Since coronary artery lesions (CALs) are the most severe complication of Kawasaki disease (KD), clinically speaking, early prediction of CALs is crucial. The authors aimed to investigate the predictive value of C-reactive protein (CRP) in predicting CALs in KD patients.
MethodsKD patients were divided into the CALs group and the non-CALs group. The clinical and laboratory parameters were collected and compared. Multivariate logistic regression analysis was used to determine the independent risk factors of CALs. The receiver operating characteristic curve was applied to determine the optimal cut-off value.
Results851 KD patients who met the inclusion criteria were studied, including 206 in the CALs group and 645 in the non-CALs group. Children in the CALs group had significantly higher CRP levels than the non-CALs group (p < 0.05). Multivariable logistic regression analysis showed that incomplete KD, male, lower hemoglobin, and higher CRP were independent risk factors for predicting CAL (all p < 0.05). The optimal cut-off value of initial serum CRP for predicting CALs was 105.5 mg/L, with a sensitivity of 47.57% and a specificity of 69.61%. In addition, KD patients with high CRP (≥105.5 mg/L) had a higher occurrence of CALs than those with low CRP (<105.5 mg/L) (33% vs 19%, p < 0.001).
ConclusionThe incidence of CALs was significantly higher in patients with high CRP. CRP is an independent risk factor for CALs formation and may be useful for predicting CALs in KD patients.
Kawasaki disease (KD), also known as mucocutaneous lymph node syndrome, is an acute febrile vasculitis of unknown etiology that occurs predominantly in children under five years of age.1 The incidence of KD is increasing, and the highest relative risk is in Asian children, especially in East Asia.2-5 The most common complication caused by KD is coronary artery lesions (CALs), including coronary artery dilatation, coronary artery aneurysm (CAA), and life-threatening myocardial infarction.6 KD has overtaken acute rheumatic fever as the most common cause of acquired heart disease in developed countries.7 Intravenous immunoglobulin (IVIG) effectively reduces the prevalence of coronary artery abnormalities when administered early in the course of KD and is now used regularly.8 Nevertheless, approximately 10–20% of KD patients are resistant to IVIG treatment and are at higher risk for developing CALs.9 Since the primary purpose of KD treatment is to prevent the development of CALs, early prediction of CALs is crucial for improving treatment outcomes in children with KD.
C-reactive protein (CRP) is a nonspecific and recognized inflammatory biomarker that increases in response to inflammation and plays a critical role in the acute phase of KD.10 A high CRP level (≥30 mg/L) serves as one of the criteria to confirm incomplete KD recommended in the American Heart Association guidelines (AHA)1. CALs are also frequently observed in KD patients with low CRP levels, although high CRP levels are expected in KD.11 Previous studies have shown that CRP has predictive value for IVIG resistance.12,13 However, the literature on CRP predicting CALs in patients with KD is limited,11,14 and the conclusions are debatable.15 In addition, the correlation between CRP and CALs is also controversial.16
Therefore, the authors conducted a retrospective study to evaluate the value of CRP in predicting CALs in children with KD. The purpose of the present study is to provide the literature on the use of CRP for early prediction of CALs to strengthen the prevention and treatment of KD patients as early as possible.
MethodsThe study protocol was approved by the ethics committee of Chengdu Women's and Children's Central Hospital (approval number 202214). The requirement for informed patient consent was waived. All methods were performed in accordance with the Declaration of Helsinki.
Study design, setting, and study subjectsPatients with KD were retrospectively recruited at Chengdu Women's and Children's Central Hospital between January 2017 and December 2019. The diagnosis of KD (including incomplete KD) was made according to the 2017 AHA guideline1 and confirmed by two experienced KD specialists.
The inclusion criteria: (1) patients with an initial diagnosis of KD, (2) <18 years old, (3) received standard treatment with IVIG 2 g/kg as a single infusion in the acute phase. The authors excluded patients who met the exclusion criteria: (1) patients with severe infection, allergy, or systemic juvenile idiopathic arthritis, or (2) recurrent KD, or (3) who refused treatment with IVIG or received an initial IVIG dose of less than 2 g/kg, or (4) who had received IVIG treatment in the first 3 months after enrollment, (5) patients with unavailable clinical or laboratory data.
