Kidney shortage for pediatric kidney transplantation (PKT) entails the need to use low-weight and age donors, despite the apprehension. The aim of this study was to analyze the pediatric deceased donor kidney transplantations (pDDKT) outcomes in the first year after the procedure, stratified by donor age.
MethodRetrospective cohort of pDDKTs carried out between January 2013, and January 2018, at a PKT reference hospital in Southern Brazil. Donors were divided into group 1 (≤ 6 years), and group 2 (> 6 years); the analysis of the outcomes was carried out in the same period.
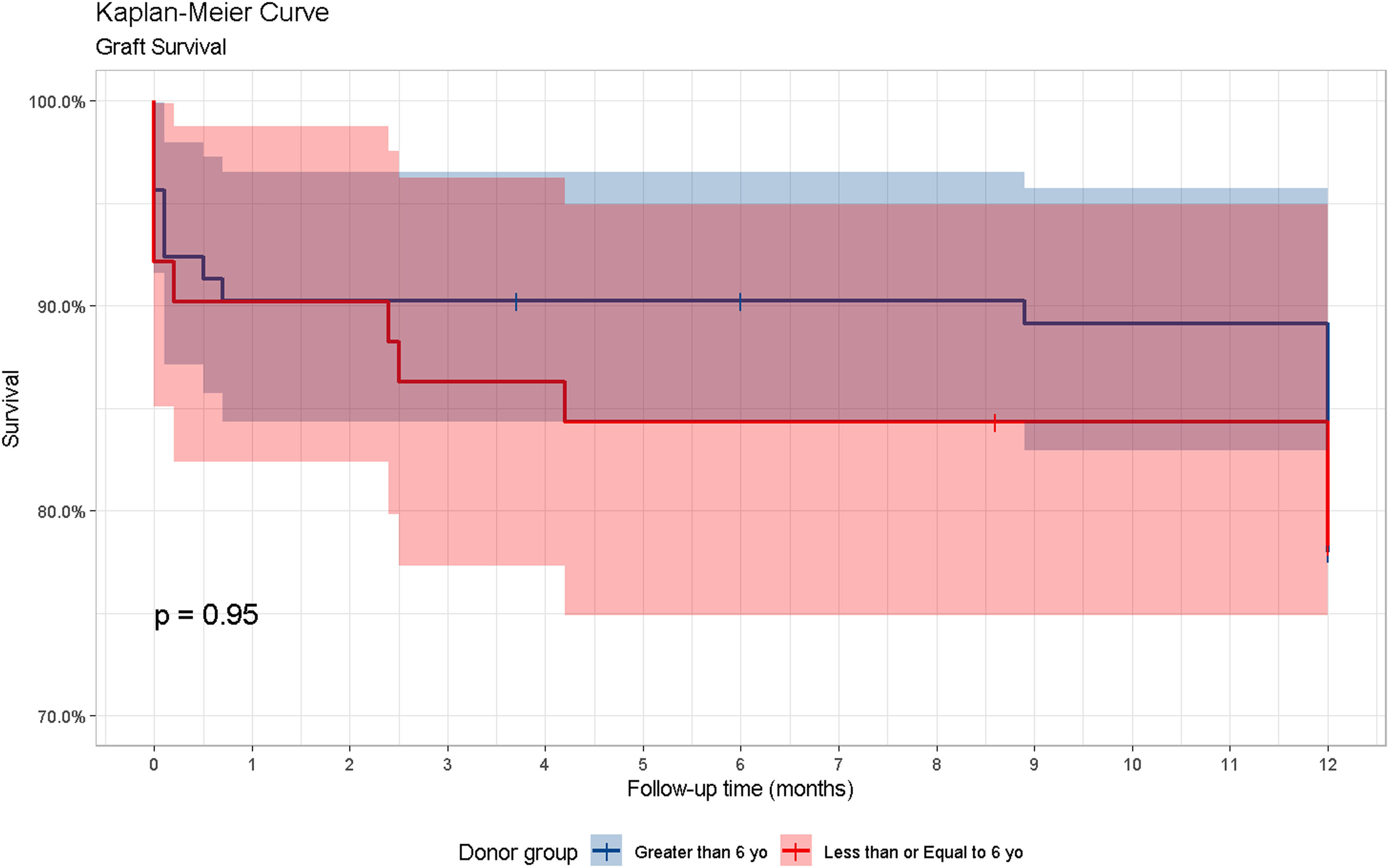
ResultsThere were 143 pDDKTs; 51 (35.66%) in group 1; and 92 (64.34%) in group 2. In both groups there were 17 graft losses (11.8%), with vascular thrombosis as the main cause (group 1: 5; group 2: 4). Among the complications, renal artery stenosis (RAS) with indication for angioplasty and stenting was more frequent in group 1 (7.8%; group 2: 2.2%). The 1-year Renal Transplant Recipients' and graft survival did not show significant differences between the groups, (p = = 0.95). However, the Glomerular Filtration Rate analysis was higher in group 2, reaching, in the 12th month, 79.3 mL/min/1,73m2, compared to 69.7 mL/min/1,73m2 in group 1(p = = 0.033).
