To review, analyze, and present the available evidence on the usefulness of point-of-care pulmonary ultrasound in the diagnosis and monitoring of community-acquired pneumonia (CAP), aiming to facilitate its potential inclusion into pediatric clinical reference guidelines.
Source of dataA non-systematic research was carried out in the MEDLINE (PubMed), LILACS, and SciELO databases, from January 1985 to September 2019. The articles that were considered the most relevant were selected.
Synthesis of dataCAP is a relevant cause of morbidity and mortality in pediatrics and its clinical management remains a major challenge. The systematic use of chest X-ray for its diagnosis is controversial because it exposes the child to ionizing radiation and there are interobserver differences in its interpretation. Recently, the use of point-of-care pulmonary ultrasound by the pediatrician has been presented as an alternative for the diagnosis and monitoring of CAP. A great deal of evidence has disclosed its high sensitivity and diagnostic specificity, with the advantages of no ionizing radiation, relatively low cost, immediate results, portability, and the possibility of repetition according to the requirements of disease evolution. Moreover, its use can help rule out possible bacterial etiology and thus prevent inappropriate antibiotic treatments that favor bacterial resistance.
ConclusionsPoint-of-care ultrasonography represents an opportunity to improve the diagnosis and monitoring of CAP. However, as an operator-dependent technique, training is required for adequate image acquisition, correct interpretation, and integration with clinical data for correct decision-making.
Point-of-care ultrasound (POCUS) is slowly being incorporated into pediatric practice, as has previously occurred in other medical specialties. It is a diagnostic or therapeutic tool that has found several applications in the hands of the clinical pediatrician, mainly in the emergency area.1,2 Adequate training in image acquisition and interpretation allows pediatricians to obtain relatively quick answers to specific clinical questions and helps in the decision-making, favoring, in many cases, the outcome in severe and urgent pathologies, as well as the monitoring of the sick child’s evolution, with low cost and zero side effects, while preventing the use of ionizing radiation.3
Initially, pulmonary ultrasound (PUS) had limited use due to the acknowledgment of the difficulty for ultrasound propagation in a medium with a large amount of air and a bone structure such as the rib cage, making it impossible to directly view the lung parenchyma. A little more than two decades ago, a different view favored its improvement in the diagnosis of pulmonary processes. Lichtenstein et al. observed and described the presence of several “artifacts” and other images, starting an interesting path in the description of these and in the semiological identification of specific pathologies.4–13 The images, according to the ultrasound appearance (hypo-, hyper-, or iso-echogenic), in different scanning modalities (B- and M-mode and Doppler), allow correlating and clinically interpreting the findings.14 The progressive experience facilitated a growing clinical management in pediatrics and the identification of some of its singularities. The child's anatomical characteristics—smaller chest wall thickness, incomplete ossification, and smaller size of the pulmonary structures—favor better ultrasonographic visualization and facilitate theevaluation.15
Nonetheless, community-acquired pneumonia (CAP) remains a relevant cause of morbidity and mortality in pediatrics and its clinical management remains a major challenge. The systematic use of chest X-ray in CAP diagnosis is controversial because it exposes the child to ionizing radiation and there are inter-observer differences in its interpretation. Recently, the use of pulmonary POCUS by the pediatrician has been shown to be an alternative for CAP diagnosis and monitoring. A great deal of evidence has disclosed its high sensitivity and diagnostic specificity, with the advantages of no ionizing radiation, relatively low cost, immediate results, portability, and the possibility of repetition according to the requirements of disease evolution. Moreover, its use can help rule out a possible bacterial etiology, thus preventing inadequate antibiotic treatments that favor bacterial resistance.
Sources of dataA non-systematic review was carried out in the MEDLINE (PubMed), LILACS, and SciELO databases covering the period from 1985 to July 31, 2019, using the following strategy: in the PubMed database with the following Boolean operators: “Ultrasonography” and its synonyms [MeSH Terms], [Title / Abstract], with“Pneumonia” and its synonyms [MeSH Terms], [Title / Abstract], with the 0–18 year-old filter, which resulted in the identification of 235 articles. In the LILACS database, the strategy (tw:((tw:(ultrasound OR ultrassonografia)) AND (tw:(pneumonia))) AND (db:(“LILACS”)) AND (tw:(child))) resulted in the retrieval of 22 articles. In the SciELO database, the strategy (subject: (ultrasound AND pneumonia)) resulted in the retrieval of 19 articles. After careful evaluation based on the association with the proposed topic, the articles that addressed the use of POCUS performed by physicians specialized in radiology or non-specialist physicians in the diagnosis and monitoring of CAP were selected.
