In this present study, the authors evaluated the predictive factors for adverse maternal-fetal outcomes in pregnancies of women with cystic fibrosis (CF). Patients were followed up by a referral center for adults in southern Brazil.
MethodsThis is a retrospective cohort study that used data from electronic medical records regarding pregnancies of women diagnosed with CF.
ResultsThe study included 39 pregnancies related to 20 different women. The main adverse outcomes were high prevalence rates of premature birth (38.5%) and maternal respiratory exacerbation (84.6%). Lower body mass index (BMI) values (< 20.8) and younger ages of CF diagnosis increased the risk of premature birth. The presence of methicillin-resistant and absence of methicillin-sensitive Staphylococcus aureus, as well as a younger age of diagnosis, increased the risk of maternal respiratory exacerbation during pregnancy.
ConclusionsConception in women with CF is often associated with maternal and fetal complications. Continuous monitoring by a multidisciplinary team should emphasize appropriate nutritional status, investigation of bacterial colonization, and immediate attention to respiratory exacerbations.
Cystic fibrosis (CF) is an autosomal recessive, multisystemic genetic disease of chronic and progressive evolution that is more common in the Caucasian population.1 Gene mutations cause a dysfunction in the cystic fibrosis transmembrane conductance regulator protein, consequently hampering ion transport on the surface of epithelial cells; this results in the thickening of secretions produced by exocrine glands.2 Clinical presentations of this disease may include a high concentration of electrolytes in sweat, deterioration of lung function, and exocrine pancreatic insufficiency.3
Recent advances in diagnosis and treatment have provided a significant increase in life expectancy for patients with CF.4 The median survival, in several countries, can exceed 40 years5-7; currently, the median survival of patients with CF in Brazil is 43.8 years.8
In view of the aging of this population, issues related to family genetics, fertility, and pregnancy are increasingly frequent.9 In 2018, 280 pregnancies were reported in the Cystic Fibrosis Foundation registry, representing approximately 4 live births per 100 women of reproductive age.10
Previous studies have shown that pregnancies in women with CF may be associated with an increased number of respiratory exacerbations, premature delivery, cesarean delivery, and low birth weight. Women with forced expiratory volume in the first second (FEV1) < 60% of the predicted values also face higher death rates 3.2 years after delivery 9,11; better results were observed in women with FEV1 > 50%, adequate nutritional status, and controlled diabetes mellitus (DM).9
Despite these findings, maternal-fetal outcomes in women with CF have not yet been exhaustively explored,12 and few Brazilian studies are available for building an arsenal of evidence capable of leading to strong conclusions.13
Improving the understanding of the impacts of pregnancy in women with CF may assist in the counseling and management of these patients. Therefore, the present study aimed to retrospectively assess predictive factors for poor maternal-fetal outcomes of pregnancies in women with CF followed up at a CF referral center for adults in southern Brazil.
MethodsThis is a retrospective cohort study that included all cases of pregnant women diagnosed with CF followed by the Program for Adolescents and Adults with CF at Hospital de Clínicas de Porto Alegre (HCPA) from January 1998 to July 2020. The study was approved by the HCPA Research Ethics Committee (number 42537415700005327) and all participants signed an informed consent form.
Data collectionPatients in this program are usually evaluated by a multidisciplinary team every 3 months. Clinical data were collected from medical records regarding the year prior to the start date of pregnancy.
Lung function data were measured using spirometry and included forced vital capacity (FVC), FEV1, and FEV1/FVC ratio expressed as a percentage of the predicted results according to age, height, and gender.14 Spirometry techniques were based on the guidelines for pulmonary function tests by the Brazilian Society of Pulmonology and Tisiology, 15 and the pre-gestational examination with the best results in the last 6 months before delivery was considered.
The authors collected results of the last 3 sputum bacteriology tests in the year prior to the pregnancy. The presence of Pseudomonas aeruginosa, methicillin-sensitive Staphylococcus aureus (MSSA), methicillin-resistant S. aureus (MRSA), and Burkholderia cepacia was assessed. Body mass index (BMI) was obtained by dividing weight (in kilograms) by height squared (in meters): BMI = weight/height2.