ConclusionsSmall donors can be considered for pDDKTs, as long as there is an expert team to perform the transplantation.
The best treatment for end-stage Chronic Kidney Disease (CKD) is PKT since It grants longer patient survival and better quality of life.1 Preferably, PKT should be performed while the dialysis treatment is initial, or even before starting, considering preemptive transplantation as the gold standard in pediatrics, since the long-term effects of dialysis in this population can cause delayed growth and development, and impaired outcomes after kidney transplantation (KT).2-4
In Brazil, the allocation of kidneys for pDDKT occurs according to Ordinance 2.600/2009, by the Ministry of Health (MS), which considers that the kidneys from donors aged < 18 years should be allocated preferentially to children in the same age group, prioritizing children and adolescents on the waiting list, favoring preemptive PKT for those with Glomerular Filtration Rate (GFR) ≤ 15 mL/min/1.73 m2, even if they are not on renal replacement therapy (RRT).2 Preemptive transplantation represents longer graft survival (SV) and better clinical outcomes, but not necessarily better GFR when compared to non-preemptive PKT. The analysis of GFR after KT is shown to be a good parameter to assess graft SV.5
Between the years 2013 and 2021, Brazil performed 3055 PKTs. In Rio Grande do Sul (RS) there were 388 (12.70%), of which 345 (11.29%) occurred in the hospital where the study was conducted.6 PKT in young children is still a challenge due to the complexities of the surgical technique and the underlying pathologies in kidney transplant recipients (RTRs). The lack of uniformity among transplant centers regarding the minimum weight and age for pediatric kidney donors (PKD), which may vary between 6 and 24 months, and between 5 and 20 kg, is also an obstacle.7-9
The use of kidneys from small donors (≤ 6 years) in PKT, however, is controversial, as previous studies have suggested an increased risk of surgical complications and graft loss, highlighting thrombosis as a major cause of graft failure.1,10-12 The overall objective of this study was to learn about the outcomes of pDDKTs performed with donors who died before their 18th birthday, throughout the first year of KT. The specific objectives were to compare patient and graft SV, GFR, and vascular complications between the pDDKTs performed with kidneys from donors under and over 6 years old.
MethodologyThis study evaluated data from a retrospective cohort, through inductive reasoning, using a quantitative analysis of pDDKTs, carried out at Hospital da Criança Santo Antônio, which belongs to the hospital complex of Santa Casa de Misericórdia de Porto Alegre, a PKT reference hospital in Southern Brazil.
Data collectionData collection began after approving the research project and prescinding free and informed consent was issued by the Ethics and Research Committee, register number 3,490,679. All pDDKTs performed with donors under 18 years old who died between January 1st, 2013, and January 1st, 2018 were included. All patients were seen by the same clinical and surgical team, and a similar protocol was applied to all during the observation period. The extraperitoneal approach was used for all surgical techniques, as already described in a previous study developed in the center. Even using small pediatric donors, the kidneys were transplanted as single grafts, and not en bloc.10 PKTs with kidneys from living donors were excluded from the study.
