Rehder et al.53 | ||
| 20–30 | ≥4000g | <0.001 | NE | Santos et al.58 | ||
| >30 | ≥4000g | <0.001 | NE | Santos et al.58 | ||
| >30 | ≥4000g | 0.048 | S | Araujo and Sant’Ana59 | ||
| 25–29 | >4000g | 0.420 | NE | Lima and Sampaio60 | ||
| 30–39 | ≥4000g | RR=2.40 (0.90–4.80) | 0.050 | SE | Oliveira et al.24 | |
| ≥25 | ≥4000g | PR=1.20 (0.60–2.40) | NE | Amorim et al.43 | ||
| ≥20 | ≥4000g | <0.001 | SE | Paula et al.23 | ||
| ≥25 | >90th percentile | 0.086 | SE | Kerche et al.42 | ||
| >35 | >90th percentile | RR=1.10 (0.70–1.80) | SE | Rehder et al.53 | ||
| <20 | >90th percentile | 0.496 | S | Kroll et al.28 | ||
| 20–30 | >90th percentile | OR=0.73 (0.39–1.35) | S | Mastroeni et al.13 | ||
| ≥30 | >90th percentile | OR=0.94 (0.47–1.85) | S | Mastroeni et al.13 | ||
| ≤30 | >90th percentile | 0.545 | SE | Farias et al.14 | ||
| Maternal age | >90th percentile | PR=1.04 (1.0–1.09) | 0.073 | SE | Castro et al.49 | |
| Parity (number of children) | ||||||
| ≥2 | ≥4000g | 0.700 | NE | Lima and Sampaio60 | ||
| ≥2 | ≥4000g | RR=3.80 (1.10–9.90) | Age, marital status, parity, NB gender, pre-gestational BMI, GWG | 0.030 | SE | Oliveira et al.24 |
| ≥2 | ≥4000g | PR=1.00 (0.50–2.00) | NE | Amorim et al.43 | ||
| ≥3 | >90th percentile | 0.136 | SE | Kerche et al.42 | ||
| 0 | >90th percentile | 0.400 | SE | Nomura et al.41 | ||
| ≥2 | >90th percentile | PR=1.41 (0.72–2.78) | 0.317 | SE | Castro et al.49 | |
| ≥3 | >90th percentile | OR=1.30 (0.77–2.19) | S | Mastroeni et al.13 | ||
| ≥1 | >90th percentile | 0.137 | SE | Farias et al.14 | ||
| Child's gender | ||||||
| Male | >4000g | <0.001 | SE | Siqueira et al.39 | ||
| Male | ≥4000g | RR=7.50 (1.00–37.60) | Age, marital status, parity, NB gender, pre-gestational BMI, GWG | 0.050 | SE | Oliveira et al.24 |
| Male | ≥4000g | 0.014 | SE | Paula et al.23 | ||
| Female | >90th percentile | 0.674 | SE | Castro et al.49 | ||
| Male | >90th percentile | 0.269 | S | Kroll et al.28 | ||
| Female | >90th percentile | OR=0.93 (0.60–1.44) | S | Mastroeni et al.13 | ||
| Maternal height (m) | ||||||
| >1.5 | ≥4000g | 0.001 | NE | Lima and Sampaio60 | ||
| 1.6–1.8 | ≥4000g | RR=1.80 (0.60–4.80) | 0.280 | SE | Oliveira et al.24 | |
| Previous macrosomia | ||||||
| >90th percentile | OR=2.37 (1.60–3.50) | <0.001 | SE | Kerche et al.42 | ||
| >90th percentile | RR=2.00 (1.20–3.40) | SE | Rehder et al.53 | |||
| >4000g | RR=3.20 (1.50–6.60) | SE | Rehder et al.53 | |||
| Arterial hypertension | ||||||
| >90th percentile | 0.126 | SE | Kerche et al.42 | |||
| >90th percentile | RR=0.80 (0.50–1.30) | SE | Rehder et al.53 | |||
| >90th percentile | 0.100 | SE | Nomura et al.41 | |||
| >90th percentile | 0.800 | S | Leal et al.57 | |||
| ≥4000g | PR=2.90 (1.10–7.90) | NE | Amorim et al.43 | |||
| >4000g | RR=1.60 (0.60–3.00) | SE | Rehder et al.53 | |||
| Cesarean delivery | ||||||
| >90th percentile | 0.100 | SE | Nomura et al.41 | |||
| >90th percentile | 0.023 | S | Kroll et al.28 | |||
| ≥4000g | <0.001 | SE | Paula et al.23 | |||
| Marital status | ||||||
| Common-law marriage | ≥4000g | 0.980 | NE | Lima and Sampaio60 | ||
