To investigate the optimal timing of initial intravenous immunoglobulin (IVIG) treatment in Kawasaki disease (KD) patients.
MethodsKD patients were classified as the early group (day 1-4), conventional group (day 5-7), conventional group (day 8-10), and late group (after day 10). Differences among the groups were analyzed by ANOVA and Chi-square analysis. Predictors of IVIG resistance and the optimal cut-off value were determined by multiple logistic regression analyses and receiver operating characteristic (ROC) curve analysis.
ResultsThere were no significant differences in IVIG resistance among the 4 groups (p = 0.335). The sensitivity analysis also confirmed no difference in the IVIG resistance between those who started the initial IVIG ≤ day 7 of illness and those who received IVIG >day 7 of illness (p = 0.761). In addition, patients who received IVIG administration more than 7 days from the onset had a higher proportion of coronary artery abnormalities (p = 0.034) and longer length of hospitalization (p = 0.033) than those who started IVIG administration less than 7 days. The optimal cut-off value of initial IVIG administration time for predicting IVIG resistance was >7 days, with a sensitivity of 75.25% and specificity of 82.41%.
ConclusionsIVIG therapy within 7 days of illness is found to be more effective for reducing the risk of coronary artery abnormalities than those who received IVIG >day 7 of illness. IVIG treatment within the 7 days of illness seems to be the optimal therapeutic window of IVIG. However, further prospective studies with long-term follow-up are required.
Kawasaki disease (KD) is an acute, self-limited disease leading to systemic vasculitis that predominantly affects children younger than five.1 KD surpasses acute rheumatic fever as a leading cause of acquired heart disease in developed countries.2 The most common complication of KD is coronary artery abnormalities (CAA), and its prevention is important to improve outcomes in patients with KD.3 Intravenous immunoglobulin (IVIG) is the mainstay of treatment in KD and has been known to be effective in abolishing vascular inflammations leading to coronary artery lesions (CALs).4 The fatality rates of KD have markedly decreased since the IVIG therapy was introduced in 1983.5
However, the association between the earlier timing of IVIG administration of disease onset and risk for IVIG unresponsiveness remains debatable.6 Moreover, the literature comparing the early and routine IVIG therapy regarding the efficacy in preventing cardiac sequelae is limited and controversial.7-12 Furthermore, several latest guidelines on the optimal timing of IVIG administration and if IVIG can be given earlier remain inconclusive.1,13,14
Given this background, the authors hypothesize that there is maybe a narrow therapeutic window of IVIG administration. Earlier or later IVIG treatment may affect the IVIG responsiveness and outcomes in KD patients. Thus, the authors performed a retrospective study to investigate the optimal timing of initial IVIG administration by comparing the outcomes of early and conventional IVIG treatment in KD patients.
MethodsThe Chengdu Women's and Children's Central Hospital Ethics Committee approved the study protocol (Approval No.B202123) and waived informed consent requirements. All methods were carried out following the Declaration of Helsinki.
Study design and participantsAll the patients with KD who were hospitalized at the Chengdu Women's and Children's Central Hospital between January 2018 and December 2020 were identified as participants in this retrospective study. The Chengdu Women's and Children's Central Hospital is a tertiary referral center that serves a catchment area of approximately 2 million people per year who live in the southwest region of China. Patients were diagnosed with KD according to the American Heart Association (AHA) guideline in 2017.1 The inclusion criteria for study subjects were as follows: (1) patients were <18 years old; (2) patients of initial onset of KD; (3) patients received standard treatment with 2 g/kg of IVIG of single infusion during the acute phase of illness. Exclusion criteria were as follows: (1) recurrent KD; (2) patients had a severe infection, allergy, autoimmune diseases, or collagen disease; (3) patients who had received IVIG in the first three months of admission; (4) patients who did not receive IVIG or initial IVIG dose was <2 g/kg; (5) patients with missing clinical or laboratory information. The AHA guideline for KD recommends starting IVIG treatment within 10 days from the onset of symptoms and, if possible, within 7 days to prevent CAA because this is when vasculitis worsens. Several retrospective studies, however, reported that IVIG use at ≤ 4 days of illness had no benefit in preventing CAA, but was instead associated with increased IVIG resistance.7,8 Therefore, the present study hypothesizes that the clinical outcomes differed among four categories (start of IVIG therapy up to 4 days, 5-7 days, 8-10 days, and more than 10 days). Thus, the remaining patients who meet the inclusion criteria were classified into four groups: those who received the initial IVIG ≤ day 4 of illness (the early treatment group), those who received IVIG on days 5-7 (the conventional treatment group), those who received IVIG on days 8-10 (the conventional treatment group), and those who were treated after day 10 (the late treatment group). The first day of fever was defined as day 1.