CAP Lower respiratory tract infections in children under 5 years of age remain the main cause of mortality from infections.16 In 2017, pneumonia caused more than 800,000 deaths in children under 5 years of age worldwide, representing 15% of all deaths,17 with an etiology attributable to Pneumococcus (55.8%), H. influenzae (8.3%), syncytial respiratory virus [SRV] (5.2%), influenza virus (1.4%), and undefined (29.3%), varying according to the regions of the world,18 depending on the epidemiological profiles related to hygiene conditions, health, nutritional status and vaccinal prevention against any of these microorganisms, among others.
CAP is pneumonia that appears in a previously healthy child, with continuous or recurrent high fever, accompanied by tachypnea and intercostal retraction, which affects outpatients two weeks before or in the first 48 h after hospital admission.19,20 However, these criteria are not very specific, since the symptoms in a child can be extremely variable, and include non-respiratory findings. This circumstance favors the performance of a chest X-ray in a large number of cases, mainly in emergency services, although it is not indicated in most cases, nor is a routine test in children with suspected CAP.19,21
For more than two decades, the World Health Organization (WHO) proposed an algorithm based on frequent symptoms in pneumonia.17 The presence of coughing and/or difficulty breathing, tachypnea (50 or more breaths per minute in children aged 2–11 months, and 40 or more between 12 and 59 months), and intercostal retraction could mean the presence of pneumonia. A recent systematic review of 23 prospective studies with nearly 14,000 children revealed that fever and tachypnea, as well as auscultatory findings, are not so strongly associated with a diagnosis of pneumonia. However, the presence of moderate hypoxemia (oxygen saturation ≤ 96%) and increased respiratory effort (nasal flaring, intercostal retractions, and rales) appear to be.22 On many occasions, clinical findings must be complemented with imaging tests, as auscultation can be negative or inconclusive.23 A chest X-ray is frequently requested and it is not indicated in most cases, nor as a routine examination in children with suspected CAP or after starting treatment, and should be reserved only for severe cases treated in the hospital or those with complications.19,21,23 The true gold standard for the diagnosis of CAP is computed tomography (CT), but it cannot be used routinely due to radio protection reasons.23 The “as low as reasonably achievable” (ALARA) principle should be applied at pediatric ages to avoid unnecessary exposure to ionizing radiation.24
PUS can provide relevant information for CAP diagnosis and monitoring, and although the chest X-ray has been accepted with all its imperfections, some sectors are still critical regarding the PUS, except in the diagnosis of pleural effusion.23
PUS technique in pediatricsThere is a variety of ultrasound equipment; they can be classified as console, portable, and ultraportable ultrasound machines.14 The ultrasound probes used for pulmonary examinations in children are usually high-frequency linear probes (6–12 MHz), which allow exploring surface structures at high resolution. However, convex and sector probes (less frequent), essential for echocardiography and deeper anatomical areas, including costophrenic areas, can be used.25–27 The depth is generally defined as between 5 and 10 cm,28 whereas other authors point to a maximum of 8 cm.29 It is highly recommended to use the smallest possible depth to improve the resolution of images obtained with these linear probes.