| Married | ≥4000g | RR=3.00 | 0.030 | SE | Oliveira et al.24 | |
| Single/other | ≥4000g | 0.004 | SE | Paula et al.23 | ||
| Single/other | >90th percentile | PR=0.87 (0.40–1.87) | 0.717 | SE | Castro et al.49 | |
| Married | >90th percentile | 0.173 | S | Kroll et al.28 | ||
| Single/other | >90th percentile | OR=0.61 (0.32–1.16) | S | Mastroeni et al.13 | ||
| Per capita income <1MW | ≥4000g | 0.350 | NE | Lima and Sampaio60 | ||
| Total family income (MW) | ||||||
| ≥1 | ≥4000g | RR=1.50 (0.50–4.20) | 0.450 | SE | Oliveira et al.24 | |
| ≥3 | ≥4000g | 0.447 | S | Kroll et al.28 | ||
| <3 | ≥4000g | OR=0.73 (0.44–1.23) | Schooling, smoking during pregnancy, age of first child, glycated hemoglobin | S | Mastroeni et al.13 | |
| Total family income | >90th percentile | PR=1.01 (1.00–1.01) | Schooling, maternal age, pre-gestational BMI, GWG, total cholesterol | 0.014 | SE | Castro et al.49 |
| Prenatal consultations | ||||||
| ≥6 | ≥4000g | 0.970 | NE | Lima and Sampaio60 | ||
| ≥7 | ≥4000g | 0.001 | SE | Paula et al.23 | ||
| <6 | >90th percentile | OR=0.69 (0.39–1.20) | S | Mastroeni et al.13 | ||
| Social security affiliation INPS/IPESC | ≥4000g | <0.01 | S | Souza et al.61 | ||
| Age at first delivery <20 years | >90th percentile | OR=1.90 (1.14–3.17) | Schooling, family income, smoking during pregnancy, glycated hemoglobin | S | Mastroeni et al.13 | |
| Anemia | >90th percentile | RR=3.40 (1.40–8.10) | 0.040 | S | Gonçalves et al.45 | |
| Level of schooling | ||||||
| <4 years | ≥4000g | 0.570 | NE | Lima and Sampaio60 | ||
| ≤4 years | ≥4000g | RR=1.80 (0.50–5.30) | 0.360 | SE | Oliveira et al.24 | |
| None | ≥4000g | 0.661 | SE | Paula et al.23 | ||
| 9–12 years | >90th percentile | 0.285 | S | Kroll et al.28 | ||
| <8 years | >90th percentile | OR=0.62 (0.32–1.20) | Family income, smoking during pregnancy, age of first child, glycated hemoglobin | S | Mastroeni et al.13 | |
| >8 years | >90th percentile | 0.519 | SE | Farias et al.14 | ||
| Interpregnancy interval (years) | ||||||
| ≥5 | ≥4000g | 0.660 | NE | Lima and Sampaio60 | ||
| ≥2 | >90th percentile | 0.459 | S | Kroll et al.28 | ||
| Family history of macrosomia | ||||||
| >90th percentile | RR=1.50 (0.90–2.30) | SE | Rehder et al.53 | |||
| >4000g | RR=1.00 (0.50–2.20) | SE | Rehder et al.53 | |||
| Smoking | ||||||
| No | >90th percentile | 0.278 | SE | Kerche et al.42 | ||
| No | >90th percentile | 0.060 | SE | Nomura et al.41 | ||
| Yes | >90th percentile | PR=0.53 (0.17–1.66) | Schooling, family income, age of first child, glycated hemoglobin | S | Mastroeni et al.13 | |
| Yes | >90th percentile | OR=0.64 (0.18–2.28) | ||||
| No | >90th percentile | 0.093 | SE | Farias et al.14 | ||
| Smoking before pregnancy | ||||||
| No | >90th percentile | 0.079 | S | Kroll et al.28 | ||
| Yes | >90th percentile | OR=0.58 (0.23–1.43) | Schooling, family income, age of first child, glycated hemoglobin | S | Mastroeni et al.13 | |
| Alcohol consumption | ||||||
| Yes | >90th percentile | PR=0.62 (0.23–1.16) | 0.348 | SE | Castro et al.49 | |
| No | >90th percentile | 0.806 | SE | Farias et al.14 | ||
