This study intends to provide a quick, easy, and inexpensive way to assess nuclear abnormalities such as micronuclei and bud frequencies; binucleated, karyorrhectic, karyolytic, pycnotic, and condensed chromatin cells in nasal scrapings of infants, which are particularly important for conducting genotoxic studies related to the inhaled atmosphere in pediatric populations.
MethodsNasal swab samples were collected from 40 infants under 12 months of age using a small cytobrush. 2,000 cells from each infant sample were analyzed and classified according to the frequency of nuclear abnormalities.
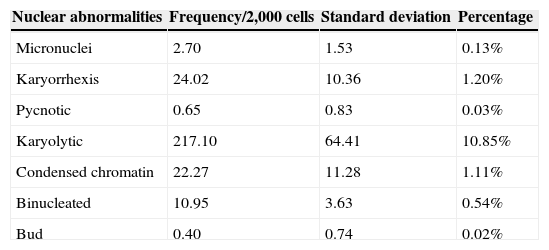
ResultsRates of nuclear abnormalities found agree with values reported in other studies of neonates and children. This study found 0.13% of cells with micronuclei; 1.20% karyorrhexis; 0.03% pyknosis; 10.85% karyolysis; 1.11% condensed chromatin; 0.54 binucleated cells; and 0.02% nuclear bud. Differences were not observed between genders or environmental passive smoking, nor was any age correlation found.
ConclusionThe assay proposed here is suitable for assessing the frequency of nuclear abnormalities from nasal cells in infants.
Este estudo pretendeu fornecer uma forma rápida, fácil e barata de avaliar alterações nucleares, como frequências de micronúcleos e brotos nucleares, células binucleadas, núcleos cariorréticos, cariolíticos, picnóticos e com cromatina condensada, em esfregaços nasais de neonatos, particularmente importante para a realização de estudos genotóxicos relacionados com a poluição ambiental em populações pediátricas.
MétodosForam coletadas amostras de esfregaço nasal de 40 neonatos com menos de 12 meses de idade, utilizando uma pequena escova citológica. Foram analisadas 2.000 células da amostra de cada neonato e classificadas de acordo com a frequência de anormalidades nucleares.
ResultadosAs taxas de anormalidades nucleares encontradas neste estudo sao compatíveis com os valores relatados em outros estudos de neonatos e crianças. Encontramos 0,13% de células com micronúcleos, 1,20% com cariorrexe, 0,03% com picnose, 10,85% com cariolise, 1,11% com cromatina condensada, 0,54 com células binucleadas e 0,02% de células com brotos nucleares. Não observámos diferenças entre os gêneros e o tabagismo passivo, assim como nenhuma correlação entre idades.
ConclusãoO ensaio proposto neste estudo é adequado para avaliar a frequência de anormalidades nucleares de células nasais em neonatos.
Studying the effects of environmental impact on exposures intends to quantify the agents present in the environment, to investigate their effect on chronic disease occurrence in exposed individuals, and to clarify the many mechanisms by which these processes occur. In this regard, there has been an increasing effort worldwide to determine the impact of environmental, genetic, and life-style factors on genomic stability.
Both in adults and children, evaluation indexes that reflect DNA damage, such as the presence of micronuclei (MN), have shown to be a useful tool that can monitor changes over increasing accidental exposures, both related to environmental and lifestyle factors. Special focus on children or infants is important, since they may have a higher sensitivity to genotoxic agents when compared to adults. Furthermore, early age genetic damage may affect the lifetime risk of adverse health outcomes. Because of that, the micronucleus assay method has been widely used to study genome damage in children after in utero and post-natal exposures in a variety of rural and urban environmental settings, resulting from maternal smoking as well as accidental industrial or technological overexposures.1–3
Through a survey of the current literature, it was observed that the frequency of nuclear abnormalities is very low at birth. However, in cases of exposure, the increase is much more pronounced when compared to adults. This corroborates the idea that there is a greater sensitivity among exposed children. The studies showed significant increases in the frequency of MN and other nuclear abnormalities in cases of children exposed to tobacco smoke indoors, living near chemical deposits, or exposed to arsenic-contaminated water and heavy metals, in addition to industrial pollutants. Despite this, the long-term action of certain chronic diseases and strong treatments like chemotherapy has not yet been conceptualized.