Induction therapy was preferably performed with basiliximabe. For hypersensitized cases, with panel-reactive antibody (PRA) > 50% or ischemia time > 24h, thymoglobulin was used. The mainstay of post-transplant immunosuppression consisted of triple therapy with prednisone, tacrolimus (converted to cyclosporine, in the presence of adverse effects or difficulty to reach adequate serum level), and mycophenolate sodium (converted to azathioprine, in case of adverse effect). Sirolimus was used if there should be refractory viral infections or neoplasms.
Regarding anticoagulation, the authors followed the protocol established at the hospital where the study was conducted, which evaluates the use of prophylactic heparin 10 IU/kg/h in the immediate postoperative period, if necessary, considering donors aged < 5 years, and recipients aged < 2 years, with previous nephrotic syndrome/focal segmental glomerulosclerosis (FSGS); and recipients with thrombophilia, previous history of vascular thrombosis (VT), and surgical peculiarities.13
Account the risks related to the large donor/small recipient situation, in the intraoperative period, the authors aimed for a mean arterial pressure (MAP) > 80mmHg before opening the clamps. In the postoperative period, an adequate and individualized MAP was maintained, with volume replacement and, volume expansion.11,13
Doppler ultrasound is a protocol after the PKT to evaluate the anatomical integrity of the graft and its vessels. Is routinely performed in the immediate postoperative period in the intensive care unit, daily for the first 3 days, monthly until the third month after PKT, and whenever necessary in case of systemic arterial hypertension (SAH) and/or increase in serum creatinine.
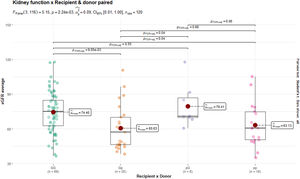
The RTRs were stratified into Group 1, which received the graft from donors ≤ 6 years old; and group 2, with donors > 6 years old. The cases that required retransplantation within the study period were kept for analysis. For the definition of small donor and recipient (s), the authors considered the infants aged 1 to 6 years; the infants aged 7 to <18 years old were considered large donors (L). The set of groups related to recipient x donor size was distributed as follows: LL (large recipient x large donor); Ls (large recipient x small donor); sL (small recipient x large donor) and ss (small recipient x small donor). To obtain the GFR, the authors used the Schwartz equation.14 The authors excluded from the GFR analysis the RTRs who deceased or presented with congenital orthopedic malformation (difficulty to measure height correctly); cases of graft loss and loss of follow-up (transferred to the region of origin or to another center, and the patients who missed appointments for more than six months) were also excluded.
Statistical analysisThe outcomes assessed were: (a) patient and graft SV in the first year, (b) GFR in 1st, 3rd, 6th and 12th months, and (c) causes of graft loss (underlying disease, VT, septic shock, death, and never functioning graft) and vascular complications.
The qualitative variables were represented as absolute and relative frequency, while the quantitative were represented by mean, standard deviation, median, and interquartile range. Normality was verified by the Kolgomorov-Smirnov (K-S) test. The association with the donor's age group was verified by the Chi-square and Fisher's exact tests. For the quantitative variables, the t-Student and, in the absence of normality, the Mann-Whitney tests were applied. The GFR was analyzed in the first year of PKT, account the donor's and recipient's size, regarding the understanding that, after this period, there could be a loss of follow-up or poor adherence to treatment. To assess the effect of the group * time interaction of the 4 GFR measurements, a mixed design ANOVA was applied. Subsequently, 4 groups were created from the combination of donor and recipient ages and average GFR, which was compared using ANOVA with Tukey's post-hoc. The RTRs and the graft SV analysis were performed by the Kaplan-Meier method. Results with p-value < 0.05 were considered significant. The analyses were performed with SPSS software, version 25.15
ResultsDuring the study, 177 pDDKTs were performed. After applying the exclusion criteria, 34 were deleted (23 were 18 years old or over, and 11 had received the kidney from a living donor). Thus, 143 pDDKTs were left, of which 10 were retransplantations. There were 2 deaths and 17 graft losses.