Sedation is generally not used, although some authors perform it.30 The child can be assessed in several positions: supine, prone, sitting, or in the lateral decubitus position.26 The transducer generally rests on two ribs (exploration of the longitudinal intercostal space), with the probe marker facing the child's head, moving slowly in the caudal direction until the diaphragm is observed, allowing the exploration of the following lines: hemi clavicular, middle, and posterior axillary lines, between the scapula and the spinal column. It is also possible to add the cross-sectional plane (the entire footprint of the transducer is supported in the intercostal space, following its main axis; this plane prevents the acoustic shadowing of the ribs and allows a larger pleural surface to be explored). The latter is used in case of abnormal findings or when the specific objective is quantifying pulmonary aeration.31 A comprehensive exploration will require observing as many intercostal spaces as possible, including the costophrenic sinuses, to rule out a pleural effusion. At this level of the pulmonary bases, it is essential to identify the so-called “bat sign” (see below) and/or the diaphragmatic muscle structure, so as not to confound basal pulmonary consolidations with solid abdominal viscera. Likewise, the presence of the thymus in babies should not be confounded with pulmonary consolidation.32–34
The lungs in children: an ultrasound overviewThe spectrum of PUS findings will largely depend on the air/liquid ratio in the parenchyma. The normal (healthy, well-aerated) lung is not visualized, only the characteristic artifacts generated by the ultrasound (non-real images). Since the air content acts as an ultrasound deflector, it reflects the waves at the level of the pleuropulmonary interface, originating the so-called “mirror image artifact.” However, as the lung becomes ill, there is a loss of aeration, with the substitution of air by the liquid component, which results in new artifacts, as is explained below. When the loss of aeration is complete, an actual visualization of the lung parenchyma (not artifacts) is attained.
The ultrasound examination of the chest in children can essentially offer the following findings35:
- •
The ribs are seen as a hyperechogenic (white) image, with posterior acoustic shadowing, although in the case of very young children, this shadowing might not exist due to incomplete ossification. The longitudinal view of two ribs with their respective acoustic shadowing and the central and upper areas determine the “bat sign.”
- •
The pleuropulmonary interface (an interface is defined as the apposition of two anatomical structures with a marked difference in their resistance when they are crossed by the ultrasound): the pleuropulmonary interface is generated by the apposition of all the structures that extend from the skin to the pleura itself (where the ultrasound travels very easily) with the pulmonary structure (where the ultrasound is deflected or passes through very slowly). This very abrupt change in the ultrasound velocity generates the so-called pleural line, actually consisting of both pleural layers (parietal and visceral) that move laterally with the respiratory cycle (“lung sliding sign”). The identification of the pleural slidingsign is essential, as it implies that both pleural layers are in contact and healthy.
- •
Regarding the pleural line, the following can be observed (Fig. 1):
- -
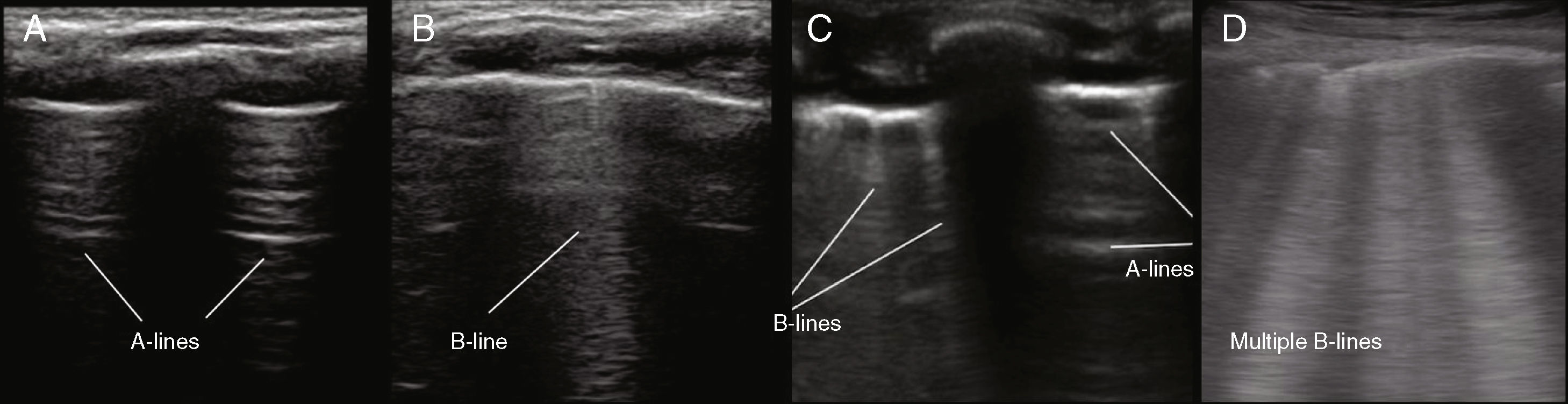
A-lines: these are artifacts caused by the reflection of the ultrasound beams at the level of the pleuropulmonary interface, whose image is replicated in this way. They are hyperechogenic, parallel, horizontal, and equidistant lines, but they differ from the true pleural line because they do not move (they do not slide). They indicate a high gas/volume ratio below the parietal pleura and can be associated with a normal lung, a hyperinflated lung, or pneumothorax.
