The association between chronic liver disease (CLD) and malnutrition, both in adults and pediatric patients, has been known for a long time.1,2 Multiple studies have also clearly demonstrated that an impaired nutritional status can be detrimental on the outcome of these patients, increasing their vulnerability to complications and reducing their life expectancy.3•6
In the last decades, it has been suggested that muscle wasting should be considered the main component of malnutrition in CLD.7 The reduction in muscle mass is nowadays better defined as sarcopenia. Sarcopenia was initially described as a physiologic process during aging, but chronic diseases may considerably anticipate this event (secondary sarcopenia). The definition of sarcopenia includes both a decrease in muscle mass and reduced muscle function8; however, up to now, a large number of studies have mainly focused on the measurement of muscle mass, disregarding the evaluation of muscle function. This could depend on the idea that measurement of muscle mass could be more objective and easier to perform than functional evaluations, the latter also being more time consuming. In adult CLD patients, total muscle area at L3 in a computed tomography (CT) or magnetic resonance imaging (MRI) is considered the gold standard for the diagnosis of sarcopenia9 and is utilized in the majority of the studies. CT and MRI are usually available in these patients for other clinical indication (before liver transplantation or to study hepatic focal lesions). The importance of focusing on muscle function to confirm sarcopenia and the need to provide clear cut-off points (for age, gender, and ethnicity) to identify patients with muscle impairment have been recently recommended by the revised European consensus on the definition and diagnosis of sarcopenia.8
Different mechanism may participate in causing sarcopenia in CLD.10 Calorie and protein intake are usually lower than those required due to low appetite, dyspepsia, and erroneous medical prescriptions, malabsorption may be present due to portal hypertension, and energy metabolism may be moderately increased during complications. Furthermore, because liver glycogen stores are depleted due to liver fibrosis, energy metabolism in these patients shows a rapid transition to a “fasting pattern‿ inducing protein catabolism to supply gluconeogenesis. Protein synthesis is also impaired in the muscle of patients with CLD for multiple reasons, such as low testosterone levels, mainly in males, reduced amino acid availability, and chronic hyperammonemia causing increased myostatin levels. For these reasons, patients with advanced liver disease undergo a progressive reduction in muscle mass, which is clearly evident either at anthropometry (arm muscle circumference) or at imaging analysis (CT or MRI). In adult patients with advanced liver disease, sarcopenia is associated with complications such as encephalopathy, infections, or refractory ascites, is a predictor of mortality in the waiting list for liver transplantation, and increases hospital length of stay and hospital costs post-transplant.9,11
The interest in sarcopenia in children with CLD has only recently been recognized in the pediatric literature12 and few studies have evaluated the impact of sarcopenia on clinical outcomes in this population. With regard to the presence of reduced muscle mass, this has been measured either by dual-energy X-ray absorptiometry (DEXA), CT, MRI, or bioelectric impedance analysis (BIA). All these methods require cooperation to stay motionless during measurements, which makes these techniques challenging in infants and young children. Furthermore, radiation exposure restricts the use of CT, if not included in clinical staging, and MRI, which is radiation free, is even more expensive. DEXA may represent a useful alternative; however, there is controversy regarding whether all-body skeletal muscle mass and appendicular lean mass perform equally well as markers of sarcopenia. Finally, age- and gender-matched normative data are not always available in healthy children, and the absence of uniform diagnostic criteria has become the main barrier in stating the presence of sarcopenia in children.
The peculiarity of assessing body composition in children is that they are growing individuals. This causes variability between males and females, and according to the child's age. A crucial event is the pubertal growth, which may also be delayed by malnutrition or the underlying disease. During puberty, females are known to gain more fat mass, while in males the increase in fat-free mass is predominant. This may obviously hamper the correct evaluation of sarcopenia.
As mentioned earlier, the assessment of muscle function is crucial for the assessment of sarcopenia since muscle strength is not linearly related to muscle mass. However, this measurement is also frequently missing in studies assessing sarcopenia in children.
Appropriate muscle function tests need to consider the development of coordination, or stability and the competence in purposeful movements in small infants. Motor function assessment scales, taking into consideration muscle strength for the evaluation of sarcopenia, have not been developed for early childhood, and a standardized assessment of muscle function for the diagnosis of sarcopenia is currently lacking in young pediatric patients. In older children or adolescents strength test utilized in adults can also be adopted (hand-grip [HG] test or six-minute walk test); however, normative values in children are not always available and measurements are therefore difficult to evaluate in an objective way.