Data collectionThe demographic, clinical outcomes, and laboratory data were extracted from the medical records. Blood samples were collected within 24 h pre-IVIG treatment. IVIG resistance was defined as recrudescent or persistent fever ≥36 h but not longer than 7 days after initial IVIG infusion.1
Treatment protocol for KD patientsIn the present study's hospital, all KD patients received the standard therapy with IVIG (2 g/kg) and aspirin (30-50 mg/kg/d during the acute phase of illness) immediately after the diagnosis. The aspirin was lowered to 3-5 mg/kg/d 2-3 days after the patients were afebrile. Other therapies, including prednisolone, were not used in the initial treatment. Combined antiplatelet and anticoagulation therapy were recommended for patients with giant aneurysms. For IVIG-resistant patients, the 2nd IVIG of the same dosage was administrated. If fever persists 36 h after the 2nd IVIG infusion, intravenous methylprednisolone (30 mg/kg/dose) was performed for 3 consecutive days. No patients received additional treatment such as infliximab, plasma exchange, and cytotoxic agents.
Complete KD was diagnosed in any children with a fever and had four or more of the following five major symptoms: (Ⅰ) erythema and cracking of lips, strawberry tongue, and/or erythema of oral and pharyngeal mucosa, (Ⅱ) polymorphous exanthema, (Ⅲ) bilateral conjunctival congestion, (Ⅳ) changes of the peripheral extremities, and (Ⅴ) unilateral cervical lymphadenopathy. Incomplete KD was defined as a child with a fever with fewer than four major symptoms and compatible laboratory or echocardiographic findings.1
EchocardiographyEchocardiography was used to detect CAA during hospitalization. Echocardiography was performed and supervised by an experienced echocardiographer and with appropriate transducers. The coronary artery abnormalities were defined as follows: (1) normal: Z score < 2; (2) only dilation: Z score 2 to < 2.5; or initial Z score < 2, Z score decline during follow-up (usually 6-12 months) ≥ 1; (3) small coronary aneurysm: Z score ≥ 2.5 to < 5; (4) medium coronary aneurysm: Z score ≥ 5 to <10, and absolute dimension < 8 mm; (5) large or giant coronary aneurysm: Z score ≥ 10, or absolute dimension ≥ 8 mm.1 Patients with a Z score ≥ 2.0 were considered to be CAA. Coronary artery aneurysms were classified into small aneurysms, medium aneurysms, and large (or giant) aneurysms.1 The Z-score was measured before IVIG administration using the formula by Dallaire and Dahdah.15 The authors chose the maximum Z-scores of the left main coronary artery, left anterior descending coronary artery, left circumflex artery, and right coronary artery.
The primary outcomes were IVIG resistance and the development of CAA in the acute phase. The secondary outcome was the length of hospitalization.
Statistical analysisThe normality of the distribution of variables was checked using the Kolmogorov-Smirnov test. Continuous variables were expressed as mean ± standard deviations or median and IQR (25th, 75th percentile) if non-normally distributed. Categorical variables were expressed by presenting the frequency and proportion in each category. To compare the difference of variables according to the timing of initial IVIG administration of disease onset (≤ day 4, days 5-7, days 8-10, and >day 10), analysis of variance (ANOVA) or Kruskal-Wallis H test was applied for continuous variables, and Chi-square analysis was applied for categorical variables. For significance found by ANOVA, Kruskal-Wallis H test, or Chi-square test, the further pairwise comparison was conducted through the Dunnett-t-test or the least-significant difference (LSD) test based on the homogeneity of data variance, or Bonferroni method for cross-table.
The multivariate regression analysis was conducted to explore the factors associated with the prevalence of IVIG resistance and coronary artery abnormalities, which were dependent variables. The independent variables for multivariate regression analysis contained both categorical and continuous variables. The continuous variables were first screened by univariate analysis, namely t-test or Mann-Whitney U test, based on the distribution normality of variables. The variables associated with dependent variables were included in the regression model. The area under the receiver operating characteristic (ROC) curve was analyzed to assess the predictive accuracy of initial IVIG administration time for IVIG-resistant and to identify the optimal cut-off point according to the Youden index. A subgroup analysis was performed by dividing CAA into dilation, small aneurysm, and medium to large (or giant) aneurysm according to the AHA guideline in 2017.1 The authors also performed a sensitivity analysis to compare the primary and secondary outcomes between the patients who received the initial IVIG ≤day 7 of illness and >day 7 of illness. A P value less than 0.05 was considered statistically significant. All statistical analyses were performed with the IBM SPSS Statistics 25.0 (SPSS, Chicago, IL, USA).
