To describe the effect of prednisolone on language in children with autism spectrum disorder. This study is based upon two hypotheses: autism etiology may be closely related to neuroinflammation; and, an effective treatment should restore the individual's language skills.
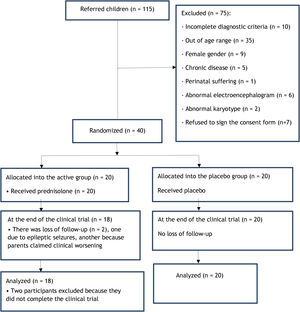
MethodThis is a prospective, double-blinded, randomized, placebo-controlled clinical trial, carried out in a federal university hospital. The initial patient sample consisted of 40 subjects, which were randomized into two parallel groups. Inclusion criteria were: male gender, 3–7 years of age, and meeting the Diagnostic and Statistical Manual of Mental Disorders – 4th edition (DSM-IV) diagnostic criteria. The final sample consisted of 38 patients, of whom 20 were randomized to the placebo group and 18 to the active group. The latter received prednisolone for 24 weeks, at an initial dose of 1mg/kg/day and a tapering dose from the ninth week onward. Language was measured on four occasions over a 12-month period by applying two Brazilian tools: the Language Development Assessment (ADL) and the Child Language Test in Phonology, Vocabulary, Fluency, and Pragmatics (ABFW).
ResultsThe side effects were mild: two patients had hypertension, five had hyperglycemia, and two had varicella. Prednisolone increased the global ADL score in children younger than 5 years of age who had developmental regression (p=0.0057). The ABFW's total of communicative acts also responded favorably in those participants with regression (p=0.054). The ABFW's total of vocal acts showed the most significant results, especially in children younger than 5 years (p=0.004, power=0.913).
ConclusionsThe benefit of prednisolone for language scores was more evident in participants who were younger than five years, with a history of developmental regression, but the trial's low dose may have limited this benefit. The observed side effects do not contraindicate corticosteroid use in autism.
Autistic spectrum disorder (ASD) has, in recent decades, become one of the most relevant neurodevelopmental disorders for pediatric practice and an urgent public health problem.1 The diagnosis depends on behavioral manifestations and is based on the presence of two essential criteria: persistent deficit in communication and social interaction; and restricted, repetitive patterns of behavior, interests, or activities.2 Recent estimates in the United States that ASD affects one in 59 children emphasize the need for scientific studies.3 ASD is known to comprise a heterogeneous group of disorders, whose complex etiology results from the interaction of genetic and environmental-epigenetic factors,4 and an early diagnosis improves prognosis by allowing the timely implementation of appropriate behavioral and educational interventions.1
Despite advances in the diagnosis and rehabilitation of children with ASD, treatment is not yet able to fully reverse the clinical picture.4 Nevertheless, patients are often treated with drugs that aim to alleviate associated behavioral symptoms, such as irritability and aggression, and comorbid disorders such as attention deficit/hyperactivity, nervous tics/Tourette's syndrome, anxiety, and depression.5,6 Studies have also evaluated interventions addressing the central manifestations of ASD with the administration of drugs7–10 and even stem cell use,11 whose preliminary results have not shown unequivocal efficacy.
Corticosteroids have emerged as a therapeutic possibility in ASD from isolated case reports describing significant improvement in language and behavioral symptoms after steroid administration for another purpose.12,13 The argument in favor of corticosteroids lies in the assumption that ASD may have an inflammatory or autoimmune etiology, and such drugs are a well-established therapeutic modality for autoimmune diseases.14 In fact, there is scientific evidence of increased risk of ASD in children with a maternal history of rheumatoid arthritis or celiac disease15 and of neuroimmune alterations in ASD.16,17 The reduction of inflammatory cytokines through immunomodulatory agents has been beneficial in some studies, suggesting the existence of an immunologically-mediated ASD subtype that could respond to specific treatments according to the individual's immune profile.18 Therefore, performing a randomized clinical trial on the efficacy of corticosteroids in the treatment of the central manifestations of ASD is opportune.