| Use of insulin | >90th percentile | 0.085 | SE | Kerche et al.42 | ||
| Previous miscarriage | ≥4000g | RR=1.02 (0.30–3.10) | 0.980 | SE | Oliveira et al.24 | |
| Gestational age (weeks) | ||||||
| 35–40 | ≥4000g | RR=0.90 (0.20–3.70) | 0.920 | SE | Oliveira et al.24 | |
| ≥42 | ≥4000g | 0.565 | SE | Paula et al.23 | ||
| White skin color | ||||||
| ≥4000g | RR=1.90 (0.60–5.00) | 0.230 | SE | Oliveira et al.24 | ||
| >90th percentile | 0.500 | SE | Nomura et al.41 | |||
| >90th percentile | PR=1.38 (0.56–3.35) | 0.481 | SE | Castro et al.49 | ||
| Age at menarche <13years | ≥4000g | RR=1.10 (0.40–3.30) | 0.810 | SE | Oliveira et al.24 | |
| Sedentary lifestyle | ≥4000g | RR=1.20 (0.20–3.10) | 0.740 | SE | Oliveira et al.24 | |
| Pre-gestational physical activity | >90th percentile | 0.102 | SE | Farias et al.14 | ||
| Preeclampsia | ≥4000g | PR=1.70 (0.60–4.70) | NE | Amorim et al.43 | ||
| Number of pregnancies ≥3 | ||||||
| >90th percentile | 0.642 | S | Kroll et al.28 | |||
| >90th percentile | OR=1.45 (0.86–2.43) | S | Mastroeni et al.13 | |||
| Maternal heart disease | >90th percentile | 0.600 | SE | Nomura et al.41 | ||
| Premature rupture of membranes | >90th percentile | 0.100 | SE | Nomura et al.41 | ||
| Collagenosis | >90th percentile | 0.700 | SE | Nomura et al.41 | ||
| Maternal energy consumption (Kcal) | >90th percentile | PR=1.00 (1.00–1.00) | 0.842 | SE | Castro et al.49 | |
| Fat consumption (mg/1000kcal) | ||||||
| Saturated: 4th quartile (11.4–18.3) | >90th percentile | PR=1.34 (0.71–2.51) | 0.362 | SE | Castro et al.49 | |
| Monosaturated: 4th quartile (7.7–20.0) | >90th percentile | PR=1.34 (0.71–2.51) | 0.362 | SE | Castro et al.49 | |
| Polyunsaturated: 4th quartile (4.2–6.8) | >90th percentile | PR=1.48 (0.80–2.73) | 0.210 | SE | Castro et al.49 | |
| Polymorphisms (allele) | ||||||
| Mutant G54D (maternal) | >90th percentile | 0.149 | S | Baggenstoss et al.27 | ||
| ADIPOQ rs2241766 mutant (NB) | >90th percentile | OR=2.01 (0.90–4.47) | Maternal age, schooling, family income, marital status, GWG, smoking before pregnancy, DM, NB gender, ADIPOQ rs2241766, LEP rs7799039, FTO rs9939609 | 0.087 | S | Kroll et al.28 |
| Wild LEP rs7799039 (NB) | >90th percentile | OR=1.98 (1.05–3.74) | Maternal age, schooling, family income, marital status, GWG, smoking before pregnancy, DM, NB gender, ADIPOQ rs2241766, LEP rs7799039, FTO rs9939609 | 0.036 | S | Kroll et al.28 |
| Mutant FTO rs9939609 (NB) | >90th percentile | OR=1.11 (0.59–2.11) | Maternal age, schooling, family income, marital status, GWG, smoking before pregnancy, DM, NB gender, ADIPOQ rs2241766, LEP rs7799039, FTO rs9939609 | 0.744 | S | Kroll et al.28 |
| Total cholesterol levels: mg/1000kcal. 4th quartile (183.5–466.7) | >90th percentile | PR=2.48 (1.31–4.66) | Maternal age, family income, pre-gestational BMI, GWG, total cholesterol | 0.005 | SE | Castro et al.49 |
| Levels of HDL-c cholesterol according to gestational age | >90th percentile | OR=0.02 (0.00–0.88) | Log of triglycerides, leptin and adiponectin, maternal age, schooling, parity, pre-gestational physical activity, blood glucose, GWG, and BMI at the beginning of the pregnancy | 0.043 | SE | Farias et al.14 |