The use of MN as a measure of chromosome damage was first proposed by Countryman and Heddle (1976)4 in peripheral blood lymphocytes, and it is the method of choice for evaluation of genotoxicity in human populations.5 However, most other investigators were interested in analyses of exfoliated cells from buccal or nasal mucosae in order to gather data for surveys of occupational exposure.6 The authors suggested the suitability of these collection sites because they are in close contact with harmful environmental agents and can be collected non-invasively, which is particularly important when conducting pediatric studies. In exfoliated cells, the presence of MN indicates extra-nuclear cytoplasmatic bodies associated with chromosomal aberrations. These are induced by a variety of substances, including carcinogenic compounds present in tobacco smoke. The induction by cells with MN of carcinogens and mutagens is a sign of the genotoxic effect of such substances.7
Through the micronucleus assay in exfoliated cells, it is also possible to evaluate some nuclear abnormalities such as breakage or loss of genetic material by measuring the presence of MN or nuclear buds (also known as “broken eggs”), cytokinesis defects represented by binucleate cells, and cell death when presenting very small nuclei (pyknosis) or condensed chromatin, or even completely losing their nuclear materials (known as karyorrhectic and karyolytic cells).8
Even though there are several studies about environmental effects upon exfoliated cells, studies that evaluate the presence of cells with MN or other nuclear abnormalities in nasal epithelium specifically in children or infants are rare. However, subjects in these age groups have been the target of many studies evaluating DNA damage in exfoliated buccal cells and/or peripheral blood lymphocytes in specific situations of exposure, such as ionizing radiation,9 biomass burning,10 or in cases of Down syndrome.11 Others used blood collection in order to assess DNA damage in healthy children.12
A recent study found no increase of anomalies that reflect chromosomal damage (MN, binucleates, nuclear buds), but significantly higher rates of nuclear aberrations that are indicative for cytotoxicity (karyolysis, karyorrhexis, condensed chromatin) were observed in the workers. These effects were more pronounced in nasal cells than in buccal cells.13
Presently, the few existing studies have shown inconclusive results in cytogenetic toxicity in humans due to different exposure levels, misleading factors, and protocol differences among laboratories. Thus, considering the importance of evaluating the effects of the environment on the health, focusing mainly on air pollution in very young children, and also the need to standardize a feasible method for researching the presence of nuclear abnormalities in exfoliated cells, this study aimed to present a quick, easy, and inexpensive way to assess nuclear abnormalities in nasal scrapings of infants.
MethodsAfter approval by the Ethics Committee (CEP/UFCSPA), nasal swab samples were collected from 43 infants under 12 months of age who were hospitalized for other conditions not affecting the airways. Children with underlying associated diseases were excluded, such as congenital heart disease, immunodeficiency, chronic lung disease, neuromuscular diseases, cytogenetic syndromes, or those who required mechanical ventilation.
A questionnaire was applied to the parents who accompanied the infant at the time of sample collection. The questions were developed based on the “Global Adult Tobacco Survey (GATS): Core Questionnaire with Optional Questions”,14 created by the World Health Organization (WHO), validated in 2010, and with changes related to pregnancy and infant exposure to tobacco smoke.
The tool chosen for collecting the samples was a small cytobrush, commercially manufactured for dental practices. It was used specifically because of its nylon bristles (important for breaking off cells easily) and suitable size for being inserted in the nostrils of such small children, and also to fit entirely in a microtube. The cell collection was performed by pediatric residents, under preceptor doctor supervision. The brushes were inserted in the nostrils of infants up to the middle turbinate, so that the bristles facing the nasal septum were bent and the bristles on the other side came in contact with the wing of the nose. In this position, they were rotated in the nasal cavity in order to obtain the largest possible amount of material cells from the epithelium. Later, the cytobrushes were stored in microtubes containing 1.0mL of saline solution (0.08 NaCl) and identified accordingly. The materials were kept refrigerated (4°C) for a maximum of 12hours.