The mechanism of action of corticosteroids that allows language function recovery in children with ASD is unclear, but seems to be related to its anti-inflammatory effects and its immunomodulatory properties through phospholipase A inhibition, which is essential for cytokine production.19,20 Such effects would result from an action on gene transcription and translation, and on enzymatic activity modulation, thus leading to the interruption of the inflammatory cascade.19,20
Deficits in language and communication skills are the most frequent manifestations in individuals with ASD and pragmatic skills are particularly affected, whereas structural skills such as semantics and syntax exhibit variable impairment.21 Pragmatic language is essential for social communication, as it includes functions such as greeting and saying goodbye to someone, alternating turns in conversation, sticking to the subject at hand, and using recovery strategies during conversation.3 It can also be defined as the conventions and rules governing the use of language for communication.22
The aim of this study is to describe the effect of prednisolone on the language of children with ASD. Two hypotheses guided this study: that the etiology of autism in part of the affected children is closely related to neuroinflammation; and that effective treatment must necessarily restore the individual's language skills.
MethodsThis prospective study consisted of a randomized, double-blinded, placebo-controlled clinical trial that contained two parallel groups. The participants were recruited from the University Hospital Outpatient Clinic and randomly assigned to receive prednisolone or placebo orally. The research project was approved by the Research Ethics Committee under number 167/07. The clinical trial was registered in the Brazilian Registry of Clinical Trials (http://www.ensaiosclinicos.gov.br/) under the number RBR-7NQ8M7 and received the universal trial number (UTN) U1111-1120-4284. Computerized randomization was based on participants’ ages and used the software BioEstat (Bioestat®, version 5.3, PA, Brazil). The researchers and the health care team were unaware of which participants comprised the two groups, because only the pharmacist who handled the trial medication had the group identification codes. Only after the completion of the fourth language assessment of all participants were randomization codes revealed.
Participant selectionThe inclusion criteria were male gender; age range 3–7 years; and diagnosis of autistic disorder defined by the two neuropediatricians who coordinated the research according to the criteria established in the Diagnostic and Statistical Manual of Mental Disorders – 4th edition (DSM-IV), in effect at the time of the study. Candidates for the clinical trial were excluded if they did not meet the inclusion criteria or if they had any of the following factors: epilepsy or electroencephalogram containing epileptiform discharges; neurodegenerative disease; brain malformation; history of meningitis or encephalitis; expansive lesion of the central nervous system; any other severe chronic disease; or gestational history suggestive of fetal or perinatal distress. Fig. 1 shows the recruitment flowchart. The recruitment period ranged from May of 2010 to September of 2012.
Initial assessmentAfter the parents or guardians signed the informed consent, the participants underwent several complementary tests to promote the homogeneity of the recruited sample and minimize the risks of using an immunosuppressive steroid for several months, namely:
- -
Prolonged electroencephalogram to detect epileptiform discharges or findings suggestive of Landau–Kleffner syndrome or continuous spike and wave during sleep (CSWS) syndrome. For this purpose, the tracing should contain at least 30min of delta-wave sleep.
- -
Magnetic resonance imaging of the brain. All examinations were performed on a 1.5T device (Magnetom Symphony; Siemens – Germany). The patients underwent a standardized protocol, which included anatomical images obtained in T1 sagittal plane, T2 axial and coronal plane, axial plane FLAIR-weighted, and axial plane diffusion;
- -
G-band karyotype;
- -
Lumbar puncture under anesthesia, with routine cerebrospinal fluid (CSF) exams;
- -
Chest X-ray;
- -
Intradermal application of purified protein derivative (PPD);
- -
Blood tests: complete blood count, electrolytes, liver and kidney function, serum calcium, magnesium, phosphate, and alkaline phosphatase levels;
- -
Serum antibody titers against the etiological agents of TORCH and VDRL syndrome;
- -
Urinalysis and urine culture test.
After randomization, the participants in the active group received prednisolone orally for 24 weeks, according to the following regimen: 1mg/kg/day for the first eight weeks. From the ninth to 16th weeks, the regimen was1mg/kg/day every other day. In the final eight weeks, gradual dose reduction was carried out on alternate days, with weekly subtraction of 10% of the dose between the 17th and 20th weeks, and of 15% between the 21st and 24th weeks. The placebo group received a syrup with identical physical characteristics, including color, odor, and consistency, during the 24 weeks of the clinical trial. Before starting the clinical trial, all participants were treated with oral albendazole for five days.