| Levels of LDL-c cholesterol according to gestational age | >90th percentile | OR=1.52 (0.80–2.88) | Log of triglycerides, leptin and adiponectin, maternal age, schooling, parity, pre-gestational physical activity, blood glucose, GWG, and BMI at the beginning of the pregnancy | 0.203 | SE | Farias et al.14 |
| Levels of triglycerides according to gestational age | >90th percentile | OR=1.0e+43 (0.00-9.5e+88) | Log of triglycerides, leptin and adiponectin, maternal age, schooling, parity, pre-gestational physical activity, blood glucose, GWG, and BMI at the beginning of pregnancy | 0.067 | SE | Farias et al.14 |
| Log of leptin concentration in the first trimester of pregnancy | >90th percentile | OR=3.92 (1.18–12.95) | Log of triglycerides, leptin and adiponectin, maternal age, schooling, parity, pre-gestational physical activity, blood glucose, GWG, and BMI at the beginning of the pregnancy | 0.025 | SE | Farias et al.14 |
| Log of adiponectin levels in the first trimester of pregnancy | >90th percentile | OR=0.54 (0.16–1.83) | Log of triglycerides, leptin and adiponectin, maternal age, schooling, parity, pre-gestational physical activity, blood glucose, GWG, and BMI at the beginning of the pregnancy | 0.321 | SE | Farias et al.14 |
| Presence of urinary tract infection | >90th percentile | 0.220 | S | Leal et al.57 | ||
| Presence of sexually transmitted disease | >90th percentile | 0.370 | S | Leal et al.57 | ||
p-value from the chi-square test. When p-value for OR, RR or PR was present, it was added. WHO, World and Health Organization; IADPSG, Association of Diabetes in Pregnancy Study Groups; ADA, American Diabetes Association. NB, newborn; INPS/IPESC, National Institute of Social Security/Institute of Social Security of Santa Catarina; BMI, body mass index; RR, relative risk; PR, prevalence ratio; OR, odds ratio; GWG, gestational weight gain; DM, diabetes mellitus; GDM, gestational diabetes mellitus; LDL-c, low-density lipoprotein cholesterol; HDL-c, high-density lipoprotein cholesterol; GP, glycemic profile; GTT, glucose tolerance test; IB, daily hyperglycemia – GTT 100g normal and altered glycemic profile; IIA, altered GTT 100g and normal GP; IIB, altered GTT 100g and GP; N, North; S, South; SE, Southeast; NE, Northeast; MW, Midwest.
Of the 15 studies that assessed excess GWG as a risk factor for excess birth weight,13,24,28,32,34,40,42–50 only three showed that excess GWG was not associated with excess birth weight.24,47,50
Pre-gestational BMITwelve studies investigated pre-gestational BMI as a risk factor for excess birth weight.13,14,24,25,28,41–43,45,49,51,52 Of these, two studies did not find a significant association with the evaluated outcome.13,28 Additionally, excess weight at the last consultation,43 excess weight during pregancy,53,54 obesity at delivery,41 excess weight at the start of pregnancy,47 and the association between pre-gestational overweight and excess GWG13 also demonstrated association with excess birth weight.