Standard treatment protocolAll patients were treated with 2 g/kg IVIG as a single infusion within 12 h of KD diagnosis and received aspirin at a dose of 30–50 mg/kg/day during the acute phase. Aspirin (3–5 mg/kg/day) was administered for 6–8 weeks until all signs of inflammation had resolved or regression of CAL was observed on two-dimensional echocardiography. Additional IVIG (2 g/kg) was administered if patients had a persistent fever for more than 36 h or had recurrent fever associated with KD symptoms after an afebrile phase. Other therapies, including prednisolone, were not used in the initial treatment. IVIG resistance was defined as recurrent or persistent fever for at least 36 h but no longer than 7 days after initial IVIG treatment.1
Data collection and group assignmentAll data were obtained from medical records, including demographic data, laboratory data, and clinical outcomes. Laboratory data were collected before the initial IVIG treatment. Coronary artery diameter was measured by echocardiography before the first IVIG treatment.
Patients were divided into two groups according to whether they were complicated with CALs in the acute KD phase: the CALs group and the non-CALs group. CALs were defined based on the normalization of body surface area dimensions as Z-scores as follows according to the 2017 AHA guideline1: (1) no involvement (Z-score <2.0); (2) dilation (Z-score ≥2.0 to <2.5), (3) aneurysm (Z-score ≥2.5 to <5 for a small aneurysm; Z-score ≥5 to <10 for a medium aneurysm; Z-score ≥10 for a giant aneurysm) of the coronary arteries, based on the maximum internal diameters of the right coronary artery, left anterior descending artery and left circumflex coronary artery.1,17 All KD patients underwent standardized echocardiograms by an experienced ultrasonographer during the acute phase.
Statistical analysisThe normality of the distribution of variables was checked using the Shapiro–Wilk test. Continuous variables were expressed as means ± standard deviations or median and IQR (25th, 75th percentiles) if non-normally distributed. Categorical variables were expressed by presenting the frequency and proportion in each category. Chi-square analysis was applied for categorical variables, and the Student-t-test or Mann–Whitney U test was used based on the normality of variables for continuous variables.
The univariable and multivariable logistic regression models were constructed, and results were expressed as odds ratios (OR) with a 95% confidence interval (CI) to identify independent risk factors for CALs. To assess the discriminatory capacity of CRP in predicting CALs, the authors performed the receiver operating characteristic (ROC) curve analysis. The authors performed subgroup analysis by dividing the CALs group into several subgroups based on CRP and hemoglobin (HB) according to Z-score. The value of independent risk factors at different Z-score levels is using ANOVA or the Kruskal–Wallis test based on the normality of distribution. The chi-square test was also applied to compare the ratio of the different Z-score groups in each independent risk factor, and the Bonferroni test was used for further pairwise tests. A p-value <0.05 was considered statistically significant. All statistical tests were performed using SPSS 12.0 for Windows XP (SPSS, Inc, Chicago, USA).