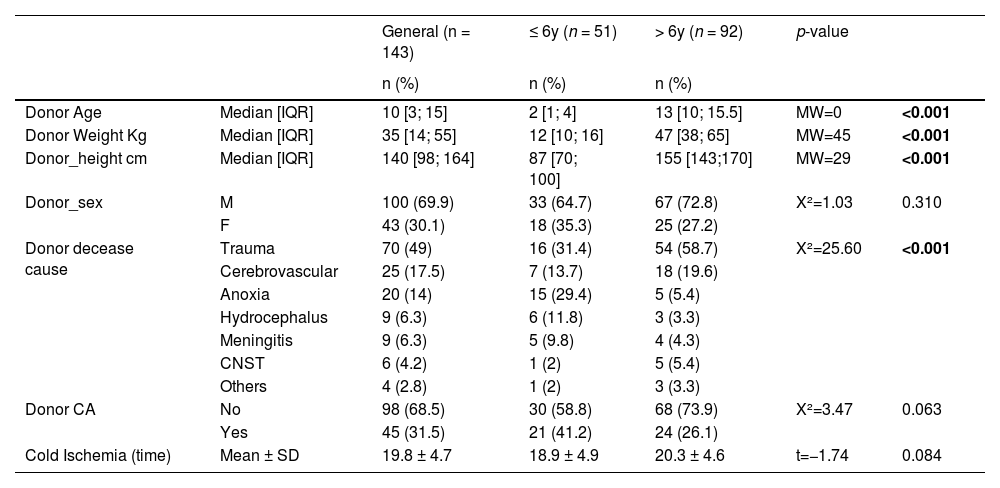
DonorsThe mean age of PKDs was 2 years in group 1 (min:1 and max:4), and 12 years in group 2 (min:7 and max:17), while the mean weight in groups 1 and 2 was 13kg (min:8 and max:30) and 49kg (min:13 and max:81), respectively. The most prevalent cause of death in both groups was traumatic brain injury (TBI) (group 1: 31.4%; in group 2: 58.7%). There was a significant association between cause of death and group; anoxia and hydrocephalus were associated with group 1, while TBI was associated with group 2 (Table 1). There was no significant difference between the occurrence of cardiac arrest (CA) and ischemia time between the groups.
Demographic and clinical characteristics of PRDs, divided into group 1(donor ≤ 6 years) and group 2 (donor > 6 years).
IQR, interquartile range; SD, standard deviation; CNST, central nervous system tumor; CA, cardiopulmonary arrest.
The mean age of RTRs was 8 years in group 1 (Min:1.5 and Max:17), and 11 years in group 2 (Min:2 and Max:17); the mean weight was 22 kg (Min:7.5 and Max:59) and 34 kg (Min:9.6 and Max:82), respectively. Automated peritoneal dialysis (APD) was the predominant pre-pDDKTs treatment in group 1 (37%); and hemodialysis, in group 2 (31%). Among the preemptive pDDKTs, 29% belonged to Group 1, and 30%, to Group 2 (Table 2).
Demographic and clinical characteristics of the RTRs, divided into group 1 (donor ≤ 6 years) and group 2 (donor > 6 years).
HD, Hemodialysis; CAPD, continuous ambulatory peritoneal dialysis IQR, interquartile range; SD, standard deviation; GFR, Glomerular Filtration Rate; RLT, Retransplantation. *excluded from the analysis 2 deceases, 17 graft losses, 3 wheelchair users, and 1 loss of follow-up.