Diabetes mellitusOf the six studies13,28,41–43,55 that investigated the association between DM and the nutritional status of newborns, three studies showed a significant association between the presence of DM and excess birth weight.41,43,45 In relation to gestational DM (GDM), three26,43,56 of five studies26,42,43,56,57 showed a significant association between the presence of GDM and excess birth weight. Only one study showed a significant association between the risk factors: (1) family history and obstetric history of DM, (2) glycemic index (total glycemic mean ≥120mg/dL and postprandial blood glucose ≥130mg/dL), and (3) Rudge classification (IB or IIA+IIB) with excess birth weight.42
Maternal ageThirteen studies assessed the association between maternal age and nutritional status at birth.13,14,23,24,28,42,43,49,53,58–60 Of these, three showed that maternal age was significantly associated with excess birth weight: ≥20 years,23 20–30 years,58 and >30 years.58,59
ParityEight studies investigated the association between parity and nutritional status,13,14,24,41–43,49,60 and only one showed that mothers who had more than two children were significantly associated with excess birth weight.24
Child's genderSix studies13,23,24,28,39,49 investigated the association between gender and nutritional status at birth. Of these, two studies showed that male gender and excess birth weight were significantly associated.24,39
Maternal heightOnly one60 of the two studies24,60 that investigated maternal height and nutritional status showed that women with height >1.5m were significantly associated with excess birth weight.
History of fetal macrosomiaTwo studies showed a significant association between history of fetal macrosomia and excess birth weight.42,53
Arterial hypertension (AH)Four studies assessed the association between AH and nutritional status,42,43,53,57 and only one study showed a significant association between the presence of AH and excess birth weight.43
Type of deliveryThree studies23,28,41 investigated the association between type of delivery and nutritional status at birth, and two studies showed that the cesarean section and excess birth weight were significantly associated.23,28
Socioeconomic characteristicsMarital statusSix studies13,23,24,28,49,60 evaluated the association between marital status and nutritional status at birth. Two studies showed that excess birth weight was significantly associated with married24 and single/widowed/divorced23 marital status.
Family incomeOnly one49 of four studies13,24,28,49 showed that an increase in family income was significantly associated with excess birth weight.
Prenatal consultationsOf three studies13,23,60 involving the number of prenatal consultations, only one study23 showed that having at least seven prenatal consultations was significantly associated with excess birth weight.
Other characteristics associated with excess birth weightThe characteristics: social security affiliation_National Institute of Social Security/Institute of Social Security of Santa Catarina (INPS/IPESC),61 age at first delivery <20 years,13 presence of anemia during pregnancy,45 newborns carrying the wild genotype (“GG”) of the LEP-rs7799039 polymorphism,28 total cholesterol levels between 183.5 and 466.7mg/dL49 and low levels of HDL-c and high levels of maternal leptin14 were significantly associated with excess birth weight.
Characteristics not associated with excess birth weightThe following characteristics were not significantly associated with excess birth weight: maternal schooling,13,14,23,24,28,60per capita income,60 interpregnancy interval,28,60 family history of DM,42 maternal history of GDM,53 family history of fetal macrosomia,53 smoking before and during pregnancy,13,14,28,41,42 alcohol consumption,14,49 fasting blood glucose,24,42,53 insulin use,42 previous miscarriage,24 gestational age,23,24 skin color,24,41,49 age at menarche,24 physical activity during and before pregnancy,14,24 preeclampsia,43 number of pregnancies,13,28 maternal heart disease, premature rupture of membranes and collagenosis,41 maternal energy consumption (Kcal), consumption of saturated, monounsaturated, and polyunsaturated fats,49 maternal G54D, ADIPOQ rs2241766 polymorphisms, and FTO rs9939609 in the newborn,27,28 maternal levels of LDL-c, triglycerides, and adiponectin,14 and urinary tract infection/sexually transmitted diseases.57
Region of the country (South, Southeast, North, Northeast, and Midwest)The 67 described risk factors were reported by studies developed in the five regions of the country. However, the South and Southeast regions showed the highest number of studies (n=23, 69.7%) and, consequently, a higher number of risk factors associated with excess birth weight. Only one study was conducted in the Midwest region (3.0%), and five studies (15.2%) were carried out in the north/northeast regions. Finally, four (12.1%) of the 33 studies were carried out with databases from four regions: South, Southeast, North, and Northeast.
