To verify the presence of anti-rotavirus serotype G9P[5] SIgA and the virus neutralization capacity of milk samples from Brazilian women.
MethodsSIgA antibody levels reactive to rotavirus G9 were determined in 30 maternal milk samples by enzyme-linked immunosorbent assay (ELISA) using purified virus suspensions. The samples’ capacity to neutralize rotavirus G9P[5] was analyzed using the MA-104 cells neutralization assay.
ResultsGreat individual variations were observed regarding the SIgA levels and neutralization titers, but all samples showed some G9P[5] neutralizing ability. A highly significant positive correlation was observed between antibody levels and neutralization titers.
ConclusionsThe high correlation between anti-rotavirus antibody levels and neutralizing capacity of the milk samples suggests a possible protective role of these antibodies against infection. These results also support the encouragement of the breast-feeding practice.
Verificar a presença de SIgA anti-rotavírus sorotipo G9P[5] e a capacidade de neutralização do vírus de amostras de leite de mulheres brasileiras.
MétodosForam determinados os níveis de anticorpos IgAS reativos contra rotavírus G9 em 30 amostras de leite materno por ELISA usando suspensões purificadas do vírus. A capacidade das amostras de neutralizarem o rotavírus G9P[5] foi analisada em ensaio de neutralização utilizando células MA-104.
ResultadosForam observadas grandes variações individuais referentes aos níveis de IgAS e títulos de neutralização, mas todas as amostras mostraram certa capacidade de neutralizar o G9P[5]. Verificamos uma correlação positiva altamente significativa entre os níveis de anticorpos e os títulos de neutralização.
ConclusõesA alta correlação entre níveis de anticorpos anti-rotavírus e a capacidade neutralizante das amostras de leite sugere um possível papel protetor desses anticorpos contra a infecção. Esses resultados também apoiam o incentivo à prática do aleitamento materno.
Rotaviruses are among the major causes of gastroenteritis affecting young children worldwide.1 The causative rotavirus agent has a capsid structure with two protein layers. The outer capsid is composed of two structural proteins that determine the rotavirus serotype classification: VP7 (glycoprotein, G types) and VP4 (protease-sensitive, P types); both induce neutralizing and protective antibodies. The inner capsid contains the structural protein VP6, which determines the rotavirus serogroup.2 The G9 serotype is frequently detected in humans, and was the second most frequent G type among rotavirus diseases and strains circulating in Latin America and the Caribbean from 1990 to 2009.3 High occurrences of the G9 strain have been detected in Brazil since 1998.4,5
Children have an immature immune system, and they depend on the antibodies that they receive from their mothers through the placenta during pregnancy or from the colostrum and breast milk after birth to protect them against infections.6 However, there is no current consensus regarding the role of maternal antibodies against rotavirus infections. Some studies have reported lower infection rates among infants that have been exclusively breast-fed, when compared to non-breast-fed infants,7,8 while other studies have failed to detect any protective effect of breast-feeding.9,10
Other reports investigating anti-rotavirus antibodies in human milk have suggested that they may interfere with anti-rotavirus vaccine efficiency by neutralizing the virus particles present in the vaccine before they can multiply within the intestinal cells, thus diminishing the immunological potential of the vaccine.11,12
A pentavalent human-bovine rotavirus vaccine containing the serotypes G1, G2, G3, G4, and G9 is currently under development at the Butantan Institute (Brazil) for human use. The aim of this study was to investigate the presence of SIgA antibodies reactive to the rotavirus serotype G9P[5] in milk samples from Brazilian mothers and their capacity to neutralize virus particles, since it may affect the immunization efficiency of vaccines containing the G9 serotype.
MethodsThe presence of SIgA anti-rotavirus serotype G9P[5] (human vaccine strain) was analyzed by enzyme-linked immunosorbent assay (ELISA) in 30 milk samples collected from healthy nursing mothers, aged between 19 and 38 years, who had given birth at the Hospital Universitário da Universidade de São Paulo, which mostly attends to patients from lower socio-economic levels, a group that is highly representative of the Brazilian female population. All samples were manually collected in the morning, between breast-feedings. Informed consent from the patients and approval from the Ethics Committee of the Universidade de São Paulo were obtained prior to the collections. The milk samples were collected from nursing mothers with good nutritional and health condition, who were not receiving any medications that might have interfered with the lactation, and had negative serology for HIV, hepatitis B, and syphilis. ELISA tests were performed by coating half of the microtiter plates with 5μg/mL of viral antigen (supernatant of MA-104-infected culture cells that had been ultracentrifuged onto a 30% sucrose cushion) and the other half with the same concentration of control antigen solution (the supernatant of ultracentrifuged non-infected cells). A milk pool was prepared from equal volumes of 50 different milk samples from the Milk Bank of the Hospital Universitário da Universidade de São Paulo and used as a positive control. The antibody levels were calculated by the difference between the optical density readings obtained with viral and control antigens. The final titers were reported as relative titers, in percentages, considering the milk pool as 100%.