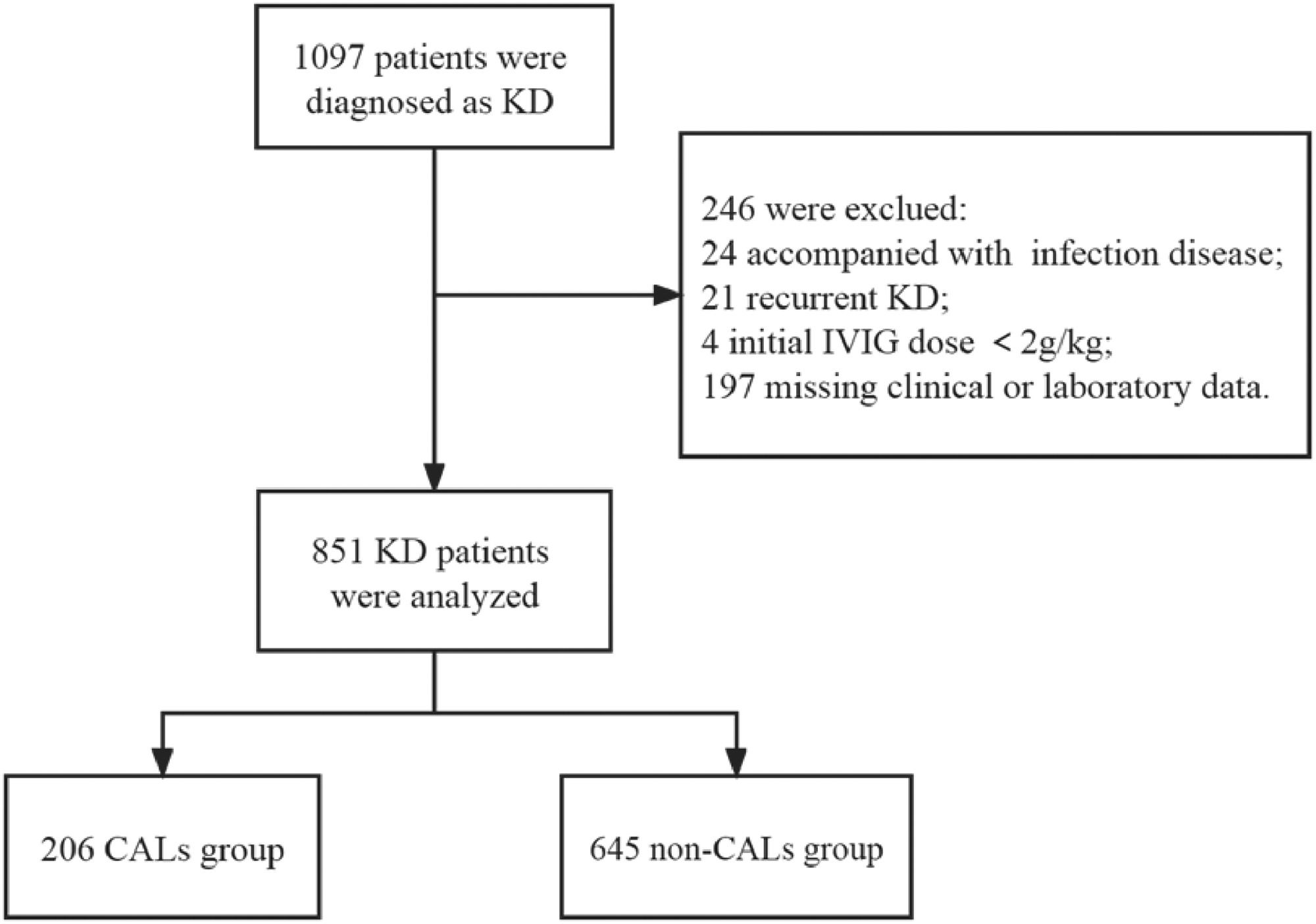
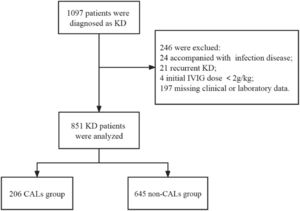
ResultsA total of 1097 patients were diagnosed with KD (including incomplete KD), of whom 246 were excluded because 24 were accompanied by severe infectious disease, 21 had recurrent KD, 4 received an initial IVIG dose <2 g/kg, and 197 lacked laboratory data on serum CRP levels and coronary artery size (Z scores). Finally, 851 patients were enrolled in the study and divided into two groups: the CALs group (n = 206) and the non-CALs group (n = 645). The flowchart is shown in Figure 1.