- -
B-lines: they appear perpendicularly from the pleural line as hyperechoic reverberations that reach the lower edge of the image on the screen, erasing the A-lines in their path and moving synchronously with the pleural sliding.36 They depend on the proportion of air and water in the lung parenchyma, decreasing their number during aeration and increasing with the increase in extravascular water content.37–39 They have a special significance in the newborn. Three or more B-lines per intercostal space (pattern B) should lead the clinician to consider the presence of interstitial syndrome.
- -
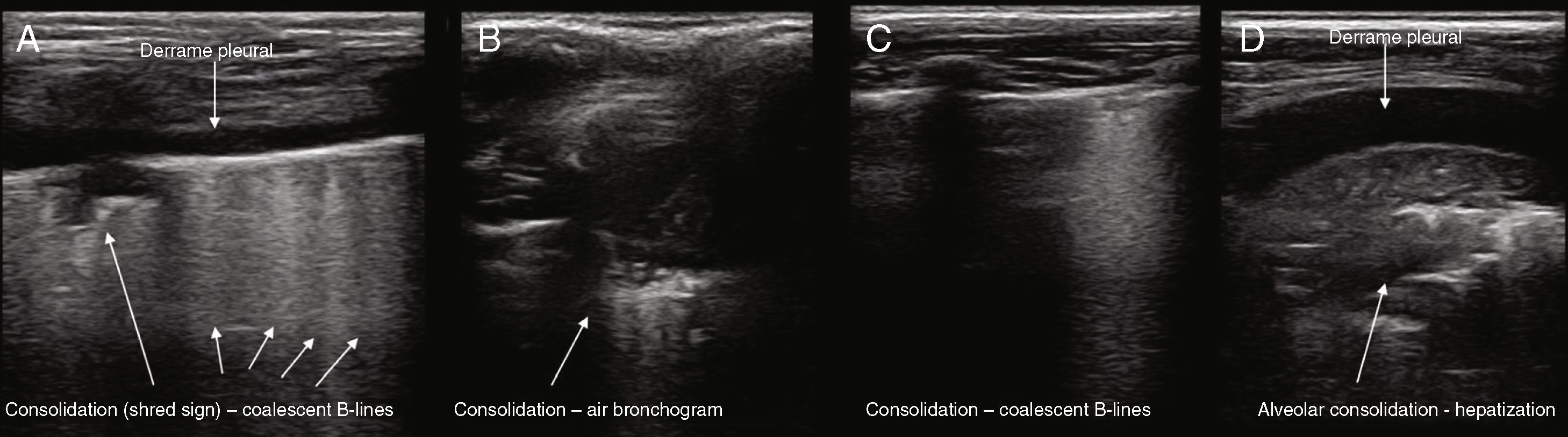
Actual images: in case of consolidation, the pulmonary image is real. The parenchyma appears as a tissue-like image (pattern C), when at least two pulmonary segments are affected. If only one segment is affected, there is a loss of linearity of the pleural interface, which will appear deviated (shred sign).
Figure 1.Frequent exploratory findings of pulmonary ultrasonography in children. A, A-lines, showing the “bat sign” (pattern A, healthy lung); B, B-line (with continuity of the pleura through the cartilaginous structure in young children); C, Coexistence of A-lines and B-lines in a single window; D, Several B-lines (B pattern, interstitial syndrome).