The milk samples were also tested for their G9P[5]-neutralizing ability using MA-104 cell cultures. Equal volumes of milk sample serial dilutions and a suspension containing 100 TCID50/mL of G9P[5] rotavirus were mixed. After 30minutes of incubation, the mixtures were added to a confluent monolayer of MA-104 cells grown in 96-well microplates. Neutralizing antibody titers were considered as the highest dilution of the samples that showed more than 60% cytopathic effect inhibition after 48hours of incubation.
The correlations between the milk samples SIgA levels and the neutralization titers were calculated using the Spearman correlation coefficient. The confidence interval was set at 95% (95% CI) and p-values < 0.05 were considered to be statistically significant.
ResultsSIgA anti-rotavirus and neutralization titers, with maximum and minimum values, 75th and 25th percentiles, mean, and median are presented in Fig. 1. Great individual variations were observed within the SIgA titers obtained by ELISA. The neutralization titers determined by the neutralization assay also varied widely. The highest titer obtained (160) was 16 times greater than the lowest titer (10), and one sample showed a discrepant value.
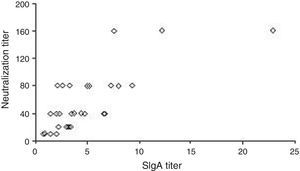
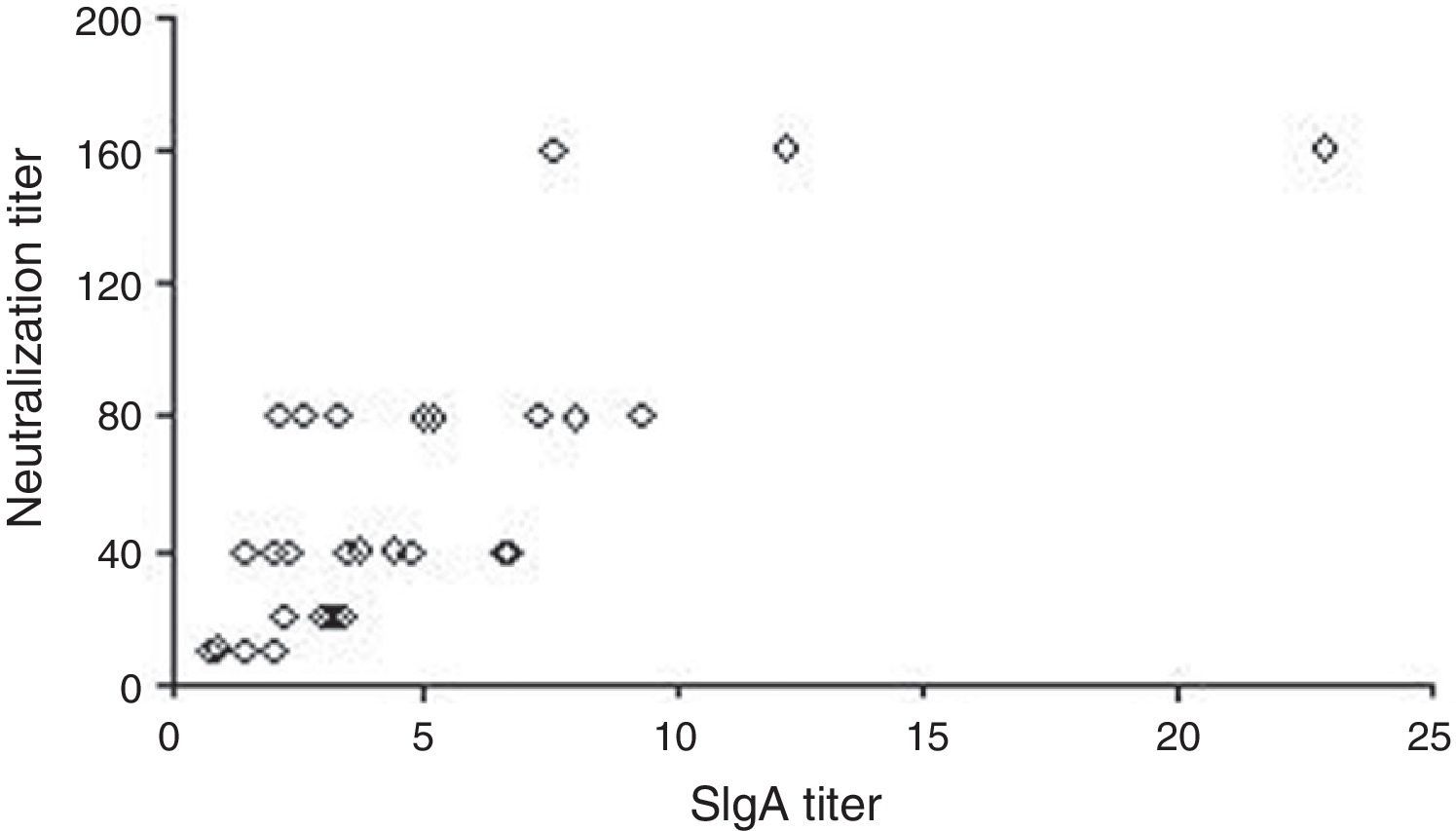
Fig. 2 shows the correlation between SIgA and neutralization titers. One sample showed very high IgA and neutralization titers discrepant from the others. A high significant and positive Spearman correlation coefficient (r=0.740, p<0.0001) was observed in the statistical analysis comparing the two parameters.
DiscussionThe levels of protection induced by currently available anti-rotavirus vaccines appear to vary depending on the locality where they have been administered. The protection conferred by vaccination diminished significantly in many countries in Africa and Asia compared to more developed nations, such as Finland.13 However, the factors that influence the low immunogenicity of these vaccines are not yet clear. One possible explanation could be the presence of anti-rotavirus antibodies in the milk of nursing mothers in developing countries where breast-feeding is a more common practice. Due the presence of these antibodies, if the infants are nursed within short periods of time either before or after oral vaccination, the immunological benefits could be compromised.11,12 Thus, it becomes important to study the anti-rotavirus antibody repertoire present in the breast milk that may potentially interfere with the immunogenicity and efficacy of the vaccine that is currently under development in Brazil.