In the laboratory, the microtubes were centrifuged for 10minutes at 1,500 rotations per minute (RPM), followed by three washes of the pellets with 1.5mL of Carnoy's fixative solution (methanol 3:1 acetic acid), always suspending the material. In the last washing, the suspension of the pellet was performed with only 1.0mL of Carnoy's fixative solution in order to further concentrate the cells. At the end of the process, three slides were made for each patient, each with approximately 0.3mL of the cell suspension material. The slides were pre-washed and stored in 70% ethanol in order to obtain better adhesion. When in use, the slides were washed again with tap water and then with distilled water. While they were still wet with distilled water, 0.3mL of the solution containing cells in suspension was dripped onto the slides. Then, they were allowed to dry at room temperature and, later, they were stained with a 10% Giemsa solution in phosphate buffered saline (PBS) pH 7.0. Finally, the slides were rinsed in tap water and air-dried.
The slide analyses were performed with an optical microscope at 400X and 1,000X magnification with the addition of mineral oil. Only basal and differentiated cells were considered in the analysis. Two thousand basal and differentiated cells from each infant sample were analyzed and classified according to the frequency of micronuclei, nuclear buds, binucleated, pycnotic, karyorrhectic and karyolytic cells, and condensed chromatin, following the evaluation criteria suggested by Knasmueller et al.8 and Thomas et al.15
The studied variable departed significantly from normality and therefore the non-parametric Mann–Whitney U-test was applied to data. The associations between two variables were analyzed by Spearman correlation. The level of significance was considered as p ≤0.05. All analyses were conducted using the SPSS for Windows (IBM Corp, Armonk, USA), version 17.0.
ResultsThe age of the infants ranged from 14 days to 12 months (mean 4.28±3.14 months). 28 (70.0%) were males. No reactions of infants to the procedures were observed. No cases of irritation were observed. Discomfort at the time of collection occurred due to the infant's fright and, in most cases, crying.
The mean frequency of nuclear abnormalities is presented in Table 1. Only three slides were ruled out for their low cellularity (<2,000; thus, the analysis considered 40 infants. There was no significant correlation between nuclear abnormalities and age (in months) (Spearman's correlation), nor differences between genders (Mann-Whitney U-test).
Frequency of nuclear abnormalities from infant nasal cells.
| Nuclear abnormalities | Frequency/2,000 cells | Standard deviation | Percentage |
|---|---|---|---|
| Micronuclei | 2.70 | 1.53 | 0.13% |
| Karyorrhexis | 24.02 | 10.36 | 1.20% |
| Pycnotic | 0.65 | 0.83 | 0.03% |
| Karyolytic | 217.10 | 64.41 | 10.85% |
| Condensed chromatin | 22.27 | 11.28 | 1.11% |
| Binucleated | 10.95 | 3.63 | 0.54% |
| Bud | 0.40 | 0.74 | 0.02% |
Regarding exposure to passive smoking, according to the questionnaire data, only 11 mothers indicated cigarette smoking before and after pregnancy, including nine who admitted smoking during pregnancy. In the family room, 20 mothers admitted that there were other household residents who smoke. Statistical analysis (Mann-Whitney U-test) also showed no differences on nuclear abnormalities among infants who were passively exposed to cigarette smoke and those who were not.
DiscussionIn the context of genotoxicity analysis in humans, most studies employ analyses of peripheral blood lymphocytes and the buccal epithelium. Reticulocytes or nasal cells are rarely used.1 Instead, this study assessed exfoliative cells, since they have been more utilized due to the non-invasive collection methods and to the fact that the frequency of nuclei abnormalities directly reflects the damage rate in the target tissues.15,16 The nasal cells analysis through the micronucleus assay presented itself as adequate once the visualization of complete cells became possible, capable of reflecting nuclear alterations.