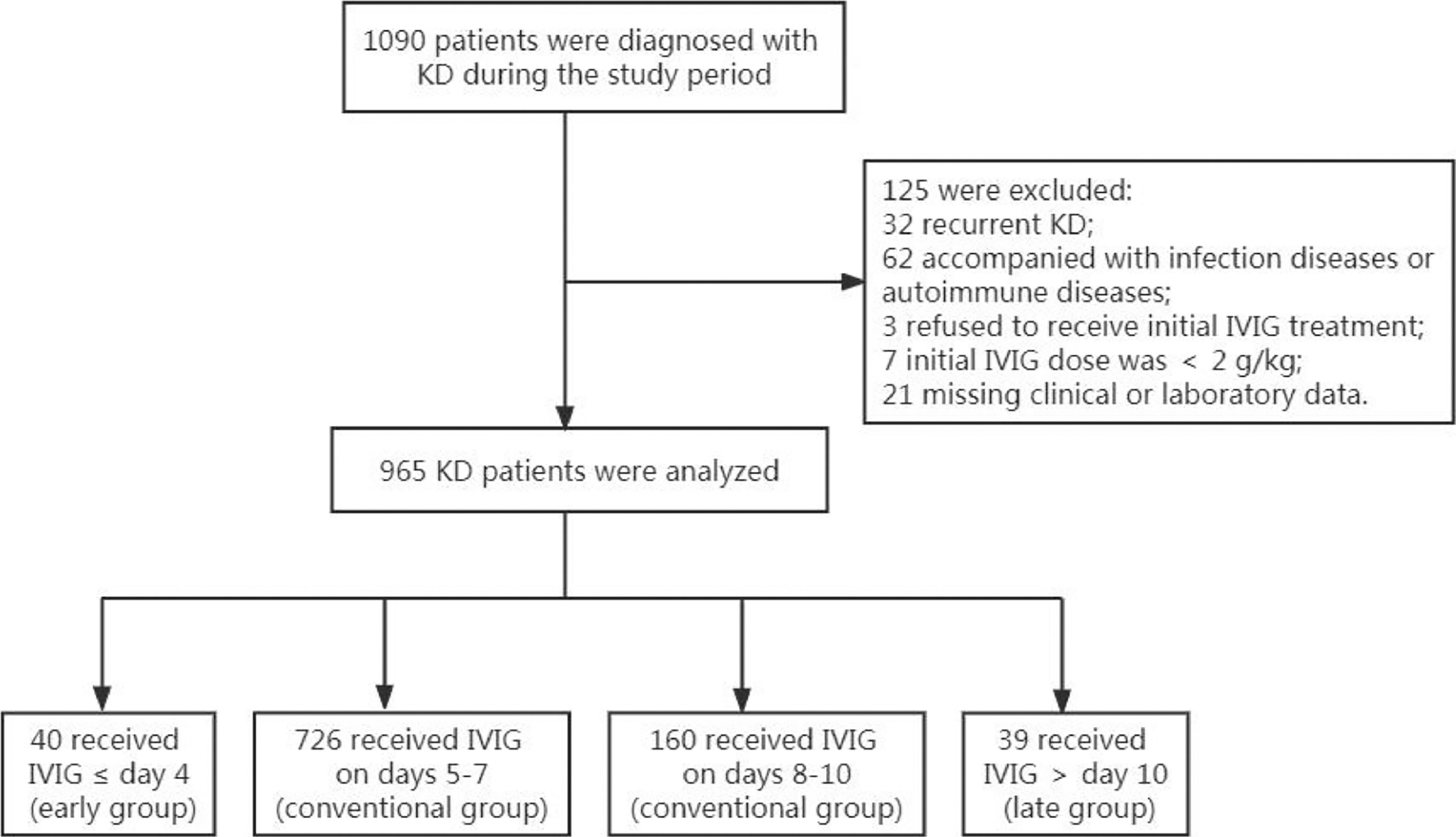
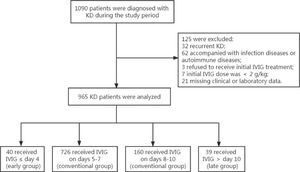
ResultsCharacteristics of included KD PatientA total of 965 KD patients who meet the inclusion criteria were included (Fig. 1), with a median age of 28.10 ± 19.79 months, ranging from 1 to 132 months. The ratio of males to females was 1.53:1. All patients received initial IVIG treatment once the KD diagnosis was made. There were 883 patients diagnosed as complete KD, accounting for 91.50% of the total KD patients. Forty patients were classified into the early group (≤ 4 days), 726 into the conventional (5-7 days) group, 160 into the conventional (8-10 days) group, and 39 into the late group (> 10 days). There were no significant differences in age. A slight difference (p = 0.039) was observed in gender among the four groups. The proportions of complete KD in early and conventional (5-7 days) groups were significantly higher than patients in conventional (8-10 days) and late groups (p = 0.000). The detailed information on the characteristics of included KD patients can be seen in Table 1.