Even though the circulation of the G9 serotype is variable in developing countries (depending on the time of year and the locality), this serotype has been found to be quite prevalent in Brazil since 1998.4,5 As such, the Brazilian population has been widely exposed to this serotype, which could influence the repertoire of antibodies present in the milk of nursing mothers. This repertoire is a result of the humoral immune response to antigens to which they were exposed. The large variations in the levels of anti-rotavirus SIgA antibodies that have been observed in these women may reflect the different degrees of their exposition to the G9 serotype.
The capacity of maternal milk to neutralize the G9P[5] serotype was also evaluated. All samples showed some ability to neutralize the virus, with a broad variation in neutralization titers. The positive correlation observed between the SIgA anti-rotavirus and neutralization titers suggest that anti-rotavirus G9 antibodies play a role in virus neutralization.
However, the fact that some samples showed high SIgA titers (≥ 80) and low neutralization titers (< 5), while other presented high neutralization titers but low SIgA titers, cannot be ignored. As such, the possibility that other components of the mothers’ milk are also important in viral neutralization must be considered. For instance, lactadherin has been observed to inhibit rotavirus infection in vitro.14
The presence of SIgA anti-rotavirus G9 in mothers’ milk should not be, by any means, considered an argument against nursing. To the contrary, the antibodies present in maternal milk can potentially protect nursing infants against rotavirus infections in early age, and for this reason the practice should be encouraged, particularly in developing countries. Additionally, aiming to improve the effectiveness of immunizations, an interval between breastfeeding and vaccination should be advised.
FundingSão Paulo Research Foundation (Fundação de Amparo à Pesquisa do Estado de São Paulo - FAPESP), grant 2004/07694-9.
Conflicts of interestThe authors declare no conflicts of interest.
We thank Dr Neuza Frazatti Gallina (Butantan Institute) for providing rotavirus G9 strain.
Please cite this article as: Santos SM, Ferreira TL, Quintal VS, Carbonare SB, Tino-De-Franco M. Milk from Brazilian women presents secretory IgA antibodies and neutralizes rotavirus G9P[5]. J Pediatr (Rio J). 2013;89:510–3.