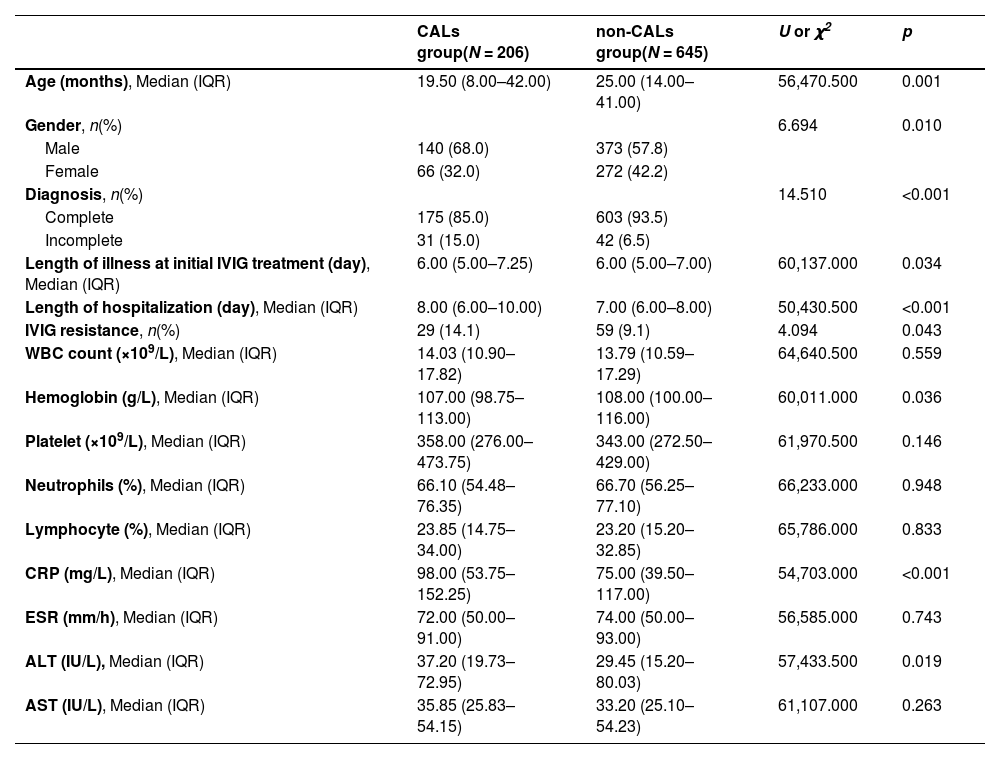
Basic demographic, clinical and laboratory characteristicsA total of 851 KD patients were included, 60.28% of whom were men (n = 513). Of the 851 cases, 206 had CALs (Z ≥ 2), accounting for 24.21%. The general characteristics and laboratory indicators of patients with and without CALs are shown in Table 1. There were no significant differences in white blood cell (WBC), platelet, neutrophils (%), lymphocyte (%), alanine transaminase (AST), and erythrocyte sedimentation rate (ESR) between these two groups.
Basic characteristics in KD patients with CALs and non-CALs.
KD, Kawasaki disease; IVIG, intravenous immunoglobulin; WBC, white blood cell; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; ALT, alanine transaminase; AST, aspartate transaminase.
The CALs group had a longer hospitalization time and younger age than the non-CALs group (p < 0.05). In addition, children in the CALs group had a higher proportion of male gender, incomplete KD, and IVIG resistance than children in the non-CALs group (all p < 0.05). Of the routine laboratory inflammatory indicators, CRP and ALT levels were significantly higher in the CALs group than in the non-CALs group (p < 0.05).