Baseline characteristics of included KD patients.
Results are presented in n (%), median (25th, 75th percentile).
The neutrophils%, C-reactive protein (CRP), White Blood Cell (WBC) count, and Hemoglobin (HB) in early and conventional (5-7 days) groups were significantly higher than those in conventional (8-10 days) and late groups (p 0.05), suggesting inflammatory reaction is severe in early and conventional (5-7 days) groups. Platelet in early and conventional (5-7 days) groups were significantly lower than in conventional (8-10 days) and late groups (p 0.05). AST and ALT in the conventional (5-7 days) group were higher than in the conventional (8-10 days) group. The four groups showed no significant difference in albumin and erythrocyte sedimentation rate (ESR) (Appendix S1).
IVIG resistance, development of Coronary artery abnormalities, and the length of hospitalizationA total of 101 patients had IVIG resistance, accounting for 10.47% of the included KD patients. The early group had a higher IVIG-resistance rate (17.5%) than the other three groups. The conventional (5-7 days) group had the lowest IVIG-resistance rate (9.92%) among the 4 groups. However, there were no significant differences in IVIG resistance rate among the 4 groups (p = 0.335) (Table 2). The length of hospitalization of the late group was significantly longer than the two conventional groups (p = 0.000). There were 213 patients involved in CAA, accounting for 22.07%. The late group had the highest rate of CAA (38.46%). The rate of CAA in the late group was significantly higher than in the conventional (5-7 days) group (p < 0.05) (Table 2).
The clinical outcomes of patients with Kawasaki disease in 4 groups.
Results are presented in n (%), median (25th, 75th percentile).
In the subgroup analysis, there was no significance in the proportion of dilation between each group. The conventional (5-7 days) group had the lowest proportion of small aneurysms (11.71%) and medium to large aneurysms (1.93%). Moreover, the early group has a proportion of small aneurysms significantly higher than the conventional (5-7 days) group (p < 0.01), while the late group has a proportion of medium to large aneurysms significantly higher than conventional (5-7 days) group (p < 0.01) (Table 3). The sensitivity analysis also confirmed no difference in the IVIG resistance between those who started the initial IVIG ≤day 7 of illness and those who received IVIG >day 7 of illness. There was a significant difference in the higher proportion of CAA (OR = 0.680; 95%CI = 0.476-0.972, p = 0.034) and longer length of hospitalization (p = 0.033) for those who started IVIG administration more than 7 days from the onset (Appendix S2).
Sub-group analysis of coronary artery abnormalities for patients with KD in 4 groups.
Results are presented in n (%), median (25th, 75th percentile).
According to the ROC analysis, the optimal cut-off value of initial IVIG administration time for predicting IVIG resistance was >7 days, with a sensitivity of 75.25% and specificity of 82.41% (area under the curve was 0.785, 95% confidence interval was 0.758-0.811; p < 0.001) (Appendix S3).
According to the multivariable logistic regression analysis, age and albumin were independent predictors of IVIG resistance in patients with KD (Appendix S4). Incomplete KD, Gender, CRP, and albumin were independent risk factors of CALs in KD patients (Appendix S5).
DiscussionThis study aimed to investigate the optimal time option for initial IVIG treatment in KD patients. There were no significant differences in IVIG resistance among the 4 groups in the present study, although the early group had the highest IVIG resistance rate. The sensitivity analysis also confirmed no difference in the IVIG resistance between those who started the initial IVIG ≤ day 7 of illness and those who received IVIG >day 7 of illness. However, patients who received IVIG administration more than 7 days from the onset had a higher proportion of CAA and longer length of hospitalization than those who started IVIG administration less than 7 days. Thus, IVIG therapy within 7 days of illness is more effective for reducing the risk of coronary artery abnormalities than those who received IVIG >day 7 of illness.