OutcomesThe primary outcome of the clinical trial was the measurement of abovementioned language scores. The secondary outcome was the Childhood Autism Rating Scale (CARS-BR) score.23 The CARS scale was applied twice, before and at the end of the clinical trial, by a neuropediatrician who remained blind to the randomization codes.
Language assessmentTwo Brazilian instruments were used to quantify the participants’ language function: the Child Language Test in Phonology, Vocabulary, Fluency, and Pragmatics (Teste de Linguagem Infantil nas Áreas de Fonologia, Vocabulário, Fluência e Pragmática [ABFW]) and the Language Development Assessment (Avaliação do Desenvolvimento da Linguagem [ADL]). All evaluations were performed by two speech therapists with extensive experience in the assessment and rehabilitation of autistic children and who were not part of the participants’ direct care team; therefore, they remained totally blind to the patients’ clinical information, as well as to the randomization groups. Each participant underwent four language measurements: just before the start of the trial, between the second and third months of the clinical trial, at the end of the 24-week trial, and six months later. Details of the ABFW's application were previously described in another sample of 31 children.24
The ADL test was created based on a North-American instrument, the Preschool Language Scale – 3rd edition, and is applicable to children aged 1–7 years. It generates three scores – the gross scores for receptive, expressive, and global language.25 The scales are applied separately, starting with the receptive scale and, subsequently, the expressive scale. The child's basal level is defined by three consecutive hits and the test ceiling is defined by five consecutive errors or absence of response.
The ABFW instrument is applicable to children from 2 to 12 years old. In this study, the authors used the sub-item that analyzes pragmatic language. The test defines three communicative means – verbal, vocal, and gestural – for each of 20 communicative functions, which were subdivided by the instrument's author herself into 13 more interpersonal actions, e.g., “action request,” “recognition of the other,” and seven less interpersonal actions, e.g. “protest,” “exploratory function.”26 Each assessment yielded four main scores – total communicative acts, and verbal, vocal, and gestural means – and scores for the 20 functions, as well as the proportions of more interpersonal and less interpersonal functions.27 The application of the instrument consisted in videotaping for at least 30min the child's interactions in a dyad with an adult in a structured context, where the child and the adult sit at a table and interact with a series of toys placed in front of them. The best 15min were used for language function analysis and communicative acts score.
Assistance during the clinical trialThe participants had outpatient appointments every 15–21 days and monthly laboratory tests to monitor for complications of continued prednisolone use. The medication vials of the two groups were indistinguishable. The participants received medication at the consultations, and the label on each vial contained only the child's name. The dose was adjusted according to the child's body weight.
Statistical analysisTwo software programs were used: Epi-Info (Epi-Info, version 7.1.3.0, Division of Health Informatics & Surveillance (DHIS), Center for Surveillance, Epidemiology & Laboratory Services (CSELS), USA) and SPSS Statistics (PASW Statistics for Windows, Version 18.0. Chicago, USA). To compare numerical data between the active and placebo groups, this study used analysis of variance (ANOVA) or, when the data did not show normal distribution according to the Kolmogorov–Smirnov test, the nonparametric Mann–Whitney test was used. To evaluate the interaction of the independent variables, the two-way ANOVA test and a multivariate linear regression model were used.
The analysis of the ADL and ABFW instrument scores on four occasions required a generalized linear mixed model with repeated measures, which allowed quantifying with greater accuracy the influence of the active drug, the age group, and the presence or absence of developmental regression on the primary outcome measurements. The level of statistical significance was defined as p<0.05. To evaluate the effect size of the results, the partial eta squared parameter was used, provided by SPSS. The effect size was classified into three categories: <0.13=small; from 0.13 to 0.25=average; ≥0.26=large.28 For the purposes of this study, an observed statistical power value ≥0.6 was considered relevant.
ResultsThe study sample consisted of 40 participants: 12 were diagnosed at recruitment and 28 had received a previous diagnosis, which was confirmed by the researchers. The randomization regarding the time of diagnosis was reasonably homogeneous, as of the 12 newly diagnosed individuals, seven were allocated to the active group and five to the placebo group; 13 formerly diagnosed children were allocated to the placebo group and 15 to the placebo group. The median age±standard deviation of the prednisolone group was 58.0±12.3 months, while that of the placebo group was 55.0±13.7 months (p=0.914). All children were males and received a diagnosis of classic autism, according to the DSM-IV criteria. Twenty children had a clinical history compatible with the presence of developmental regression. Two participants, both from the active group, left the study at their parents’ sole discretion, respectively after four and 12 weeks of the start of the clinical trial, so the final sample consisted of 38 participants. The parents of one participant concluded that the test medication was harming their child, while the parents of the second participant decided to drop him out of the study after the child had two epileptic seizures since the start of the trial.