The patient SV in group 1, during the first year, in the 1st, 3rd, 6th, and 12th months were 100%, 100%, 100%, and 95.2%. In group 2, the results were 97.7%, 97.7%, 97.7%, and 96.5%, respectively (p = 0.8) (Figure 1). Group 1 presented a graft SV of 90.2%, 86.3%, 84.3%, and 78.3%, while the SV in group 2 was 90.2%, 90.2%, 90.2%, and 78%, in the same period (p = 0.95). Considering the 143 pDDKTs, there were 17 (11.8%) graft losses, 5 (9.80%) cases in group 1, and 7 (7.60%) in group 2; 12 cases occurred in the first month after transplantation. The most frequent cause of graft loss in both groups was VT, with 5 (9.80%) cases in group 1, and 4 (4.34%) in group 2 (p = 0.868). Among the graft losses caused by VT, when the dialysis modality of the RTRs was analyzed, 5 cases occurred in Group 1, of which 3 performed CAPD, 1 HD+CAPD, and 1 was preemptive. Concerning the occurrences in Group 2, 3 performed HD, and 1, CAPD. There was no significant difference between donor size and the dialysis modality with which recipients were treated.
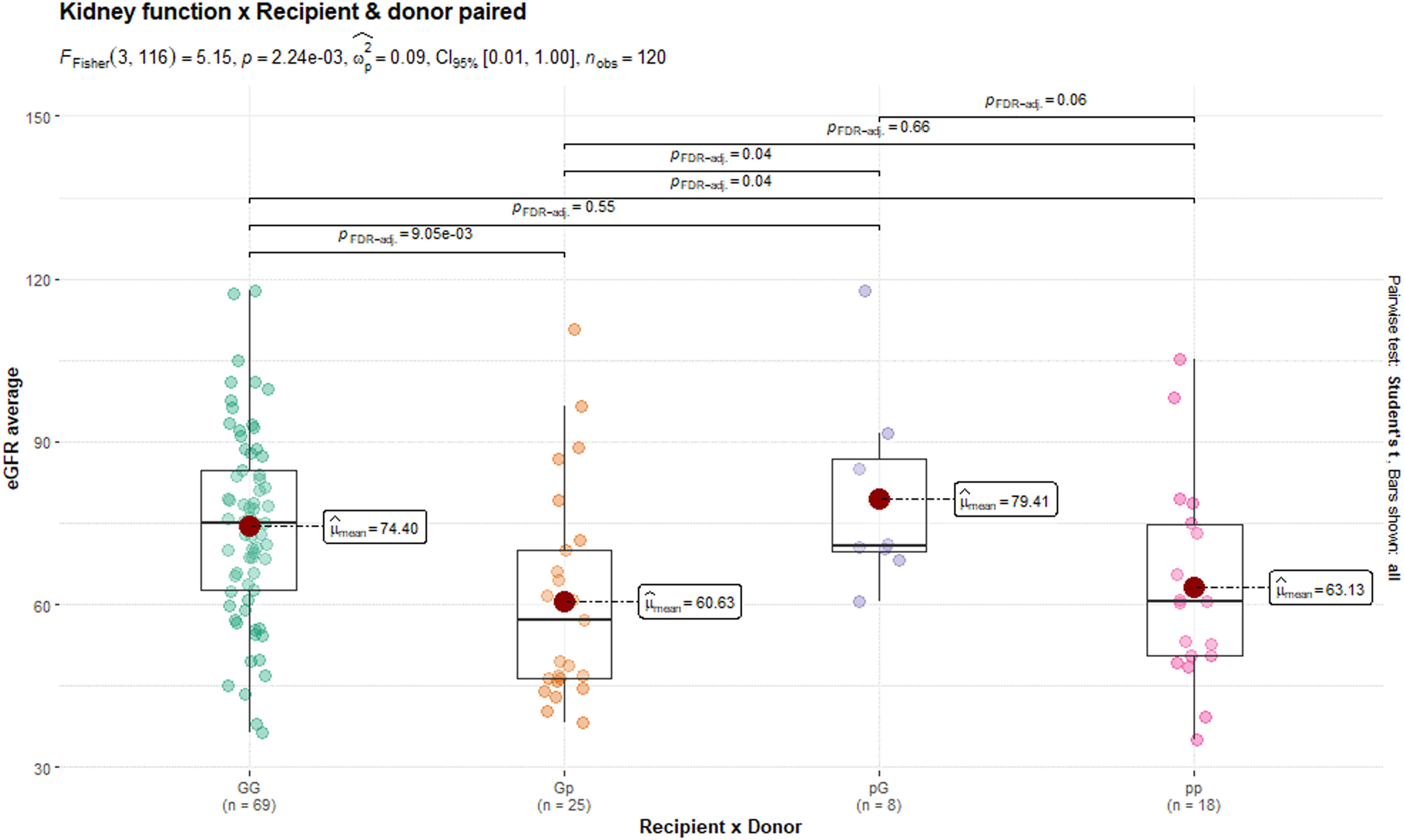
Prophylactic anticoagulation with heparin was used in 100% of the cases in which thrombosis and graft loss occurred in group 1, and in 77.8% of the cases in group 2. During the period under review, 17 (11.8%) graft losses were followed up; 10 (6.9%) patients, 1 (1.96%) from group 1, and 9 (9.78%) from group 2, rejoined the list and underwent a new pDDKT within the same period. There was a significant difference in the analysis of the GFR in the 1st, 3rd, 6th and 12th month after the pDDKT, and it increased in both groups over the months, however, with a better rate in the group with large donors (group 1: 53.1 ml/min/1.73m2; 59.5; 64.3; 69.7; and group 2: 69.5 ml/min/1.73m2; 73.5; 77.4 and 79.3) (Table 2). The GFR mean in the first year of pDDKT, concerning recipient size x donor size, showed a better rate in transplants performed with kidneys from large donors (sL and LL), regardless of recipient size (p = 0.002) (Table 2).
According to Tukey's test, the LL group had a higher GFR mean than the Ls group. However, the GFR was higher than 60 ml/min/1.73m2 in both groups at 12 months of TR (Figure 2). There was no significant difference in either group related to RAS; 4 (7.84%) cases occurred in group 1, and 2 (2.17%) in group 2. There were 2 deaths in group 2. One of them was due to septic shock, which happened 3 days after pDDKT, with Nephrotic Syndrome as the underlying disease. The other death was due to massive cavernous sinus thrombosis with diffuse cerebral edema, 4 days after KT, in a patient diagnosed with FSGS (Table 2).
DiscussionIn view of the donor shortage and highlighting that, in Brazil, the allocation of kidneys from pediatric donors is directed to patients under 18 years old, there are challenges in the use of small donors, considering their restrictions or optimization. As limitations of the study, the authors point out the intrinsic restrictions of the retrospective design, the small sample size, the short post-transplant follow-up time, and the lack of investigation regarding the transoperative period information.