The late and conventional (8-10 days) groups had higher proportions of incomplete KD than the early and conventional (5-7 days) groups in the present study. A higher proportion of incomplete KD may experience significant delays in diagnosis,16 which leads to the late IVIG therapy. Furthermore, it is not surprising that patients treated earlier had a higher level of CRP, neutrophils%, WBC, and lower platelet counts. It was suggested that the patients in the early and conventional (5-7 days) groups might likely have more typical symptoms and more severe cases than conventional (8-10 days) and late groups, leading to an early initial administration of IVIG.17
IVIG has been the mainstay of treatment in KD to reduce the prevalence of CAA. However, about 10–20% of patients show resistance to IVIG treatment and present a higher risk of coronary vasculitis.18 Initial IVIG resistance is also associated with an increased risk of coronary artery aneurysms.19,20 The criteria for when to provide IVIG are unclear and differ from previous studies.7,12,21-23 The 2004 AHA and 2018 Italian Society of Pediatrics guidelines stated that IVIG should be used within 10 days after the onset of disease and, if possible, within 7 days. In the latest 2017 AHA guideline, it is suggested that IVIG should be instituted as early as possible within the 10 days of illness onset of fever; However, there is no suggestion on the optimal timing of IVIG and if it can be given earlier. Several studies reported that IVIG use at ≤ 4 days of illness is associated with increased IVIG resistance.7,21,22,24 These results must be interpreted with caution. Muta et al.7 reported early treatment is likely to result in a greater requirement for additional IVIG. Still, they did not perform multivariate analysis because of limited data associated with the need for IVIG retreatment. The present results are similar to previous studies that showed earlier IVIG treatment within 4 days may not increase the higher incidence of IVIG resistance.12,23 The early group showed a high rate of IVIG resistance because there might be more patients with severe inflammation or atypical clinical course, respectively.
The IVIG resistance may be associated with the dynamic activation status of macrophages and the level of serum cytokines during the acute phase of KD.25 Inappropriate time options for IVIG treatment may affect the balance of pro-inflammatory and counter-inflammatory cytokines and subsequently the patient's clinical outcome, including additional treatment and development of CAA. Future studies should focus on the pharmacokinetics of IVIG and the influence of immunological changes during the acute phase of KD on treatment response. The ROC analysis showed the optimal cut-off value of initial IVIG administration time for predicting IVIG resistance was >7 days. Multivariable logistic regression analysis revealed that age and albumin were independent predictors of IVIG resistance, which is compatible with the previous studies.10,26
The most common sequela of KD is CAA, which is speculated to be caused by acute systemic inflammation.27 In the present study, the late group had the highest rate of CAA. It was significantly higher than the conventional (5-7 days) group, indicating the IVIG should be administrated within 10 days after the disease onset to prevent the CAA. The results of the subgroup analysis were similar - the late group has a rate of medium to large aneurysms significantly higher than the conventional (5-7 days) group. Although the early group has a higher proportion of small aneurysms than the conventional (5-7 days) group, one possible reason is that this was due to a bias caused by including severe cases with full clinical symptoms as early as day 4 after onset. The sensitivity analysis also showed that patients who began IVIG treatment more than 7 days of illness onset might develop CAA more frequently than those treated on days ≤ 7. To minimize cardiac sequelae, it is crucial to avoid any delay in IVIG treatment because earlier inflammatory suppression may contribute to avoiding developing CAAs.28 Multivariate regression revealed that the incomplete KD, male gender, higher level of CRP, and lower level of albumin were independent risk factors of CALs, in line with other reported studies.26,29,30
LimitationsThis study has several limitations. First, it was a retrospective observational study with potential selection and information bias. Second, all the KD patients came from Sichuan, southwest China, limiting the findings' generalization. Third, the study results could have been limited by incomplete data caused by patients being lost in follow-up after discharge from the hospital. A rigorous prospective study with long-term follow-up patients from other districts is needed.
ConclusionIVIG therapy within 7 days of illness is found to be more effective for reducing the risk of coronary artery abnormalities than those who received IVIG >day 7 of illness. All patients meeting diagnostic criteria for KD should be treated as soon as the course of illness as the diagnosis can be established. IVIG treatment within the 7 days of illness seems to be the optimal therapeutic window of IVIG. However, further prospective studies with long-term follow-up are required.
FundingThis manuscript did not receive any funding.
Ethical approvalThe Chengdu Women's and Children's Central Hospital Ethics Committee approved the study protocol (Approval No.B202123) and waived informed consent requirements.