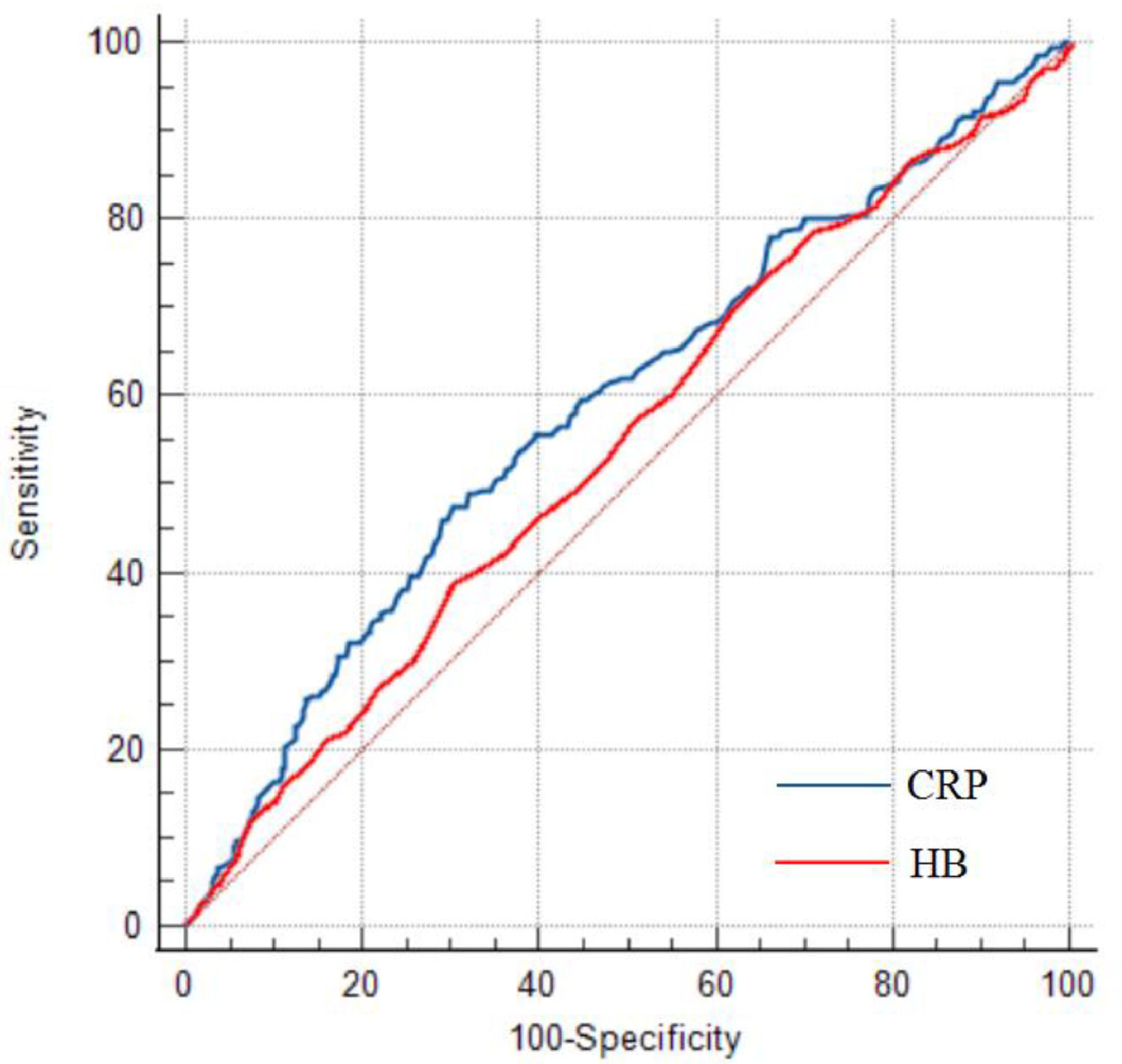
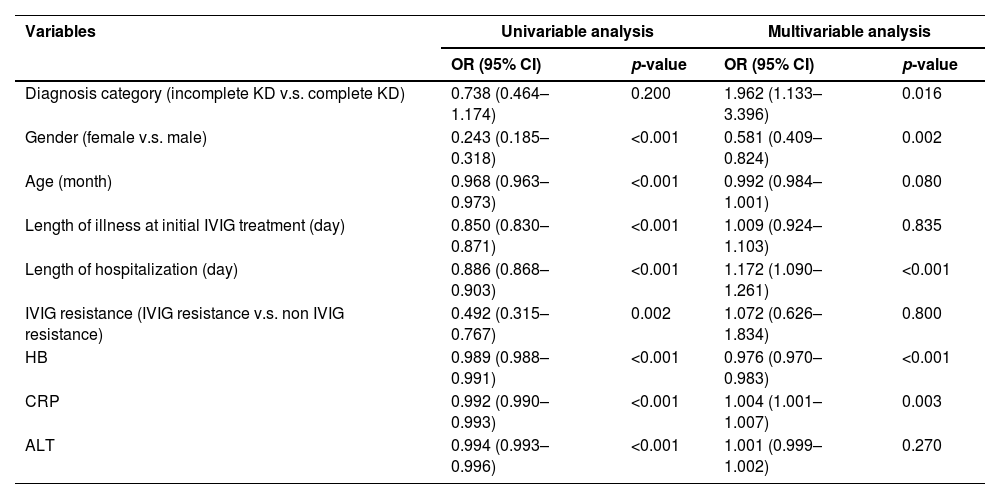
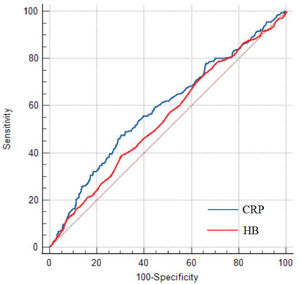
Prediction for CALs development and ROC analysisThe univariable and multivariable logistic regression models were constructed to identify independent risk factors for CALs. The result is shown in Table 2. The multivariable logistic regression analysis showed that incomplete KD, male, lower hemoglobin (HB), and CRP were independent risk factors for CALs in KD patients (all p < 0.05). The results of ROC curve analysis for CRP to predict CALs are shown in Figure 2; the area under the ROC curve (AUC) was 0.588 (95% CI = 0.554–0.622, p < 0.001). The sensitivity and specificity in predicting CALs were 47.57% and 69.61%, respectively, with a cut-off level of 105.5 mg/L determined by the Youden index. For HB, the sensitivity and specificity in predicting CALs were 38.83% and 70.08%, respectively, with a cut-off value of 102 g/L.
Univariable and multivariable logistic regression analysis for predicting CALs in KD patients.
CALs, coronary artery lesions; KD, Kawasaki disease; IVIG, intravenous immunoglobulin; HB, hemoglobin; CRP, C-reactive protein; ALT, alanine transaminase.
The authors also performed subgroup analysis by dividing the CALs group into multiple groups by Z score to compare CRP differences among subgroups with different degrees of CALs. As shown in Appendix S1 and Appendix S2, there was no significant difference in CRP or HB between the subgroups with different degrees of CALs.