Information related to age, ethnicity, age at CF diagnosis, and comorbidities (DM related to CF, pancreatic insufficiency) was collected. In order to measure maternal outcomes during pregnancy, the patient's age, whether or not she received counseling by the CF team, as well as complications (gestational DM, eclampsia/pre-eclampsia, hospitalization, respiratory exacerbation, and conception route) were recorded.
The analysis of neonatal outcomes was performed through the collection of data on gestational age (GA), weight, fifth minute Apgar score, investigation for CF, breastfeeding, complications at birth, and length of stay (days). The GA was measured in weeks and classified as preterm birth (GA < 37 weeks) or term birth (GA between 37 and 42 weeks).16 The fifth minute Apgar score considered values ≤ 6 as a risk for the newborn.17
In the study's consultations, women who have a desire to gestate are routinely informed about the clinical factors that increase the likelihood of complications during pregnancy, such as inadequate nutritional status, the decline in lung function, the presence of liver disease, and CF-related DM.
Statistical analysisData were organized in an Excel (version 16.0) spreadsheet and analyzes were performed using SPSS version 20.0. Continuous variables were presented as means ± standard deviations when data presented normal distributions or as median and interquartile range in case of non-normal distributions. Categorical variables were expressed in absolute and relative frequencies.
Considering the particularity of the variable “age of diagnosis”, it was analyzed only once in the case of multiparous women. The other clinical variables are changeable over time and for this reason, were analyzed separately for the 39 pregnancies.
Prevalence ratio, estimated by using Poisson regression with robust variance, was used to verify the association between clinical variables and negative maternal-fetal outcomes.18 Analyzes based on receiver operating characteristic (ROC) curves were used to determine the cutoff points for each variable for better sensitivity and specificity. A statistical significance level of less than 5% (p < 0.05) was adopted for all models.
Results41 pregnancies were studied in 25 women with CF between January 1998 and July 2020. One woman had twin pregnancies and one pregnancy was conceived by in vitro fertilization.
Two cases of spontaneous abortion were excluded from the study. There was no abortion or termination of pregnancy for medical reasons. There were two neonatal deaths and no maternal deaths related to pregnancy.
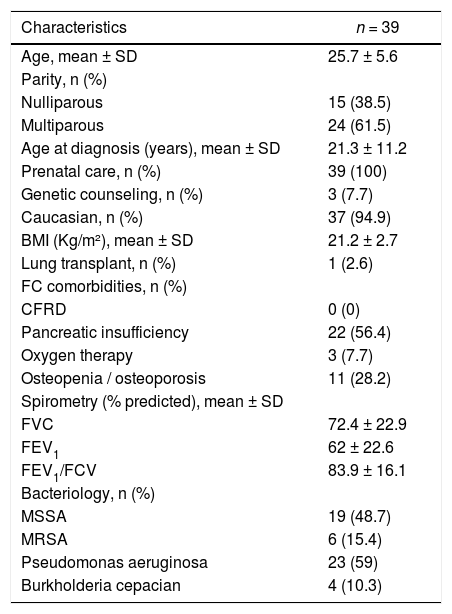
The sample consisted of 94.9% Caucasian women, with a mean age of 25.7 years, a mean FEV1 of 62% the predicted value, and a mean BMI of 21.2 Kg/m2. The general characteristics of women with CF included in this study in the pre-pregnancy period are shown in Table 1.
General characteristics of women with cystic fibrosis included in the study in the pre-pregnancy period.
SD, standard deviation; BMI, body mass index; CFRD, cystic fibrosis-related diabetes; FVC, forced vital capacity; FEV1, forced expiratory volume in the first second; MSSA, methicillin-sensitive Staphylococcus aureus (oxacillin); MRSA, methicillin-resistant S. aureus (oxacillin).
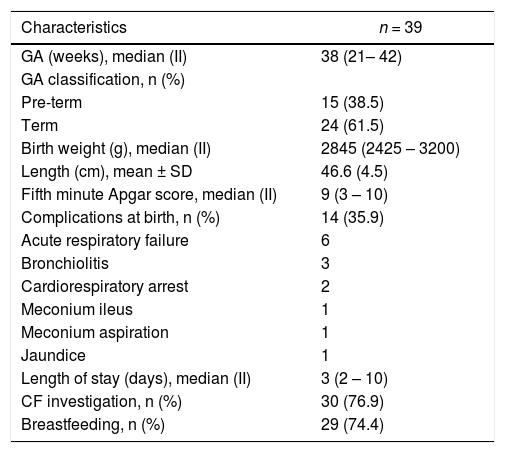
The most prevalent neonatal outcome was birth with a GA of fewer than 37 weeks, characterizing premature delivery in 38.5% of cases. Fourteen newborns presented some type of complication at birth, the most prevalent being related to the cardiorespiratory system (71.42%). Table 2 shows the fetal outcomes of neonates included in the sample.