DiscussionIn this pioneering systematic review involving only studies conducted with the Brazilian population, 33 articles were assessed and 67 risk factors for excess birth weight were found, of which 31 were significantly associated with the outcome. The 33 studies were carried out in the five regions of Brazil. Among the biological risk factors, GWG, pre-gestational BMI, and DM were the main predictors of excess birth weight, also corroborating studies carried out in other countries.62–64
Brazil is a country with continental dimensions, with more than 200 million inhabitants distributed unevenly in the five different geographic regions. The authors believe these characteristics influence the different risk factors for the birth of children with excess body weight. These factors include cultural characteristics, distribution of federal/state government resources, availability of healthy foods, access to health care (public/private), income, and schooling. Notably, all these factors have been more prominent in the South and Southeast regions, the two richest regions of the country.65,66 Although in this study it was not possible to establish the effect of the region on excess birth weight development, GWG was the only risk factor identified in all five regions of the country. Regarding pre-gestational BMI and DM, they were identified in all regions except the Midwest.
Regarding the type of health system described in the assessed studies, either public or private, most of them (90.9%) was performed in the public system. However, due to the regional inequality of the articles assessed in this review, it was not possible to perform any analysis about the health system used by the population.
Describing and evaluating the effect of factors that lead to excess birth weight in different cultures and populations is crucial to preventing the potential occurrence of noncommunicable diseases throughout the child's life. Some studies have shown that the negative effects of excess birth weight, both in childhood and adolescence, as well as in adult life, have significantly contributed to the development of several chronic noncommunicable comorbidities, such as morbid obesity, DM, neoplasia, and cardiovascular diseases.67,68 These results show that maternal follow-up during the gestational period is a mandatory strategy to prevent the development of these diseases.
The establishment of a scenario where the mother has pre-gestational excess weight, excess GWG, and DM seems to be related to difficulties regarding the implementation of public health policies aimed at maternal follow-up before and during pregnancy. It is noteworthy that these factors can be modified before and during the gestational period,69,70 and that they reflect the complex sociodemographic, economic, political, and cultural conditions of each country and between the different regions of each country.33,65,71
Since the 1990s, Brazil has undergone a period of intense nutritional transition, characterized by a reduction in the prevalence of childhood malnutrition and an increase in the prevalence of obesity in different age groups.10,72 Among the main factors causing this nutritional transition is the population's nutritional standard, as a result of changes in the individual diet.24,73 This change in the Brazilian food habits includes the adoption of a diet rich in fats, sugar, and refined foods, and a reduction in the consumption of complex carbohydrates and fibers.24,74 Together with the progressive decline in physical activity and stimulated mainly by the excess use of electronic equipment, the predominance of a sedentary lifestyle has substantially contributed to the increase of obesity in the country.24,73 Additionally, the reduction in family size, the increase in food availability, the greater concentration of individuals in the urban areas, where they spend less energy and have access to numerous types of industrialized foods,24,75 and the increase in social benefits are aspects that influence the nutritional transition process in Brazil.
Studies carried out in Brazil and in other countries have shown that the constant and adequate multidisciplinary monitoring/intervention for pregnant women and women of reproductive age with excess body weight is a simple preventive measure, specific to primary health care, which is essential to minimize the negative effects of excess birth weight for the mother–child pair.69,76 In addition to preventing the birth of macrosomic newborns, favoring natural childbirth and preventing several other problems caused by an LGA newborn, the monitored practice of physical activity and/or diet are possible interventions to be adopted to prevent excess gain during pregnancy.69 However, Brazil does not seem to be able to prevent the spread of overweight/obesity in the country. Data from the Brazilian Institute of Geography and Statistics (Instituto Brasileiro de Geografia e Estatística [IBGE]) show that between 1979 and 2009, the prevalence of overweight and obesity in adult women increased from 28.7% to 48.0%, and from 8.0% to 16.9%, respectively.77 In the same period, the prevalence of obesity in children aged 5–9 years increased from 2.4% to 14.2%,77 disclosing the challenge to prevent the progression of obesogenic conditions among the population.