Results of CALs in patients with high CRP or lower HBThe authors use the cut-off value determined by Youden's index to compare the incidence of CALs in KD patients with different levels of CRP or HB. There were 556 patients with a CRP level of <105.5 mg/L, 108 of whom developed CALs (32 dilations and 76 CAA). There were 295 patients with a CRP level ≥105.5 mg/L, and 98 of them had CALs (26 dilations and 72 CAA). Meanwhile, there were 80 KD patients with CALs in the lower HB group (<102 g/L) and 126 KD patients with CALs in the higher HB group (≥102 g/L). KD children with a higher CRP level (≥105.5 mg/L) or a lower HB value (<102 g/L) had a higher incidence of CALs than children with a lower CRP (<105.5 mg/L) (33% vs. 19%, p < 0.001) or higher HB (29% vs. 22%, p < 0.05). Further Bonferroni test showed that KD patients with a higher CRP level (≥105.5 mg/L) or a lower HB level (<102 g/L) had a higher incidence of small aneurysms than children with a lower CRP (<105.5 mg/L) or higher HB (≥102 g/L) (p < 0.05) (Appendix S3).
There were 122 KD patients with a higher level of CRP (105.5 mg/L) and lower HB level (<102 g/L), 43 of whom developed CALs. There were 729 patients with a CRP level ≥ of 105.5 mg/L, and 163 had CALs. Similarly, KD patients with a higher level of CRP and lower HB had a higher incidence of CALs than children with a lower CRP and higher HB (35% vs. 22%, p = 0.01) (Appendix S4).
DiscussionIn the present retrospective study, the authors aimed to investigate the predictive value of CRP in predicting CALs in KD patients. The results showed that CRP is an independent risk factor for the development of CALs in KD patients. KD patients with CALs had a significantly higher CRP level than those without CALs. In addition, KD patients with a high CRP level (≥ 105.5 mg/L) had a higher incidence of CAL than those with a low level. The present study suggests that CRP is an independent predictive marker for CALs and may be useful in predicting CALs in KD patients.