Fetal outcomes of neonates included in the sample.
GA, gestational age; SD, standard deviation; II, interquartile range; CF, cystic fibrosis.
The first case of fetal death occurred with a newborn at 540 days of life. The mother had preeclampsia during pregnancy, which occurred in the first year after a bilateral lung transplant. The neonate was born at 35 weeks, weighing 1752 g, had surgical meconium ileus and respiratory symptoms that required mechanical ventilation was diagnosed with cystic fibrosis, and remained hospitalized from birth until the date of death. The second case of death involved a newborn with 34 weeks of GA who developed cardiac arrest shortly after birth.
During the gestational period, the most common negative maternal outcome was respiratory exacerbation (84.6%), requiring oral antibiotics in 10 cases (25.6%) and intravenous antibiotics in 11 cases (28.2%). Three women were admitted to an intensive care unit during pregnancy, one of whom required invasive mechanical ventilation. The hospital length of stay ranged from 3 to 180 days. Only 3 pregnancies (7.7%) were accompanied by the occurrence of pre-eclampsia, and 1 (2.6%) woman presented gestational DM. The most prevalent mode of delivery was cesarean section (56.4%).
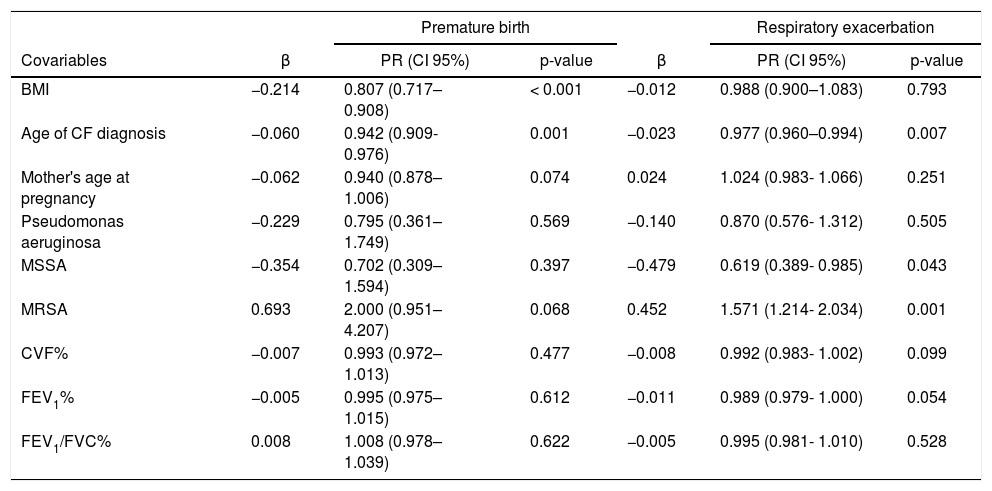
The associations between dependent variables (premature birth and maternal respiratory exacerbation) and covariables are shown in Table 3. The BMI cutoff point of 20.8 kg/m2 was established through the ROC curve for predicting premature birth, with a specificity of 0.300 and sensitivity of 0.692.
Results of the Poisson regression for premature birth and maternal respiratory exacerbation as dependent variables during the gestational period.
CF, cystic fibrosis; β, standardized regression coefficient; PR, prevalence ratio; CI, confidence interval; BMI, body mass index; FVC, forced vital capacity; FEV1, forced expiratory volume in the first second; MSSA, methicillin-sensitive Staphylococcus aureus (oxacillin); MRSA, methicillin-resistant S. aureus (oxacillin). p-values of less than 0.001 are highlighted in bold.
The present study analyzed the maternal-fetal outcomes of 39 pregnancies monitored by the HCPA team. The present study's results indicated high prevalence rates of premature birth and maternal respiratory exacerbation outcomes (38.5% and 84.6%, respectively). In addition, BMI values below 20.8 kg/m2 and early CF diagnosis increased the risk of premature birth. The presence of MRSA, absence of MSSA, and younger age of diagnosis contributed to an increased risk of respiratory exacerbation during pregnancy.