In the present study, more than a third of the donors were small (35.66%), similar to another study that analyz ed donor age and risk of complications in pDDKTs, which had 41.95% of donors aged ≤ 6.1 The present study emphasizes the importance of using small donors in pDDKT, with good GFR in both groups, but with better renal function (RF) in the one with large donors.
This study found similar patient and graft SV in both groups, regardless of donor size. A recent study looking at the use of kidneys from small donors showed a patient SV of 97% in the first year for small donors (< 15Kg), and 98% for large donors (> 15Kg), while graft SV in both groups was 93% and 94% respectively.16 Studies that followed pDDKTs with small and large donors found no significant difference in graft SV between them, which throws light on the use of grafts from small donors, and the importance of using these kidneys, especially in the face of organ shortages for this population.1,16,17 Graft losses were more frequent in the first month of KT, with VT as the main cause, without a significant difference when comparing small and large donors. The post-PKT complications, known as vascular, include VT and RAS.18 Advances in immunosuppressant treatments have reduced the incidence of graft rejection, making vascular complications a relevant factor in graft loss, which may occur in the renal artery of the donor, the recipient, or in the suture region.19
In this study, VT was the most frequent cause of graft loss in both groups. A previous study from the same service,10,11 found 4 (6.4%) cases of VT in pDDKTs in small recipients (< 15Kg). Similarly to the present results, other studies found 4.08% to 6.6% cases of VT with small donors, and 3.6% to 3.27% with large donors. Although some studies do not associate donor size with a risk factor for VT,1,16 others claim that the use of kidneys from small donors under 20 kg may present a greater risk of VT, vascular complications, and acute tubular necrosis.20
VT is the most common cause of early graft loss or failure, occurring in 2% to 11% of pDDKTs. Its development has been associated with young recipient age, prior CAPD, donor and recipient blood vessel size, ischemia time, hypotension, graft hypoperfusion, and documented difficulties in hemostasis.21,22 Researchers describe higher risks for small RTRs with small donors.20,21 However, other studies, similar to the results previously obtained by this center, found no significant difference between VT cases and donor size.1,10,11,16 Prophylactic anticoagulation with heparin was used in patients who thrombosed and lost the graft in 100% of the cases in group 1, and 77.8% in group 2, with no significant difference (p = 0.471) related to graft loss. Even the administration of anticoagulants to the RTRs, according to the protocol, did not prevent thrombosis in either group. A recent study points out the use of heparin or enoxaparin in pre-established protocols for pDDKTs only in specific and rare cases of increased risk for VT, present in clinical history or laboratory findings.16 Such findings point to the need for studies on the application of anticoagulation protocols.