- -
In the last decade, there has been an increase in the number of studies supporting the use of ultrasound in the treatment of respiratory pathologies,23,40–46 with specific original research on pneumonia in the pediatric age range in different parts of the world,35,41,47–80 meta-analyses and specific analyses,22,52,68,69,81–85 and other related publications.53,59,86,87
These studies have provided information on several aspects associated with the potential use and effectiveness of PUS for pneumonia diagnosis and monitoring. Thus, in relation to the location of CAPs, in adults 98.5% of cases are in contact with the pleura10 and it is believed that a lower number occurs in children,65 which favors ultrasound diagnosis. However, Reissiget al.86 state that in 8% of cases, pneumonic lesions might not be detectable by PUS because they do not reach the pleural line or are hidden behind bone structures. The areas of error for the ultrasound diagnosis can be retro-scapular and apex areas, which do not reach the subpleural area, or other areas of difficult access, such as the posterior apical regions hidden by the scapula and supraclavicular fossa, perihilar areas, and the axillary region.47,52,57,61,65,74 In another area, the left lung base, the confluence of the spleen and air in the stomach may appear to be a pulmonary consolidation with bronchogram on PUS.49 Miliner et al. observed in children aged between 1 and 7 years old that pneumonia consolidations are located in the following areas: 54.3% in the bases, 37.3% in the medial area, and 8.3% in the apical area, with 46.4% completely in the posterior thoracic plane (24.8% on the left and 21.7% on the right) and 53.7% in the anterior and lateral planes.
Lesion size is another relevant factor. Shah et al.49 found pulmonary consolidations smaller than 1 cm located by ultrasound, with negative radiography; they indicated a limit close to 1.5 cm for the detection of pulmonary consolidation by radiography. Claes et al.54 observed consolidations of 0.5–1.6 cm by PUS, which were negative at the X-ray. Similarly, it must be taken into account that atelectasis may appear similar to pulmonary consolidation and, in small areas, differentiation might not be possible.68 Lovrenski et al.63 state that, for the diagnosis of pulmonary consolidation in children by auscultation, it must be at least 30 mm in size, since the identification is significantly reduced with smaller sizes (false-negative results with smaller diameters). Lung ultrasound can detect smaller sizes.
The specific ultrasonographic findings observable in CAP in children are diverse and depend on several factors, including the infectious agent, location, degree and size of pulmonary involvement, and perhaps the most relevant: the degree of the pulmonary process evolution in children at the degree when the evaluation is being performed. Xin et al.,83 in a recent meta-analysis, highlighted the four most frequently observed findings: pulmonary consolidation, air bronchogram, pleural abnormalities, and pleural effusion.
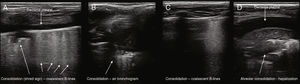
Ianello et al.61 reported that the consolidation zones appear as hypoechoic areas with blurred margins and that they observed an air bronchogram in all cases. Copetti and Catarrosi35 describe the consolidations as a hypoechogenic area with ill-defined borders, with less echogenic pleura in the area affected by consolidation and reduced or absent pleural sliding. Weinberg et al.88 made the first description of an air bronchogram by ultrasound in pulmonary consolidation in children. Its presence is variable: between 36.3% and 95.3%, according to several studies,47,55–67,60,70 therefore being a less specific sign. Yilmazet al.70 described the presence of pleural irregularities (100%), B-lines (99.9%) (multiple focal points in 69.1% and confluent ones in 78.5%), air bronchogram (95.3%), subpleural consolidation (95.3%), fluid bronchogram (32.2%), and pleural effusion (3.4%). Fig. 2 shows several patterns of pulmonary consolidation in pneumonia.