In the present study, the adverse fetal outcome with the highest prevalence rate was premature birth (38.5%). A similar result was found in a retrospective cohort of 2 178 954 pregnancies, of which 77 involved women with CF. In the comparative analysis, results indicated that women with CF were more susceptible to giving birth to newborns with less than 37 weeks of GA (18.2% versus 8.9%, p = 0.008).19 This seems to be a frequent finding since previous studies have reported rates of premature birth ranging from 10% to 48%.20,21
The association analyses demonstrated that women with lower BMI values in the pre-conception period had a higher risk of premature delivery. For each additional unit in terms of maternal BMI, the risk of premature birth was reduced by 19.3%. The nutritional recommendation for women with CF is to maintain a BMI ≥ of 22 kg/m222 for better management and prevention of respiratory infections. Additionally, evidence suggests that severe malnutrition, determined by a BMI < 18 kg/m2, is considered a relative contraindication for pregnancy in this population.23 The authors’ findings corroborate the current guidelines, as BMI values < 20.8 kg/m2 showed better sensitivity and specificity for predicting the occurrence of premature birth.
The main adverse maternal outcome observed in the present study was respiratory exacerbation during pregnancy (84.6% prevalence rate). Similar results have been reported by previous studies,13,24 and only one case-control study indicated no difference (p > 0.2) between rates of respiratory exacerbation between pregnant and non-pregnant women with CF. However, the sample size was small and modest differences between these groups may have been lost.12
Spontaneous vaginal delivery is indicated in clinical guidelines for women with CF, except for maternal or fetal complications. In the present study, the rate of cesarean sections was high (56.4%). This finding is justified by the increased frequency of exacerbations, pulmonary complications, and complications related to pregnancy (gestational DM, pre-eclampsia) in this population.25
The age of early CF diagnosis acted as a risk factor for exacerbations and premature birth; each additional year in the age of diagnosis reduced the risk of exacerbation by 2.3% and premature delivery by 5.8%. Such findings can be explained by the direct association between late diagnosis and atypical forms of the disease, which have milder clinical presentations. In these cases, symptoms are usually manifested in only one organ and the number of hospitalizations throughout life is significantly lower when compared to patients diagnosed early with the typical forms.26
The National Neonatal Screening Program was only instituted in Brazil in 2011, a fact that influences the highest average age of diagnosis found in the present study (21.3). As screening in the country expands, diagnoses become more precocious and this ceases to act as a risk factor for negative outcomes in the population.27
In general, women with CF with FEV1 > 60% tolerate pregnancy well.9 In the present study, the mean FEV1 of 62% and the small sample size may justify the absence of associations between lung function and negative maternal-fetal outcomes.
Similarly, the authors’ findings indicated that pregnant women colonized by MSSA had a lower risk of respiratory exacerbation. In contrast, those colonized by MRSA had a higher risk of presenting the same outcome. Colonization by MRSA has been associated with several negative clinical outcomes in patients with CF, including an accelerated decline in lung function, an increased number of hospitalizations, and early mortality.28,29 Bacterial colonization by Burkholderia cepacia has also been associated with a rapid decline in lung function in previous studies conducted with pregnant women with CF.20,30
Altogether, it is known that sepsis of respiratory origin can generate potential complications for pregnant women and fetal development. Therefore, the authors believe that the present study's findings strengthen the current consensus recommendations and clinical guidelines to closely monitor pregnant patients with CF and introduce, early and rigorously, treatment of respiratory exacerbations.9,30
This study was retrospective, of small sample size, and single-centered, which may limit the applicability of the present study's findings to different populations. Additional studies and larger sample sizes are needed to identify long-term adverse outcomes for pregnant women with CF. Despite these limitations, this is the first study that examined the predictors of adverse outcomes in pregnant women with CF in Brazil.
The authors conclude that the present study's findings add to the current body of evidence that indicates that conception in women with CF is often associated with maternal and fetal complications. Continuous monitoring by a multidisciplinary team must emphasize the appropriate nutritional status, as well as investigate bacterial colonization and provide immediate attention to respiratory exacerbations.
This work was performed at Hospital de Clínicas de Porto Alegre, Porto Alegre, RS, Brazil.