The occurrence of CALs has dropped to 4% of KD patients with timely standard IVIG and aspirin treatment.1 CALs may lead to ischemia, cardiogenic shock, and even sudden cardiac death.18 Therefore, it is imperative to identify the risk factors for CALs in KD patients. In the present study, incomplete KD, male gender, low HB, and high CRP were found to be independent risk factors for CALs in KD patients. There are several North American19 and Japanese risk-scoring systems (e.g., Harada score,20 Kobayashi score,21 Egami score22) for predicting CAA or IVIG resistance. Both the North American and the Japanese risk models showed that CRP and younger age were independent predictors of CAL occurrence, which was consistent with the present results. Moreover, higher CRP and younger age were also highly associated with IVIG resistance in a North American1 or Asian population.22 These results highlight the importance of vigilance for KD at a young age with higher CRP. The present results showed that low HB was an independent risk factor for predicting CALs (p < 0.001). However, the present study showed that HB had good specificity but low sensitivity (38.83%), which is less predictive than CRP for predicting CALs in Chinese KD children. The present results were in line with previous investigations, which reported similar findings.23,24 The persistent and ongoing systemic inflammatory reactions affect all the medium-sized arteries, especially the coronary arteries, and in multiple tissues and organs, which eventually cause the development of CALs in KD patients.1 Therefore, high CRP levels are generally observed in KD patients.
CRP is a widely recognized acute-phase reactant protein that exhibits elevated expression in response to infection and inflammation.25 CRP levels generally correlate with the degree of inflammation, i.e., a higher level indicates a higher inflammatory status.26 Previous studies have shown that elevated CRP levels in adult patients with coronary artery disease may predict myocardial ischemia or sudden death.27,28 The present results showed that KD patients with CALs had significantly higher CRP levels than those without CALs. Furthermore, the authors use the cut-off value to compare the incidence of CALs in KD patients with a higher or lower CRP level. KD patients with a high CRP level (≥105.5 mg/L) had a higher incidence of CAL than those with a low level. Further Bonferroni test showed KD patients with a CRP level ≥ of 105.5 mg/L had a higher risk of small aneurysms than patients with a lower CRP level <105.5 mg/L.
Therefore, the present data are consistent with the hypothesis that the inflammatory process is involved in CALs formation in KD patients. The present findings suggest that CRP levels are not only elevated in KD patients with CALs but also reflect a continuing condition of vasculitis and may predict the size of CAL. Previous studies also reported that the risk of CALs formation was higher in KD patients with severe vasculitis and inflammatory responses (higher CRP level).14,29 Thus, physicians should pay more attention to monitoring the clinical course of KD kids with a high level of CRP in terms of CALs development, especially coronary artery aneurysm formation.
However, subgroup analysis showed no significant difference in CRP levels among the different CALs classifications. Therefore, it should be noted that CRP alone does not always reflect the severity of KD, although CRP is known to reflect the extent of systemic inflammation.30 Nevertheless, CALs were also found in KD patients with low serum CRP levels, suggesting that patients with low CRP levels may not represent a homogeneous group. One possible explanation would be that an increase in CRP levels occurred after the initial sample test, but further studies are needed to test such a hypothesis. The low sensitivity of CRP (47.57%) in predicting CALs, indicating the initial serum CRP level alone may not have better predictive power in the context of KD.
LimitationsThis study has several limitations. First, the retrospective design limits the present research, which inevitably leads to selection and information bias. Second, the authors could not evaluate inflammatory markers other than CRP and WBC. Third, all KD patients were Chinese, which limits the generalizability of the results. Therefore, further prospective studies are needed to confirm the present results.
ConclusionsIn conclusion, the occurrence of CALs was significantly higher in patients with a high level of CRP. CRP is an independent risk factor for CALs formation and may be useful for predicting CALs in KD patients. The clinical course of KD patients should be carefully monitored.
FundingThis manuscript was supported by the Chengdu Medical Research Project [2022065].