The presence of pleural effusion is also variable and can be detected by PUS as small effusions at costophrenic angles, not visible on X-ray. Iuri et al. observed twice as many cases detected by US as compared to chest X-ray.47 Similarly, in pneumonia complicated by stroke, US facilitates the identification of floating fibrin and the presence of septa, to distinguish between exudate and transudate.60
In the case of the newborns, pneumonia can disclose abnormalities in the pleural line (disappearance, irregularities, interruption, and blurred appearance), pulmonary consolidation (hepatization of subpleural tissue, with air or fluid bronchogram), disappearance of the pleural sliding, and the lung point (a non-essential criterion).43,89
Another relevant aspect is the visualization of changes in the pneumonic process according to clinical evolution, offering a wide range of findings that will depend on the evolution phase: subpleural consolidation with bronchogram branching, confluent focal B-lines, subpleural hypoechoic areas without air bronchogram (called “hepatization”) that can be combined with adjacent B-lines and small 5-mm hypoechoic subpleural areas (probably small atelectasis areas).23
Guerra et al.90 describe pneumonia as a hypoechoic subpleural area and/or an area characterized by hepatization, both with irregular borders and an air bronchogram inside. They say that both models can be part of the same infectious process, but at two different evolution stages: as the air decreases and is replaced by the inflammatory fluid, the hepatization process increases sharply. Caiulo et al. observed the following in children with pneumonia at diagnosis on the first day: air bronchogram in 78% and numerous confluent B-lines in 66% (when interpreting these B-lines adjacent to consolidation, they are probably an expression of perilesional inflammatory edema), 20% with abnormalities in the pleural line, and 18% with pleural effusion. At a first control between three and six days, coinciding with clinical improvement, 91.6% of the subpleural hypoechoic areas decreased in size or disappeared, and 100% of the B-lines disappeared, as well as the pleural effusion in the present cases. Omran et al.,74 five days after the diagnosis, observed that consolidation showed evolution, disappearing in 26.5%and decreasing in 55.1% of cases (totaling 81.6%), coinciding with clinical improvement; in other cases, it remained the same size (4.1%) or increased in size (14.3%). The air bronchogram present in 78% decreased to 52%. Ianello et al.,61 also after five days, observed disappearance of the consolidation in 46.7% and decrease in size of the consolidation in 38.3% (total of 85%); the remaining 15% showed an insignificant decrease in size. Therefore, the possibilities offered by this technique are remarkable, allowing monitoring of the evolution, not only measuring the size of the consolidation, but also the decrease in the air bronchogram and the pleural effusion volume, if there is any.55
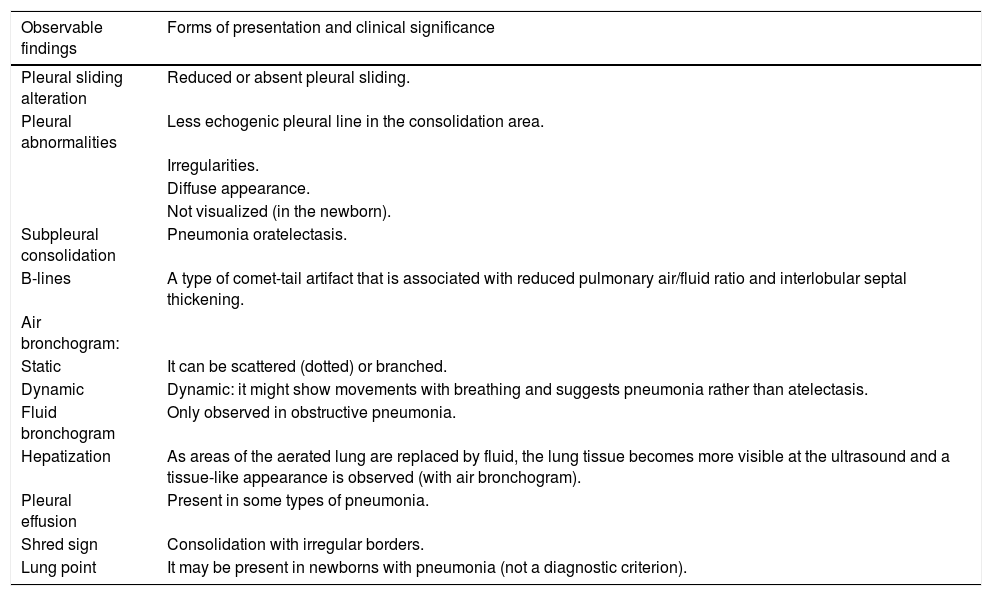
Another element of interest is the association between ultrasound findings and etiology, which is currently one of the aspects that may require the greatest research effort. The confluent B-lines and small subpleural consolidations without air bronchogram are interpreted by some authors as having a viral origin.40,62,91,92 The sub-centimeter pulmonary condensations observed by PUS in the diagnosis of pneumonia can be semiologically associated to viral processes: viral pneumonia, bronchiolitis, and even other non-infectious processes, such as asthma40,45,62,91 or atelectasis.75 Their incorrect interpretation can support unnecessary antibiotic use. Thus, when observing these small pulmonary consolidations with air bronchogram and a clinical picture of pneumonia, it can be treated with antibiotics from the beginning of a bacterial process, or observed closely while awaiting the evolution. Therefore, the PUS can be a diagnostic tool that contributes to the differentiation between bacterial causes and viral.78Table 1 summarizes the ultrasound findings found in CAP.35,43,47,56,57,91,93,94
Summary of ultras the pediatric age group.35,43,47,56,57,90,93,94.