Of the 38 participants, 25 were under 5 years of age, of whom 13 received prednisolone and 12, placebo. The 13 participants older than 5 years were randomized, so that five received prednisolone and eight, placebo. Of the 20 participants who had developmental regression, 14 were younger than 5 years old, seven received prednisolone and seven, placebo, while six were older than 5 years – five received placebo and only one received prednisolone.
Forty-seven children underwent a prolonged electroencephalogram, but none were diagnosed with Landau–Kleffner syndrome or CSWS. However, six had frank epileptiform discharges, which led to exclusion from the study, and one was excluded after the examination due to severe obesity.
The 40 participants in the initial sample underwent brain magnetic resonance imaging; three were diagnosed with an arachnoid cyst and 24 exhibited Virchow-Robin perivascular space ectasia.
The G-band karyotype was normal in all participants. The remaining initial examinations were considered normal.
The PTONI nonverbal intelligence test was applied by a psychologist to the 38 participants of the final sample. Only eight children from the active group and six children from the placebo group were able to complete the test. The median score of the active group was 61, with a standard deviation of 31.6 and range of 46–143, while the placebo group showed a median of 56, standard deviation of 16.3, and range of 46–86, so there was no statistically significant difference between the groups (p=0.437).
Side effectsThree participants showed typical symptoms of CSF hypotension two to three days after lumbar puncture, which resolved with rest and hydration. Two participants contracted chickenpox of inconsequential intensity, which responded to oral acyclovir use. One participant had impetigo, which responded to antibiotic therapy. Two children treated with prednisolone experienced a significant increase in blood pressure (systolic or diastolic pressure>95th percentile), whereas five had hyperglycemia in the first two months of prednisolone treatment, but these side effects resolved without treatment.
The median weight gain over the 24 weeks of the study was 3.6kg in the active group vs. 1.9kg in the placebo group (p=0.043), but as there was a significant difference in median body weight between the two groups at baseline (placebo group=25.1kg vs. active group=21.35kg), the weight gain promoted by continuous use of prednisolone was not sufficient to reveal the allocation of each participant in either group.
Mild-to-moderate irritability was present at certain times during the clinical trial in several participants who received prednisolone, but in only one of them was it significant to the point of aggressiveness, which resolved over time.
Rehabilitation and schoolingAll participants received multidisciplinary rehabilitation in public institutions, except two children in the active group and two children in the placebo group. All participants attended school, except for two children in the active group and three in the placebo group. Therefore, there was no significant difference between the active and placebo groups.
Analysis of the primary outcomeThe supplementary material (Tables 1 and 2) show the level of statistical significance, effect size, and observed power of the variables ‘prednisolone use,’ ‘age range,’ and ‘developmental regression,’ as well as their interactions on the language scores.
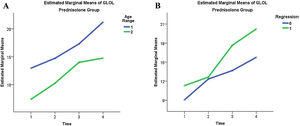
The global language score of the ADL scale was close to statistical significance (p=0.057), with an observed power of 0.615 and an effect size between medium and large when the interactions of the abovementioned independent variables were evaluated. In the active group, those with regression had a mean score of 15.466 vs. 12.689 of the participants without regression, while in the placebo group the former had a mean score of 8.071 and the latter, of 8.967. Participants under the age of 5 years in the active group attained an average score of 16.551 when compared with 11.594 for those older than 5 (Fig. 2), but the same scores in the placebo group were respectively 6.042 and 10.996.
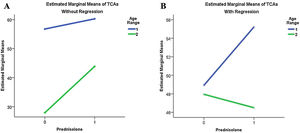
The total number of ABFW communicative acts nearly reached statistical significance (p=0.054), with an observed power of 0.623 and effect size of 0.243. Fig. 3 shows that in participants under 5with developmental regression, prednisolone use provided a greater benefit in the language score.