Antithrombotic prophylaxis, is part of the protocol for the prevention of vascular complications in patients at intermediate and high risk of VT, by the use of sodium heparin in continuous infusion 10 IU/kg/h, or Acetyl Salicylic Acid 2 mg/kg. Perioperative and postoperative anticoagulation is still controversial. The advantages and disadvantages of its use should be balanced according to the increased risk of bleeding.21 The use of anticoagulation protocols is an important tool in the prevention of VTs in pDDKTs, and should be evaluated by the transplant team, considering each child individually. A study conducted in 80 PKT centers, spread over 37 countries, pointed out that the use of anticoagulation or antithrombotic prophylaxis is still not uniform, however, heparin is the preferred medication in the early postoperative period, while ASA was chosen as prophylaxis.23
In this study, the cases of RAS requiring angioplasty and stenting were more frequent in the group with pDDKT performed with small donors, with no graft losses by RAS. The study found 7 (8.3%) cases of RAS among vascular complications in 84 pediatric patients submitted to pDDKT.24 The literature shows that RAS is a common vascular complication after transplantation that occurs between 3 months and 2 years after KT.19,25 The forms of treatment for RAS are percutaneous transluminal angioplasty (PTA), stent placement or direct surgical revascularization.25 The implementation and organization of routines in the postoperative period suggest imaging exams (such as Doppler) to evaluate the graft. A study on the management of arterial complications after transplant highlights the importance of post-surgery follow-up programs so that there can be early detection of RAS since late diagnosis can compromise revascularization and prevent full recovery of graft function25.
In this study, GFR was increasing in both groups over the first year of PKT, maintaining a GFR > 60 1 ml/min/1.73m2. However, it was significantly better in the large donors' group, similar to the previous study, which found a GFR of 76.1 ml/min/1.73m2 in the first year of transplantation in recipients who weighed < 15Kg10,11. On the other hand, a recent study evaluating pDDKTs with small donors showed, in the first year, an increase of 14 ml/min/1.73m2 in GFR with small donors (< 15Kg), while the GFR of large donors (> 15Kg) remained stable.16 Kidneys from pediatric donors tend to show a relatively stable increase in GFR over the first year, during the growth of the child and the transplanted kidney, a fact that does not occur with the kidneys from adult donors when transplanted in the pediatric population, which show a reduction in GFR without recovery, not keeping up with the needs of the growing child.16
Survival of the graft and the RTR, showed similar results in the use of kidneys from small and large donors. Graft losses due to vascular factors occurred independently of donor age, with no significant differences between the groups. The pDDKT with the use of small donors needs to be appreciated and pondered by the expert transplant teams, considering the low offer of organs for this population and the satisfactory results that have been presented for renal transplants with small donors. New studies should be conducted to analyze the use of anticoagulation protocols, in order to support and provide reliable data about their application in pDDKT.
This research did not receive any specific grant from any public, private, or non-profit funding agency; all expenses were covered by the authors' own funds.