| Observable findings | Forms of presentation and clinical significance |
|---|---|
| Pleural sliding alteration | Reduced or absent pleural sliding. |
| Pleural abnormalities | Less echogenic pleural line in the consolidation area. |
| Irregularities. | |
| Diffuse appearance. | |
| Not visualized (in the newborn). | |
| Subpleural consolidation | Pneumonia oratelectasis. |
| B-lines | A type of comet-tail artifact that is associated with reduced pulmonary air/fluid ratio and interlobular septal thickening. |
| Air bronchogram: | |
| Static | It can be scattered (dotted) or branched. |
| Dynamic | Dynamic: it might show movements with breathing and suggests pneumonia rather than atelectasis. |
| Fluid bronchogram | Only observed in obstructive pneumonia. |
| Hepatization | As areas of the aerated lung are replaced by fluid, the lung tissue becomes more visible at the ultrasound and a tissue-like appearance is observed (with air bronchogram). |
| Pleural effusion | Present in some types of pneumonia. |
| Shred sign | Consolidation with irregular borders. |
| Lung point | It may be present in newborns with pneumonia (not a diagnostic criterion). |
Sensitivity and specificity in the diagnosis of pneumonia by ultrasound vary widely, according to published studies. They depend on many factors, although perhaps the two most important are the type of selected patients (age, hospital emergency or not, primary care, etc.) and the examining physician’s training in PUS. The meta-analysis by Balk et al.,82 analyzing 1510 children in 12 pneumonia studies, comparing PUS with chest X-ray, showed a sensitivity of 95.5% (greater than that of X-ray) and similar specificity for both procedures. Again, the differences in sensitivity and specificity should be emphasized, depending on whether consolidations <1 cm are considered.49
Pereda et al.81 stated that sensitivity and specificity are better in the hands of experienced users, but are also adequate in less experienced hands. Recommendations for the training of general pediatricians in PUS for the diagnosis of pneumonia can have an important impact on different clinical situations, especially in low-resource countries and in small primary care clinics where chest X-ray may not be available.
The time of exploration should also be emphasized. Several studies have shown a great variability in the duration of PUS with the objective of diagnosing pneumonia in the pediatric population: ranging from three to 15 min,43,45,49,53,54,63–65,69,77,79 with an average of approximately 7.4 min, according to a recent report of 18 series with more than 2000 children.45
Training and education for the diagnosis of ultrasound pneumoniaSeveral published studies on the use of ultrasound for the diagnosis of pneumonia in the pediatric population include the type of training received by doctors who performed the ultrasound, but only a few analyzed the potential impact of this training and previous experience on the obtained results.
Shah et al.51 offered training with videos and human models before their study and focused on ultrasound identification and tracking the signs of pneumonia, presence of viral suspicion elements, pleural effusion, atelectasis, and/or pneumothorax. These authors did not find any significant differences between those who had experience less than or equal to 25 previous ultrasound exams and those with more experience, although the sensitivity and specificity were slightly higher in the latter group (83% and 88%, vs. 92% and 91%). Jones et al.62 applied the training protocol of Shah et al.,49 also with good results.
Guerra et al.90 achieved good results in their study, in which the previous training of pediatricians consisted of at least 40 PUS explorations prior to the study. Zhan et al.95 showed that residents with minimal experience in ultrasonography and no supervision were able to identify pulmonary consolidations with good specificity, although with less sensitivity. Samson et al.75 analyzed the diagnostic results of PUS in a pediatric emergency room and observed that, with pediatricians trained in ultrasonography who had performed more than 25 previous exams, sensitivity and specificity were 84.3% and 97.2%, respectively, compared to 91.2% and 90.7% in pediatricians with previous experience and a lower number of exams. Corsini et al.43 also observed that, in a previous training with 25 supervised ultrasound exams and six other exams in the case incorporation phase, results similar to those observed by radiography can be obtained in the diagnosis of neonatal respiratory pathology. Pereda et al.,81 in their meta-analysis, also observed good diagnostic accuracy in the hands of non-specialist ultrasonographists.