Comparison of the total communicative acts (TCAs) estimated marginal means of the Child Language Test in Phonology, Vocabulary, Fluency, and Pragmatics (ABFW) between the active (‘Prednisolone’=1) and placebo (‘Prednisolone’=0) groups, stratified by age group (‘1’ <5 years; ‘2’ ≥5 years) and absence (A) or presence (B) of developmental regression.
The supplementary material (Table 2) shows the scores for some of ABFW's 20 communicative functions, as well as more interpersonal and less interpersonal functions. Some functions, such as “naming” and “shared play,” showed favorable results for prednisolone.
The total vocal acts of the ABFW was the score that showed the most expressive results (supplementary material). The interaction between prednisolone and age reached a p-value of 0.004, with a power of 0.913 and an effect size of 0.378.
DiscussionThis is the first prospective study in the medical literature based on a double-blinded, randomized, placebo-controlled clinical trial on the treatment of ASD with corticosteroids. Aside from isolated case reports, only one study by Duffy et al. compared 20 autistic children treated with prednisolone and 24 untreated children.14 Those authors employed a daily dose of 2mg/kg for at least four months and described behavioral and language improvement, but the study was retrospective, which certainly compromises the external validity of their data.
The side effects observed throughout the clinical trial did not affect the study conclusion and were manageable without any major difficulties. A daily dose of 1mg/kg/day and daily use for only eight weeks contributed to the favorable side effect profile.
The scores of the two language measuring instruments obtained during four assessments over the 48-week period, including the 24 weeks of the clinical trial and the subsequent six months, progressively increased in both groups, a likely effect of natural developmental evolution and the rehabilitation programs that almost all participants attended.
However, when data are analyzed between the active and placebo groups, statistically significant differences in score evolution appear, with greater increases in the prednisolone-treated group. Such differences are more pronounced in favor of the active group in the age range below 5 years and in the presence of developmental regression, as shown in Figs. 2 and 3.
In the present sample, 20 of 38 children (52.6%) were identified with developmental regression, a higher proportion than the traditionally cited rate of 30%.29 One possible explanation is that the selected sample included individuals with more intense clinical pictures. However, Ozonoff and Iosif argue that the regression of developmental milestones is far more common in children with ASD than previously believed, because in prospective studies with close observation of participants from birth, the detection of loss of social and linguistic developmental milestones occurred in the vast majority.30
The authors believe that the results attained in this study warrant further clinical trials with larger numbers of participants and broadening of the randomization criteria to include the presence of developmental regression.
The pioneering spirit of the present study does not diminish its limitations. The prednisolone dose of 1mg/kg/day used during the first eight weeks of the trial is low, considering the current literature. It is assumed that a higher dose would achieve more favorable intervention outcomes. The number of sample participants (n=38) was relatively small, which compromised the power of statistical analysis and made it difficult to compare subgroups stratified by age and developmental regression. Additionally, randomization should have considered the presence or absence of developmental regression in addition to age, which would have allowed an equitable distribution of subgroups for comparison.
FundingThe clinical trial was partially funded by a CNPq research grant, through contract number 575332/2008-5, which covered approximately 10% of expenses.
Conflicts of interestThe authors declare no conflicts of interest.
The authors would like to thank pharmacist Nilo Jorge Piccoli for performing reliable and confidential handling of the trial drugs, radiologist Dr. Teresa Cristina de Castro Ramos Sarmet dos Santos for performing and interpreting brain magnetic resonance imaging (MRI) scans with her usual competence, and neurologist Dr. Fabio Emilio Brandão for the impeccable performance and interpretation of prolonged electroencephalograms. They also would like to thank all resident physicians who participated daily in assisting the clinical trial participants.
Please cite this article as: Brito AR, Vairo GP, Dias AP, Olej B, Nascimento OJ, Vasconcelos MM. Effect of prednisolone on language function in children with autistic spectrum disorder: a randomized clinical trial. J Pediatr (Rio J). 2021;97:22–9.
This clinical trial was registered in the Brazilian Registry of Clinical Trials (http://www.ensaiosclinicos.gov.br/) under number RBR-7NQ8M7 and received the following universal trial number (UTN): U1111-1120-4284.
Study conducted at Universidade Federal Fluminense (UFF), Hospital Universitário Antônio Pedro (HUAP), Niterói, RJ, Brazil.