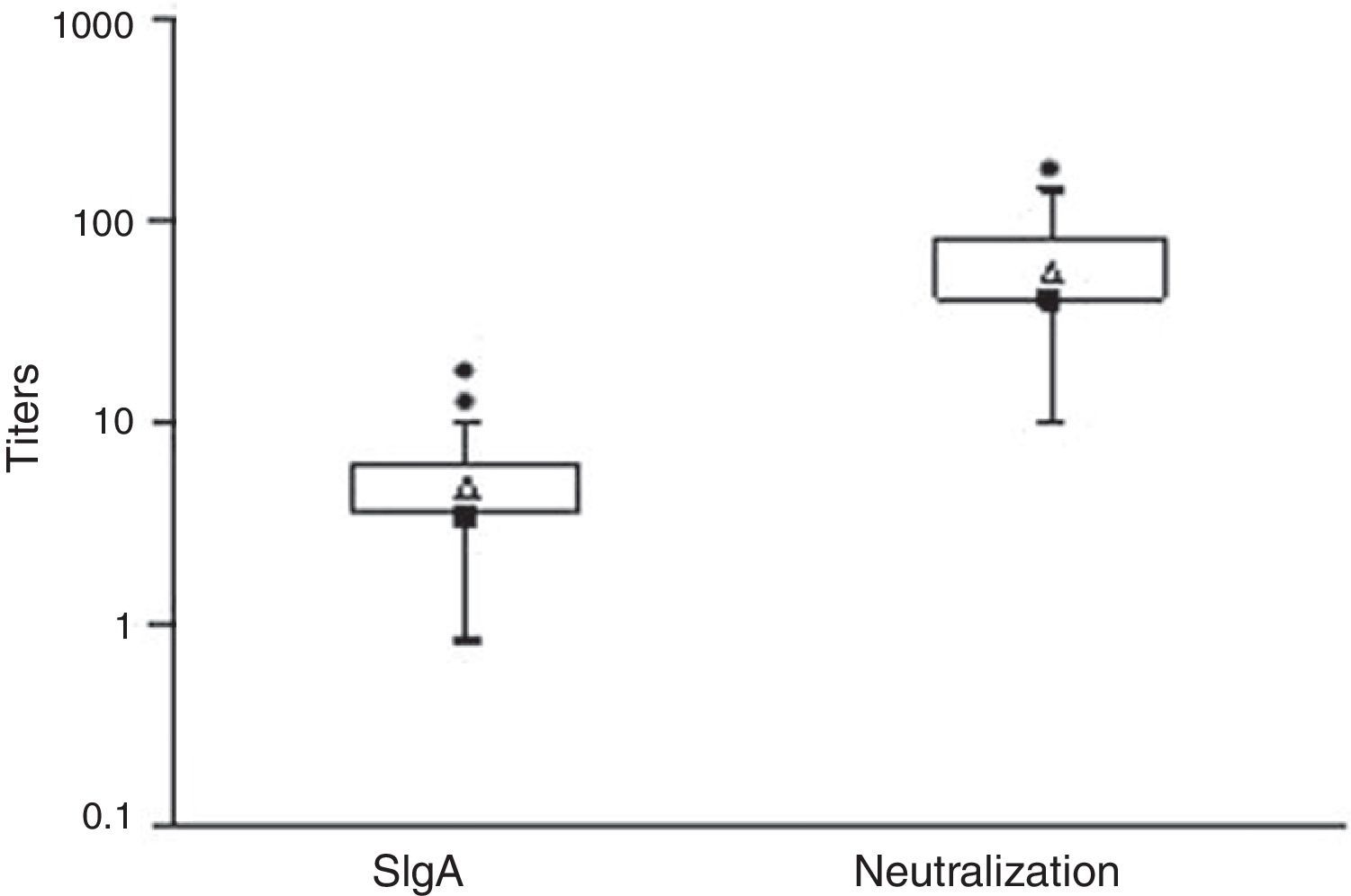
![Anti-rotavirus SIgA antibodies and neutralizing titers in milk samples. Box plots: [■] - medians; [▵] - means; [●] - discrepant values; bottom edge of the box: 25th percentile; top edge of the box - 75th percentile; bottom short line - lowest value; top short line - highest value. Anti-rotavirus SIgA antibodies and neutralizing titers in milk samples. Box plots: [■] - medians; [▵] - means; [●] - discrepant values; bottom edge of the box: 25th percentile; top edge of the box - 75th percentile; bottom short line - lowest value; top short line - highest value.](https://static.elsevier.es/multimedia/00217557/0000008900000005/v6_201502280259/S0021755713001162/v6_201502280259/en/main.assets/thumbnail/gr1.jpeg?xkr=ue/ImdikoIMrsJoerZ+w90K3EErzaXq47TPDDaeTjoE=)