In short, the training should allow the acquisition of sufficient skills and capacities to capture and interpret ultrasound images of the lung in children, in addition to integrating the results to the clinical decision-making. Evidence shows that the learning curve may require less time than in other ultrasound exams, although it is necessary to continue investigating the minimum requirements for correct accreditation.
It is relevant to highlight the importance that this “operator-dependent” technique can have in low- and middle-income countries. The findings of Chavez et al.54 deserve to be emphasized, who observed that the WHO algorithm for the diagnosis of pneumonia does not facilitate the correct diagnosis of pneumonia in more than one-third of the studied children. By adding PUS to the approach with this algorithm, sensitivity and specificity increase, whereas the time to diagnosis and prescription is reduced, contributing to the reduction in complications, mortality, antibiotic resistance, and treatment costs. The use of PUS can be a cost-effective solution for the diagnosis of CAP in low-income areas.66 The training of general pediatricians in PUS for the diagnosis of pneumonia in children can have a significant impact on low-income countries and in small primary care clinics.81
Final considerationsPUS shows good diagnostic accuracy for consolidation when compared to clinical and radiological findings.80 Thus, it can be considered the fifth element of the clinical examination in pediatrics: inspection, palpation, percussion, auscultation, and insonation.
The incorporation into clinical practice is slow and it is not included in the clinical practice guidelines for CAP in children.68 It could be the first tool to obtain an image58 and be part of the so-called “personalized treatment.”96 Its use in pediatric clinical practice is not limited to the diagnosis and follow-up of CAPs, but also in severe acute patients, helping in the differential diagnosis of respiratory failure (asthma, pneumonia, acute respiratory distress syndrome, pulmonary thromboembolism, acute cardiogenic edema, etc.), follow-up of the treatment response, pulmonary aeration assessment, helping in pulmonary recruitment maneuvers, the ideal PEEP (positive end-expiratory pressure) titration and in the assessment of the chances of successful extubation, as well as many other applications, whether or not associated with functional echocardiography and other point-of-care examinations.
Some of the main characteristics of POCUS application in the diagnosis of pneumonia are highlighted: rapid acquisition and interpretation of images, mobility, easy access to the patient and the option of performing strict monitoring, repeating the examination as often as necessary, without exposing the patient to ionizing radiation.
The need for specific training in this field makes it necessary to incorporate, both in undergraduate and specialization courses, the necessary content for the essential skills to be attained, similarly to others such as auscultation. The availability of portable and ultraportable devices (the latter fit in the doctor's lab coat pocket) favors its learning and use. It is essential to recognize normal patterns at different pediatric ages to detect pathological alterations, which is essential in PUS. The acquisition and interpretation of images, as well as their integration into diagnosis and decision-making should be the objective. Similarly, as observed, the training must be accredited27 and offered, alone or together with other POCUS trainings, to general pediatricians, emergency responders, intensivists, and pulmonologists who treat the pediatric population, adding quality, effectiveness, and assertiveness to the differential diagnosis and treatment.
ConclusionsThe progressively improving results with the use of POCUS for the diagnosis of pulmonary pathology in pediatrics, particularly of CAP, affirm that this tool should become an essential ally in assessment, both for the initial diagnosis and for the monitoring of the pathology. However, it is necessary to continue the studies in the pediatric population, due to the great heterogeneity of the studies published to date.
FundingThe Carolina Foundation, Spain.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Toro MS, Martínez JL, Falcão RV, Prata-Barbosa A, Cunha AJ. Point-of-care ultrasound by the pediatrician in the diagnosis and follow-up of community-acquired pneumonia. J Pediatr (Rio J). 2021;97:13–21.
Study conducted at Universidad de Sevilla, Sevilla, Spain.