In contrast, some authors have shown promising results regarding the lifestyle changes in the Brazilian population. The increase from 33.0% to 35.2% in the consumption of fruits and vegetables in the period between 2008 and 2016 in adults suggests a potential change in the diet of the Brazilian population.75 The frequency of the regular consumption of fruits and vegetables in 2016 was higher in women (40.7%) than in men (28.8%).75 In the same period, in both genders, the regular consumption of fruits and vegetables increased with age and with the level of schooling.75 Regarding the practice of physical activity during leisure time, there was an increase from 30.3% in 2009 to 37.6% in 2016 in the adult population, also suggesting a possible change in the population's lifestyle.75
It is imperative that public policies aimed at controlling/monitoring women's health also consider the cultural, sociodemographic, economic, and even regional conditions of the country. Very often, the cultural influence of family and close friends can be a determinant in the nutritional status of the mother–child pair. It is essential to involve family members in the strategies to improve family quality of life, especially regarding the regular practice of adequate physical activity and diet.13
From the perspective of public health, it seems evident that primary health care and its constant monitoring should be offered to women before, during, and after the gestational period. Even if the woman starts her pregnancy with excess pre-gestational BMI, interventions to return to the appropriate nutritional status are more effective when performed in the first months of pregnancy, when adherence to regular physical activity and dietary control are more effective. If excess weight gain occurs during pregnancy, specific strategies implemented by a multidisciplinary team make it possible to adjust the woman's weight to prevent the occurrence of potential comorbidities and the birth of macrosomic or LGA newborns. The success of an intervention aimed at improving the nutritional status of the mother at any moment of her pregnancy is directly associated with the involvement of the family, rather than the mother alone.
Among the strengths of this study are the extensive literature review involving five databases, including cross-sectional and longitudinal studies. The review was not limited to language and year of publication, and thus covered four decades worth of studies. Another noteworthy point is related to the organization of data, which were presented aiming to reduce the heterogeneity between the studies and facilitate the analysis. Finally, because this represents the first systematic review to describe several risk factors for excess birth weight in Brazilian children, it will substantially contribute to the creation of public policies aimed at improving the quality of life at birth.
Some limitations regarding this systematic review should be considered. First, the different reference standards78–87 for excess birth weight used by the studies made it difficult to compare the data, limiting a more robust data analysis, such as meta-analysis. Second, the absence of the reference criterion for the nutritional status classification in some articles made it impossible to exactly identify how many and which definitions were used. This is an important issue, since some countries use their own classification criteria and, therefore, caution should be taken when comparing the studies. Third, the different criteria used to assess the association (chi-squared, RR, PR, OR) between the outcome variables and the study predictors made it difficult to compare the results, since the magnitude of each criterion used is not the same. Fourth, the impossibility of developing a meta-analysis in this study prevented the authors from assessing the effect of the region on the different identified risk factors. Most of the studies included in the review were carried out in the South and Southeast regions, exactly because they are the regions where the distribution of resources for teaching and research remains greater. In this sense, the presented data may not accurately reflect the characteristics of the other regions (North, Northeast, and Midwest). Finally, the absence of a single tool capable of assessing the risk of bias in the different study designs also made it difficult to analyze the bias between studies.
Final considerationsGestational weight gain, pre-gestational BMI, and DM were the main predictors of excess birth weight in Brazilian children. The determinant factor to ensure the establishment of adequate nutritional status in the gestational period and even after delivery appears to be the quality and frequency of the follow-up of mothers and their children by health care agencies. It should be remembered that the data presented and discussed in this review were based on the 33 identified studies. The disproportionate distribution of these studies according to the region does not allow the generalization of the results to the entire country.
FundingCoordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES); Fundo de Apoio à Pesquisa da Universidade da Região de Joinville.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Czarnobay SA, Kroll C, Schultz LF, Malinovski J, Mastroeni SS, Mastroeni MF. Predictors of excess birth weight in Brazil: a systematic review. J Pediatr (Rio J). 2019;95:128–54.