In the present study, nuclear abnormalities were easily observed in cells from smears stained with the 10% Giemsa solution in PBS pH 7.0. However, bacteria and cell debris are misleading factors that may mask the effect of the micronuclei or buds as compared to other staining tests like Papanicolau's, Feulgen's, or Wright's stain.6,17–19 Nevertheless, this difficulty can be resolved using microscope resources. The bacteria stained with Giemsa and observed through the microscope light differ from micronuclei or buds because they look brighter, are smaller, and are grouped together with a stronger color. Likewise, cell debris can be differentiated from micronuclei or buds by adding PBS, which provides a neutral pH and also because the debris reacts differently when stained compared to the main nucleus.
Nuclear abnormality frequency was analyzed in 2,000 cells similar to other studies, which have analyzed 2,000 cells or more.19 Only three slides were ruled out for their low cellularity (<2,000). Despite the lower number of cells, in the present study it was possible to assess the presence of nuclear abnormalities in cells from the nasal cavities of infants, which are smaller than those of adult individuals, and also are more difficult to collect since the brush may be uncomfortable for the children and they may have to be controlled by their parents.
Many other studies employed micronucleus assay in child populations using invasive methods such as blood samples,1,20,21 or in newborns using umbilical cord blood.22 Despite using Giemsa as stain, the findings presented here correspond with others in the literature, considering comparisons with older children who already were more significantly exposed to environmental factors, because none of the current studies linked the presence of MN or other nuclear abnormalities in the first year of life with environmental factors.
At the present time, it is difficult to define the real biological significance of nuclei abnormality frequency in infants. Comparisons regarding gender only emphasize the lack of differences between males and females, especially among children so young and not yet suffering the action of hormonal factors. However, their predictive value for disease requires studies combining data from different exposure, such as the environmental factors of the infant and the mother's lifestyle.1 Therefore, the frequency of nuclei abnormalities in nasal cells from infants was not associated with smoking habits of same-residence inhabitants.
There are no studies of nasal cells from infants, although rates of nuclear abnormalities found here agree with values reported in other studies19 of neonates and children (0.13% micronuclei; 1.20% karyorrhexis; 0.03% pyknosis; 10.85% karyolysis; 1.11% condensed chromatin; 0.54 binucleated cells; 0.02% nuclear bud). Evaluating lymphocytes and buccal cells, other authors suggest that micronuclei frequency was low at birth and increased in children 1-4 years of age.23 According to literature data, there is still controversy over the baseline frequency of micronuclei and other nuclear abnormalities, and it is less clear when evaluated in exfoliated epithelial cells, mainly in regard to age and gender.24
The mean micronuclei levels in exfoliative buccal cells reported by two other studies25,26 in 0- to 6-year-old children differed by more than 0.2-fold, suggesting a major impact of environmental factors and technical variability on buccal cell micronuclei frequency in human studies. In another study that involved lymphocytes and buccal cells of children and their mothers, no statistically significant difference by age was observed, due to a broad range of inter-individual variability.15
Atmospheric air pollution effects appear to be especially significant in children, who are more sensitive than adults because their bodies are still in a development stage.27 Interestingly, in a study with children of various ages, the strongest effect of air pollution on micronuclei frequency was observed in the youngest individuals.28 This observation is consistent with the potentially greater sensitivity of children to environmental exposures.29
This is the first infant biomonitoring study to identify and quantify nuclei abnormalities in nasal cells from infants. The results presented here may be precursors to new perspectives in understanding the environmental effects in nasal cells, and effects related to respiratory diseases in the pediatric population. Although this study did not find differences in the frequency of nuclear abnormalities among infants who lived with exposure to cigarette smoke, the micronucleus assay of nasal cells was also able to detect other nuclear abnormalities.
The assay proposed here is suitable for assessing the frequency of MN in infants. Therefore, the authors suggest that further studies should be conducted in this field of evaluation, to help establish the predictive value of nuclear changes in the elucidation of disease processes and population monitoring, providing better health conditions for both infants and the elderly.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Mergener M, Rhoden CR, Amantéa SL. Nuclear abnormalities in cells from nasal epithelium: a promising assay to evaluate DNA damage related to air pollution in infants. J Pediatr (Rio J). 2014;90:632–6.
Study conducted at Universidade Federal de Ciências da Saúde de Porto Alegre (UFCSPA), Porto Alegre, RS, Brasil.