In this issue of the Jornal of Pediatria, Rendeze et al.13 report descriptive data from children who were pediatric patients with CLD followed at the clinical treatment outpatient clinics, and transplanted patients from the Department of Gastroenterology and Pediatric Hepatology at the Universidade Federal de Bahia (Brazil). There were 85 patients included (64.7% females, mean age 11.7+3.4 years, 50% in the pubertal stage) affected by various liver diseases, with biliary atresia and autoimmune hepatitis representing the most frequent etiologies. There were 28 children with a diagnosis of cirrhosis, which was Child-Pugh score A in 82.1%. Sarcopenia was diagnosed based on the simultaneous presence of muscle mass and muscle strength insufficiency. Muscle mass was analyzed by DEXA and muscle strength by HG test. The authors fixed the cutoff for these parameters at the median value obtained in the study population. The diagnosis of sarcopenia was made in 40% of patients. Muscle mass was influenced by gender (higher in males) and age group (<10 vs. >10 or <14 vs. >14 years), but not by BMI or other parameters including body weight.
This study valuably participates in exploring the paradigm of sarcopenia in children with CLD.
CLD is a relevant condition in pediatric patients, which may occur even in the first year of life for some genetic diseases, such as biliary atresia or Alagille syndrome. These patients may develop liver insufficiency and need early liver transplantation, and both malnutrition and sarcopenia may compromise their clinical outcome, as is the case in adult patients. In spite of this, few studies have been dedicated to this issue.
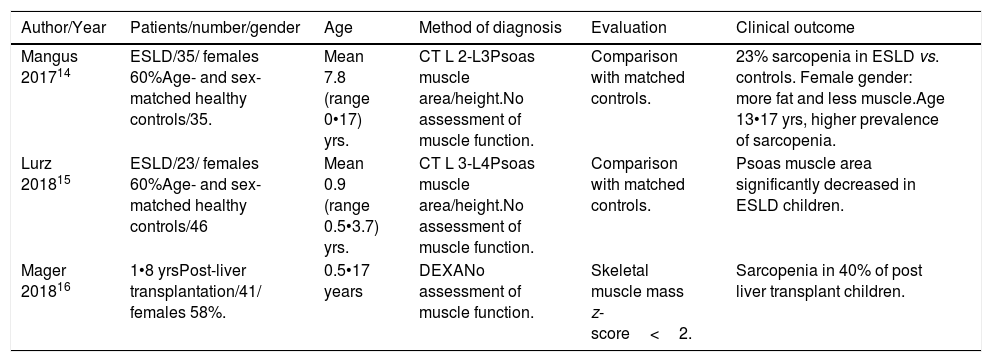
Only three retrospective studies have been published on sarcopenia in children, two in patients with CLD and one in patients after liver transplantation14•16 (Table 1). All these studies underlined that basic nutritional parameters, such as BMI or anthropometry, could underestimate the presence of sarcopenia in children. Mager et al.16 reported that the presence of sarcopenia in liver-transplanted children was associated with some relevant clinical outcomes (growth retardation, length of hospitalization, and rate of re-admission).
Studies on sarcopenia in pediatric patients with chronic liver disease.
| Author/Year | Patients/number/gender | Age | Method of diagnosis | Evaluation | Clinical outcome |
|---|---|---|---|---|---|
| Mangus 201714 | ESLD/35/ females 60%Age- and sex-matched healthy controls/35. | Mean 7.8 (range 0•17) yrs. | CT L 2-L3Psoas muscle area/height.No assessment of muscle function. | Comparison with matched controls. | 23% sarcopenia in ESLD vs. controls. Female gender: more fat and less muscle.Age 13•17 yrs, higher prevalence of sarcopenia. |
| Lurz 201815 | ESLD/23/ females 60%Age- and sex-matched healthy controls/46 | Mean 0.9 (range 0.5•3.7) yrs. | CT L 3-L4Psoas muscle area/height.No assessment of muscle function. | Comparison with matched controls. | Psoas muscle area significantly decreased in ESLD children. |
| Mager 201816 | 1•8 yrsPost-liver transplantation/41/ females 58%. | 0.5•17 years | DEXANo assessment of muscle function. | Skeletal muscle mass z-score<2. | Sarcopenia in 40% of post liver transplant children. |
ESLD, end-stage liver disease., CT, computed tomography, DEXA, dual-energy X-Ray absorptiometry.
The study by Rendeze et al.13 is the first examining both muscle mass and muscle function in children with CLD, as recommended by the recent EWGSO consensus.8 It is worth noting that although the patients enrolled were less severe (cirrhosis less than 30% compensated • the large majority Child-Pugh class A, followed as outpatients), the authors reported a prevalence of sarcopenia as high as 40%. These results demonstrate the difficulties in comparing different series when methods of assessment and diagnosis are not standardized. Indeed, the study by Rendeze et al.13 utilized an internal comparison for the diagnosis of sarcopenia so that the patients were considered sarcopenic when they were above the median values for appendicular skeletal muscle (ASM) in the enrolled population. The result is that different series may have different cutoffs, which hampers generalization of the results. It is important to emphasize that sarcopenia needs to be evaluated in children with CLD and that future research is needed to improve methods and diagnostic criteria considering the possible confounding factors. The good news is that this process has started.
Conflicts of interestThe author declares no conflicts of interest.